Abstract
Background
Glomerular filtration rate (GFR) is considered the indicator of overall kidney function, and therefore, its assessment has become an important clinical tool in the daily care of chronic glomerulonephritis (CGN) patients. Currently, practical guidelines recommend using Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations to assess GFR in CKD patients.
Methods
A cross-sectional study was performed in CGN patients. Standard GFR was measured using 24-hour urine creatinine clearance. GFR was estimated using the Cockcroft-Gault, Modification of Diet in Renal Disease, CKD-EPI equation based creatinine, cystatin C, and combined creatinine and cystatin C. The performance of GFR estimation equations were examined using bias, precision and accuracy and agreement between standard GFR and estimated GFR by calculating Cohen’s k.
Results
A total of 125 patients (74 male, 59.2%) with mean age 56.1±18.1 years were included. Mean standard GFR was 51.6±32.2 mL/min per 1.73 m2. A significant correlation was found between standard GFR and all estimated GFRs (r=0.573 to 0.660, P<0.001). CKD-EPI-creatinine-cystatin C equation had the smallest absolute bias and the significantly highest accuracy, although it was not significantly different from CKD-EPI-cystatin C equation (P=0.523). CKD-EPI-creatinine-cystatin C equation had the highest accuracy to classify CKD staging (Cohen’s k=0.345), but it underestimated GFR in 32% and overestimated GFR in 18% of the CGN patients.
Conclusion
CKD-EPI-creatinine-cystatin C equation estimated GFR with little bias, and the highest accuracy among CGN patients. This equation gave a better estimate of GFR than the equation based on serum creatinine.
Background
Creatinine clearance has been used to estimate glomerular filtration rate (GFR) and is often used for the initial evaluation of glomerular disease.Citation1,Citation2 The estimated creatinine clearance rate can also be used to monitor the response to therapy and to initiate an early transition to dialysis therapy. However, this technique is complex, time-consuming, and difficult to perform in clinical practice.Citation3 Many equations to estimate GFR have been proposed, and estimated GFR based on serum creatinine or serum cystatin C is routinely used in the general population.Citation4 Recently, the Chronic Kidney Disease Epidemiology Collaboration has developed a new equation (CKD-EPI) based on serum creatinine and serum cystatin C.Citation5 CKD-EPI has greater precision and is preferred when estimating GFR for classified chronic kidney disease (CKD) stage.Citation6 However, to date, this equation has not been evaluated in chronic glomerulonephritis (CGN) patients.
Glomerulonephritis has a tendency to progress to CGN. The condition is characterized by irreversible and progressive glomerular and tubulointerstitial fibrosis, ultimately leading to a reduction in the GFR.Citation7 Currently, a subgroup of CKD patients such as CGN shows no clear-cut advice exists regarding which equation is the most precise for optimal estimation of GFR. Because most CGN patients receive corticosteroids treatment and present with a systemic inflammatory state, there is a potential opportunity to modify the production rate and release creatinine and cystatin C during therapy.Citation8,Citation9 These are challenging issues for these patients. We assessed the performance of the creatinine and cystatin C based estimations of Cockcroft-Gault, Modification of Diet in Renal Disease (MDRD), and CKD-EPI equations compared to a 24-hour urine creatinine clearance measurement in a study consisting of CGN patients.
Methods
This cross-sectional study was approved by the Institutional Review Board, Royal Thai Army Medical Department, and all subjects participated in the study after giving informed consent. Serum samples were assayed for serum creatinine and cystatin C. CGN patients with stable renal function and proteinuria more than 0.5 g/day from the outpatient renal clinics of Phramongkutklao Hospital, Bangkok, Thailand, were recruited. All participants had their medical history reviewed with body weight, height, and body mass index measurement.
