Abstract
Background
Diagnosis of encapsulating peritoneal sclerosis (EPS) is based on clinical symptoms, radiologic findings, and macroscopic or histological criteria. Two diagnostic scores for radiologic findings in computed tomography (CT) scans of patients with EPS have been established in the past (by Tarzi et al and Vlijm et al). The macroscopic appearance of EPS has previously been separated into three types. The use of CT scan as a tool to predict different macroscopic phenotypes, leading to specific surgical techniques and different medical treatment, has not yet been investigated.
Methods
We retrospectively analyzed 30 patients with late-stage EPS who underwent major surgery with peritonectomy and enterolysis. The preoperative CT scans were scored according to the two aforementioned established diagnostic CT scores. The macroscopic phenotype, surgical procedure, and laboratory values at the time of surgery were evaluated. CT findings in the different macroscopic phenotypes were analyzed.
Results
All patients had highly predictive CT scores for EPS. The macroscopic Type III had significantly higher CT scores compared with the other macroscopic phenotypes. Patients with macroscopic Type I had significantly higher C-reactive protein values compared to EPS Type III. Operation time was significantly longer, and repeated surgery and intraoperative complications were more frequent in EPS Type I compared with EPS Type III (P<0.05). Using the CT score and CRP level, the sensitivities for prediction of EPS I and III were 78% and 87% with corresponding specificities of 67% and 93%.
Conclusion
Abdominal CT scans might help to identify patients with a higher risk of complications and provide important information for the surgical intervention prior to surgery.
Introduction
Encapsulating peritoneal sclerosis (EPS) is a rare but severe complication of long-term peritoneal dialysis (PD). In the late stages of EPS, patients present mostly with signs of bowel obstruction, abdominal distension with abdominal pain, and weight loss. The incidence of EPS ranges between 0.3% and 8.1%.Citation1–Citation6 Although therapy has improved over the years, EPS-related morbidity and mortality are still high (mortality ranges from 19% to 55%),Citation2–Citation4,Citation6–Citation8 especially in the 1st year after diagnosis.
More than 10 years ago, the International Society of Peritoneal Dialysis defined the criteria for the diagnosis of EPS. Over the following years, several working groups refined these criteria and added further aspects; clinical signs and symptoms, and radiological, macroscopic, and histological findings are the diagnostic pillars.Citation9–Citation14 Additionally, risk factors for the development of EPS have been described: time on PD, younger age at start of PD, smoking, glucose exposure, and frequency and severity of peritonitis.Citation2,Citation3,Citation6,Citation9,Citation15,Citation16
The earlier stages of EPS are difficult to detect because clinical symptoms are lacking, and histological and radiological findings are not specific. Changes in transporter status and ultrafiltration failure can be the first signs in the development of EPS.Citation2 As previously reported, the time from the first onset of symptoms to the requirement for surgery is quite short.Citation4,Citation17,Citation18 Evidence-based medical treatment options for patients with EPS are lacking, especially for those in the late stages of the disease.Citation7,Citation8,Citation19 Corticosteroids are a widely discussed treatment option, particularly in patients with EPS with signs of systemic inflammation, associated with elevated C-reactive protein (CRP) values.Citation13,Citation19 A proportion of patients will develop clinical signs of bowel obstruction and require major surgery;Citation20–Citation26 outcome was improved by this treatment when carried out in experienced centers.Citation20,Citation22
We recently described different macroscopic phenotypes of patients with EPS.Citation18 Type I represents the inflammatory type of the disease, whereas a resoluble, cobweb-like, interenteric sclerotic cover visible on top of the brown EPS membrane characterizes Type II. Type III is the classic type, with intestinal cocooning and a sclerotic capsular enclosing the whole intestine. Prior to surgery, computed tomography (CT) scans were routinely performed in all patients with EPS.Citation27 Recently, Tarzi et alCitation28 and Vlijm et alCitation29 established two CT scoring systems for the diagnosis of EPS. To date, the use of CT scans as a tool to predict different macroscopic phenotypes, leading to specific surgical techniques and different medical treatment, has not yet been investigated.
Patients and methods
We included patients with EPS from our referral center that underwent peritonectomy and enterolysis (PEEL) between June 2005 and December 2013. All patients fulfilled all criteria for the diagnosis of EPS,Citation14,Citation28–Citation31 and were in the late stages of the disease with a requirement for major surgery.
The study protocol was approved by a local ethics committee in Germany (#322/2009BO1, Eberhard Karls University Tübingen, Germany). All patients gave written informed consent before participating in the study.
CT scans
All patients with EPS underwent CT scans of the abdomen and pelvis within a few days prior to surgery. Two experienced observers blinded to the intraoperative findings analyzed the CT scans using the two established CT scoring systems;Citation28,Citation29 details of both scoring systems are tabulated in and . Using the CT scoring system of Tarzi et alCitation28 (), two groups of patients were defined according the median CT scores of the study group: one group with CT scores of 10–22 (high CT score) and one group with CT scores of 2.5–9 (low CT score). Owing to the dichotomous items used in the EPS scoring system of Vlijm et alCitation29 (), no groups could be defined for further statistical analysis.
Table 1 CT scan scoring system by Tarzi et al
Table 2 Cut-off values for a positive CT scan, using the CT scan scoring parameters of Vlijm et al
Macroscopic phenotypes of EPS
Three different macroscopic phenotypes of EPS have been characterized.Citation18 Type I represents peritoneum with a sticky fibrin coating on top of the EPS membranes that contain the brown and thick peritoneum with rare interenteric sclerotic membranes. Type II is characterized by a fragile, cobweb-like interenteric sclerotic cover on top of the brown EPS membrane. Type III is the classic type with intestinal cocooning, and a sclerotic capsule enclosing the whole intestine with a tendency to shrinkage, accompanied by interenteric sclerotic capsules.
Surgical procedure
PEEL is a surgical procedure that goes beyond simple adhesiolysis and decortication of the EPS membranes. PEEL is regularly performed as total enterolysis with adhesiolysis of the encapsulated intestine. During this procedure, decortication of the sclerotic membranes is performed. PEEL results in restitution of the intestinal passage and prevention of recurrent disease by decortication and resection of the peritoneum (deserosation), or, if full peritonectomy is not possible, partial deserosation of the peritoneal membrane. The primary aim is to avoid intestinal perforation or injury to the serosa during serosal suturing or bowel resection.
Statistical analysis
Continuous data are expressed as mean ± standard deviation. All continuous variables were tested for normality using the Kolmogorov–Smirnov test. The median with an interquartile range was used where the distribution was not normal. Comparisons between different groups were performed with the Mann–Whitney U test or Fisher’s exact test, as appropriate. Analysis was performed using GraphPad Prism statistical software package (San Diego, CA, USA). P<0.05 (two-tailed) was considered significant.
To predict EPS phenotypes based on the CT findings and the CRP value, a multiclass classification tree was constructed. To avoid an over-optimistic estimate of the misclassification rate, we judged its predictive ability by running a bootstrap method (R- package random Forest Survival, Regression and Classification). Similarly, the multiclass area under the curve (AUC) was estimated by cross-validation and with the help of the R-package receiver operating characteristic.
Results
Clinical information of the study group
In total, 30 patients with late-stage EPS with a requirement for major surgery were included in the study. Clinical data from the study population are summarized in . Mean age at time of diagnosis was 52±11.8 years. There was a male predominance (24 men and six women). PD duration at time of surgery was 73 months (range, 57.8–106.5) and time between cessation of PD and surgery was 10 (2–23) months. Time from onset of symptoms associated with EPS to surgery was 4.5 months (range, 1.0–11.3). At the time of surgery, 20 patients had already been switched to hemodialysis, eight patients were switched to hemodialysis at the time of surgery, and two patients had a functioning transplant. CRP levels were elevated in 28 out of 30 patients, with median CRP levels of 2.6 mg/dL (range, 1.3–9.6; normal range, 0.1–0.5). The American Society of Anesthesiologists physical statusCitation32–Citation34 was high in all patients (3±0.5), but no differences could be detected between the three different groups (P>0.05).
Table 3 Clinical data of study population
Regarding the surgical procedure, eleven patients required intestinal anastomosis due to bowel obstruction. Operation time was statistically significantly longer in EPS Type I than in EPS Type III (365±86.6 minutes vs 288±81.1 minutes) (P<0.05). The number of patients who needed reoperations (2±6 days after initial operation) was also higher in EPS Type I than in EPS Type III (P<0.05). Serosal defects with requirement for sutures or intestinal anastomosis (one patient) due to bowel injury (by the surgeon) was more frequently observed in patients with EPS Type I than in patients with EPS Types II and III (P<0.001).
Prior to surgery, six of 30 patients were treated with steroids, and only a small proportion of patients (two of 30 patients) received tamoxifen. One patient received immunosuppressive therapy after kidney transplantation. One patient was taking mycophenolate mofetil for treatment of systemic lupus erythematosus.
Radiological evaluation
CT scans prior to surgery were available for all 30 patients; 21 scans were performed at our hospital, while the remaining nine were performed by the referring physicians or in the transferring hospital. Twenty CT scans (67%) were performed using intravenous contrast medium (CM). The analysis of the two scoring systems is described in and . For the diagnosis of EPS, all patients had to have positive CT scores (with or without CM), using both of the established scoring systems.Citation28,Citation29 The details of the CT findings of our study population are summarized in and and . The median score was four (range, 3–5) on the Vlijm et al system,Citation29 and eleven (range, 8–12) on the Tarzi et al system.Citation28 In EPS Type I, only mild peritoneal calcification with extensive loculated ascites and adhesions of bowel loops was observed. CM enhancement of the peritoneum was a common finding in CM-enhanced CT scans in patients with Type III (). Bowel wall thickening, dilatation, and tethering of the bowel, particularly in the pelvis minor, were present in all three groups of patients (). Extensive calcification of the peritoneal membrane with extensive peritoneal thickening was a common finding in EPS Type III ().
Figure 1 Cross-sectional abdominal CT images of EPS Type I–III patients.
Abbreviations: CT, computed tomography; EPS, encapsulating peritoneal sclerosis; PEEL, peritonectomy and enterolysis; CM, contrast medium.
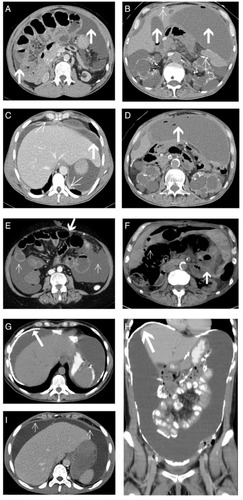
Table 4 Results of the CT scoring system in our study population using both established CT scoring systems
Table 5 CT findings in different macroscopic phenotypes of EPS (n=30)
Comparison of CT findings between the different macroscopic phenotypes
We compared the CT findings of the 30 patients with the intraoperative macroscopic phenotypes (). In all patients, bowel wall thickening, tethering of the bowel (especially in the pelvis), dilatation of bowel loops, and peritoneal thickening could be detected. In EPS Types I and II, loculated ascites was significantly more common than in EPS Type III using the Vlijm et alCitation29 score (P<0.01 and P<0.05). Furthermore, pronounced areas of calcifications of the peritoneal membrane were more common in EPS Type III than in Types II and I (P<0.01 and P<0.05, respectively) ( and ).
Using the CT score established by Tarzi et al,Citation28 patients with macroscopic Type I showed a median CT score of eight (range, 8–11), whereas patients with EPS Type II had a median score of six (range, 5–7) (P<0.05). Median CT scores of 12 could be detected in patients with EPS Type III (range, 11–15). Patients with EPS Type III had significantly higher CT scores than patients with EPS Types I and II (P<0.01 and P<0.001) (). Additionally, all patients with EPS Type III had CT scores >10 using the scoring system of Tarzi et al,Citation28 whereas none of the patients with Type II had CT scores >9. In EPS Type I, 40% of the patients were in the high CT score group. CRP levels were statistically significantly higher in these patients compared to Type III (P<0.01).
Figure 2 CT score of patients with different macroscopic phenotypes (mean ± SD) and CRP levels (median with IQR) in patients with EPS Type I–III.
Abbreviations: CT, computed tomography; SD, standard deviation; CRP, C-reactive protein; IQR, interquartile range; EPS, encapsulating peritoneal sclerosis.
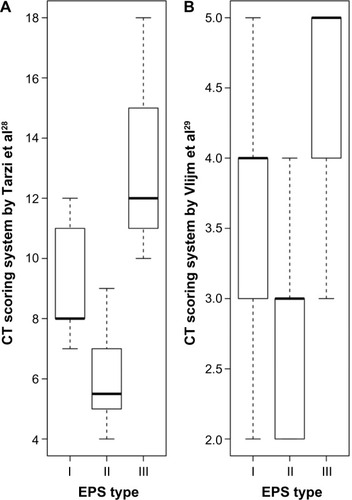
There were no significant differences in CRP levels between Types I and II or between Types II and III (P=0.9 and P=0.09).
Prediction of the macroscopic phenotype prior to surgery
Using the score from Vlijm et al,Citation29 there was an overall error rate of 50% (multiclass AUC =0.62) in the prediction of the macroscopic phenotype. After the addition of CRP levels to the radiological findings, the overall error rate decreased to 43% (multiclass AUC =0.70).
Taking into account the Tarzi et alCitation28 score, the overall error rate in the prediction of the macroscopic phenotype was 33% (multiclass AUC =0.66). Using radiological findings used in Tarzi et alCitation28 score in combination with CRP values resulted in an overall error rate of 23% (multiclass AUC =0.81).
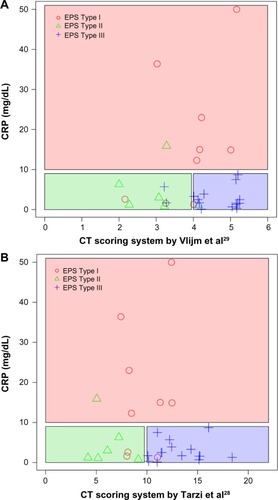
Therefore, the Tarzi et alCitation28 score in combination with CRP levels was used for further analyses. The sensitivity of the prediction of EPS Type I was 0.78 (95% confidence interval [CI] =[0.4 0.97]) with a specificity 0.76 (95% CI =[0.53 0.92]). The positive predictive value was 0.58 (95% CI =[0.28 0.85]) and the negative predictive value was 0.89 (95% CI =[0.65 0.99]), with a misclassification rate of 0.23 (95% CI =[0.1 0.42]). The sensitivity of the prediction of EPS Type II was 0.5 (95% CI =[0.12 0.88]) and the specificity was 0.96 (95% CI =[0.79 1]). The positive predictive value was 0.75 (95% CI =[0.19 0.99]) and negative predictive value was 0.88 (95% CI =[0.7 0.98]), with a misclassification rate of 0.13 (95% CI =[0.04 0.31]).
The prediction of EPS Type III had a sensitivity of 0.87 (95% CI =[0.6 0.98]) and a specificity of 0.93 (95% CI =[0.68 1]). The positive predictive value was 0.93 (95% CI =[0.66 1]), and the negative predictive value was 0.88 (95% CI =[0.62 0.98]), with a misclassification rate of 0.1 (95% CI =[0.02 0.27]) ( and ).
Figure 3 Prediction of the macroscopic phenotype based on the CT scores
Abbreviations: CT, computed tomography; CRP, C-reactive protein; EPS, encapsulating peritoneal sclerosis.
Discussion
In this retrospective single-center study, we used CT parameters from two established CT scores to investigate the differences between CT findings prior to surgery and the macroscopic phenotype in a large cohort of well-defined patients with EPS.Citation28,Citation29,Citation35 In patients with late-stage EPS with symptoms of bowel obstruction, major surgery using PEEL or using decortication and enterolysis of the peritoneum seems to be the only established treatment option.Citation4,Citation19
In the current study, statistically significant differences in the CT scores were found for the macroscopic phenotypes. In all patients with EPS, bowel wall thickening, tethering of the bowel (especially in the small pelvis of EPS patients), dilatation of bowel loops, and peritoneal thickening occur. Patients with the typical cocooning and sparse exudations of the sclerotic capsule (Type III EPS) had higher CT scores than patients with the inflammatory Type I (sticky fibrin coating on top of the peritoneum). Nearly half (40%) of patients in the Type I group had CT scores >9; in these patients, CRP levels were significantly higher than that in patients in the Type III group. Additionally, the areas of calcifications were almost exclusively detected in patients with EPS Type III. In Type I EPS, pronounced loculated ascites dominated. Therefore, the combination of CT score, CRP levels, and the presence of calcification might be a useful tool to differentiate between Types I and III EPS with a sensitivity between 78% and 87% and a specificity of 67% and 93%. Especially, the differentiation between EPS Type I and EPS Type III using simple tools (CT findings and CRP levels) has consequences in everyday clinical practice (eg, organization of the operation room plan due to different operation times depending on the macroscopic phenotype and selection of the surgeon).
In cases of requirement for major surgery, there were clear differences in the surgical techniques used for the inflammatory phenotype (Type I) and the classic phenotype (Type III), as shown by a shorter operation time for EPS Type III compared to EPS Type I.
In patients with EPS Type I, the surgical technique used is much more sophisticated because there is no separable sclerotic membrane covering the intestine, but rather a diffuse, thin, and fragile fibrin membrane, and this is reflected in the higher numbers of patients requiring suturing of serosal defects or anastomosis during surgery or reoperation in EPS Type I compared with EPS Type III. Therefore, CT scans prior to surgery should be integrated into the surgical planning process to distinguish between the different macroscopic phenotypes of EPS in order to optimize the surgical procedure.
The study has several limitations, mostly due to its retrospective character. No statistically significant differences could be detected between the groups of patients with high and low scores regarding outcome, but it should be noted that the study was not powered to find such a difference. Another limitation is the abundance of patients on hemodialysis in our cohort, and the fact that only seven patients had received a kidney transplant in the past (post-transplantation EPS). Therefore, there might be differences regarding the prediction of the macroscopic phenotype using the CT score in patients with post-transplantation EPS. As previously discussed regarding the CT score, the assessment of fluid loculation in patients still on PD or shortly after stopping PD might be challenging.
Different surgical procedures in late-stage patients with EPS have been described elsewhere.Citation22,Citation23,Citation26,Citation36 In our patient cohort, the operations were done by a single surgeon and were performed as previously described.Citation22 Therefore, differences in operation time, intraoperative complications, and the number of particular features (eg, intestinal anastomosis) might be apparent using other surgical approaches.Citation36,Citation37 Furthermore, due to the retrospective study design, we could not definitively prove that using the CT score would have changed the surgical approach. A prospective study needs to address the clinical impact of the scoring system prior to surgery. To date, the different macroscopic phenotypes of EPS have been described only in 24 patients from our referral center. There is still discussion whether these macroscopic phenotypes might represent different stages of the disease.Citation38 Nevertheless, regardless of whether the different phenotypes might represent different stages of the disease, there is growing evidence that patients should be treated individually, based on the different changes in the peritoneal membrane.Citation19 It is noteworthy that the numbers of patients included in this study are low and conclusions should therefore be treated with some caution.
In conclusion, surgical treatment of the inflammatory EPS Type I was associated with more intraoperative complications and a longer operation time with different surgical techniques. The combination of CT score, CRP levels, the presence of calcification, and the absence of fluid locations might be a useful tool to differentiate Type I from Type III prior to surgery.
Acknowledgments
The study was supported by the Robert-Bosch Foundation. DK, JL, and NB were supported by the Robert-Bosch Foundation. There were no external funding sources for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
- KawanishiHKawaguchiYFukuiHEncapsulating peritoneal sclerosis in Japan: a prospective, controlled, multicenter studyAm J Kidney Dis200444472973715384025
- BrownMCSimpsonKKerssensJJMactierRAEncapsulating peritoneal sclerosis in the new millennium: a national cohort studyClin J Am Soc Nephrol2009471222122919541815
- JohnsonDWChoYLivingstonBEEncapsulating peritoneal sclerosis: incidence, predictors, and outcomesKidney Int2010771090491220375981
- SummersAMClancyMJSyedFSingle-center experience of encapsulating peritoneal sclerosis in patients on peritoneal dialysis for end-stage renal failureKidney Int20056852381238816221244
- RigbyRJHawleyCMSclerosing peritonitis: the experience in AustraliaNephrol Dial Transplant19981311541599481732
- KorteMRSampimonDELingsmaHFDutch Multicenter EPS Study. Risk factors associated with encapsulating peritoneal sclerosis in Dutch EPS studyPerit Dial Int201131326927821454391
- BalasubramaniamGBrownEADavenportAThe Pan-Thames EPS study: treatment and outcomes of encapsulating peritoneal sclerosisNephrol Dial Transplant200924103209321519211652
- LatusJUlmerCFritzPEncapsulating peritoneal sclerosis: a rare, serious but potentially curable complication of peritoneal dialysis-experience of a referral centre in GermanyNephrol Dial Transplant20132841021103022734107
- KawaguchiYKawanishiHMujaisSTopleyNOreopoulosDGEncapsulating peritoneal sclerosis: definition, etiology, diagnosis, and treatment. International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal DialysisPerit Dial Int200020Suppl 4S43S5511098928
- KawaguchiYSaitoAKawanishiHRecommendations on the management of encapsulating peritoneal sclerosis in Japan, 2005: diagnosis, predictive markers, treatment, and preventive measuresPerit Dial Int200525Suppl 4S83S9516300277
- KorteMRSampimonDEBetjesMGKredietRTEncapsulating peritoneal sclerosis: the state of affairsNat Rev Nephrol20117952853821808281
- BraunNAlscherMDKimmelMAmannKButtnerMEncapsulating peritoneal sclerosis – an overviewNephrol Ther20117316217121458394
- HabibSMBetjesMGFierenMWEps RegistryManagement of encapsulating peritoneal sclerosis: a guideline on optimal and uniform treatmentNeth J Med2011691150050722173363
- NakamotoHEncapsulating peritoneal sclerosis – a clinician’s approach to diagnosis and medical treatmentPerit Dial Int200525Suppl 4S30S3816300270
- OulesRChallahSBrunnerFPCase-control study to determine the cause of sclerosing peritoneal diseaseNephrol Dial Transplant19883166693132642
- NakaoMYokoyamaKYamamotoIRisk factors for encapsulating peritoneal sclerosis in long-term peritoneal dialysis: a retrospective observational studyTher Apher Dial2014181687324499086
- KawanishiHHaradaYNoriyukiTTreatment options for encapsulating peritoneal sclerosis based on progressive stageAdv Perit Dial20011720020411510276
- LatusJUlmerCFritzPPhenotypes of encapsulating peritoneal sclerosis – macroscopic appearance, histologic findings, and outcomePerit Dial Int201333549550223378473
- KawanishiHSurgical and medical treatments of encapsulation peritoneal sclerosisContrib Nephrol2012177384722613913
- KawanishiHMoriishiMTsuchiyaSExperience of 100 surgical cases of encapsulating peritoneal sclerosis: investigation of recurrent cases after surgeryAdv Perit Dial200622606416983941
- KawanishiHMoriishiMIdeKDohiKRecommendation of the surgical option for treatment of encapsulating peritoneal sclerosisPerit Dial Int200828Suppl 3S205S21018552257
- UlmerCBraunNRieberFEfficacy and morbidity of surgical therapy in late-stage encapsulating peritoneal sclerosisSurgery2013153221922422981361
- KawanishiHShintakuSMoriishiMDohiKTsuchiyaSSeventeen years’ experience of surgical options for encapsulating peritoneal sclerosisAdv Perit Dial201127535822073830
- KawanishiHWatanabeHMoriishiMTsuchiyaSSuccessful surgical management of encapsulating peritoneal sclerosisPerit Dial Int200525Suppl 4S39S4716300271
- GandhiVCHumayunHMIngTSSclerotic thickening of the peritoneal membrane in maintenance peritoneal dialysis patientsArch Intern Med19801409120112037406618
- CelicoutBLevardHHayJMsikaSFingerhutAPelissierESclerosing encapsulating peritonitis: early and late results of surgical management in 32 cases. French Associations for Surgical ResearchDig Surg19981566977029845640
- UpponiSButlerAJWatsonCJShawASEncapsulating peritoneal sclerosis-correlation of radiological findings at CT with underlying pathogenesisClin Radiol201469110310924209872
- TarziRMLimAMoserSAssessing the validity of an abdominal CT scoring system in the diagnosis of encapsulating peritoneal sclerosisClin J Am Soc Nephrol2008361702171018667742
- VlijmAStokerJBipatSComputed tomographic findings characteristic for encapsulating peritoneal sclerosis: a case-control studyPerit Dial Int200929551752219776044
- BraunNFritzPUlmerCHistological criteria for encapsulating peritoneal sclerosis – a standardized approachPLoS One2012711e4864723144917
- HondaKNittaKHoritaSHistologic criteria for diagnosing encapsulating peritoneal sclerosis in continuous ambulatory peritoneal dialysis patientsAdv Perit Dial20031916917514763056
- SakladMGrading of patients for surgical proceduresAnesthesiology194119412281284
- American Society of AnesthesiologistsNew classification of physical statusAnesthesiology196324111
- KeatsASThe ASA classification of physical status – a recapitulationAnesthesiology1978494233236697075
- GoodladCTarziRGedroycWLimAMoserSBrownEAScreening for encapsulating peritoneal sclerosis in patients on peritoneal dialysis: role of CT scanningNephrol Dial Transplant20112641374137920810453
- KawanishiHSurgical treatment for encapsulating peritoneal sclerosisAdv Perit Dial20021813914312402606
- KawanishiHIdeKYamashitaMSurgical techniques for prevention of recurrence after total enterolysis in encapsulating peritoneal sclerosisAdv Perit Dial200824515518986001
- WatsonCJButlerAJBradleyJAClassification of encapsulating peritoneal sclerosis is important, but must encapsulate the entire spectrum of the diseasePerit Dial Int201333547948124133081