Standard GFR measurements
All participants performed self-directed 24-hour urine collections and underwent creatinine clearance the next day, during which blood and spot urine samples were also collected. Serum and urine creatinine was analyzed using the enzymatic method, calibrated to be traceable to isotope dilution mass spectrometry. For comparison with estimated GFR equations, the measured GFR was normalized to 1.73 m2 of the body surface area (BSA) by multiplying the measured GFR by 1.73/BSA. The BSA was calculated according to Du Bois and Du Bois.Citation10 All biochemical analyses of blood samples were conducted at the Phramongkutklao Hospital Laboratory. Stratification of measured GFR was based on the stages of CKD.
Estimated GFR equations
The prediction of GFR by the Cockcroft-Gault, MDRD, CKD-EPI equation based serum creatinine, serum cystatin C and combination of serum creatinine and cystatin C were calculated. The estimated renal functions using the (abbreviated) MDRD and the CKD-EPI equations were expressed as GFR in mL/min per 1.73 m2. Serum cystatin C was analyzed using the immunonephelemetric technique (BN; Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA). summarizes all of the equations used to estimate GFRs. Different equations estimating the GFRs were compared with the results of 24-hour urine creatinine clearance as standard GFR.
Table 1 Equations used for the estimation of glomerular filtration rate
Statistical analysis
Data were expressed as mean ± standard deviation, median and its 25 to 75 interquartile for non-Gaussian variables (Kolmogorov–Smirnov test), or number and percentage. Bias, precision, accuracy, and Pearson’s correlation coefficients with respect to standard reference were calculated. Calculation of the difference between standard GFR and estimated GFR represented bias value and the standard deviation of this difference represented precision value. Accuracy was evaluated by the percentage of patients with GFR within 30% of standard GFR. Differences in estimated GFR and absolute bias and accuracy between the equations were compared with Student’s paired t-test or McNemar test, respectively. Bland–Altman plots were made to analyze whether differences between GFR and standard GFR were related to the magnitude of GFR. Patients were classified by stages of CKD according to level of standard GFR, as well as on the basis of each equation. Agreement between the standard GFR and each estimated GFR in the different stages of CKD was assessed by calculating Cohen’s k. All statistical tests were two-sided, and P<0.05 was required to reject the null hypothesis. Statistical analysis was performed using SPSS for Windows, version 16.0 (SPSS Inc., Chicago, IL, USA).
Results
A total of 125 patients with CGN were evaluated, as summarized in . The participants were all Thais, 59.2% male with mean age 56.1±18.1 years. Body mass index was 23.8±4.4 kg/m2. The cause of CGN with median proteinuria of 1.16 (interquartile range [IQR] 0.53, 2.68) g/day included the following: diabetic nephropathy (29.6%), lupus nephritis (26.4%), IgA nephropathy (14.4%), membranous nephropathy (10.4%), focal segmental glomerulosclerosis (10.4%), minimal change disease (4%) and IgM nephropathy (2.4%) and miscellaneous CGN (2.4%). Seventy-three patients (47%) received corticosteroid, and 84 (54.2%) ACE inhibitor or angiotensin 2 receptor antagonist. Mean and median serum creatinine, and serum cystatin C levels were 2.59±2.37 (1.9, IQR 1.2, 2.8) mg/dL and 1.89±1.02 (1.6, IQR 1.15, 2.38) mg/L, respectively. Mean standard GFR was 51.6±32.2 mL/min per 1.73 m2.
Table 2 Clinical features in the study population
Absolute bias, absolute precision, accuracy within 30%, and correlation coefficient between estimated GFRs and standard GFR are summarized in . A significant correlation was found between CKD-EPI-creatinine (r=0.619), CKD-EPI-cystatin C (r=0.649), CKD-EPI-creatinine-cystatin C (r=0.660), Cockcroft-Gault (r=0.573), and MDRD equation (r=0.617) with standard GFR. All equations significantly underestimated standard GFR (P<0.001) except CKD-EPI-cystatin C equation. CKD-EPI-creatinine-cystatin C equation had the smallest significant absolute bias when compared with CKD-EPI-creatinine, Cockcroft-Gault and MDRD equation (P<0.01) (). It also showed the significantly highest accuracy when compared with CKD-EPI-creatinine, Cockcroft-Gault and MDRD equation (P<0.01), although it did not significantly differ from CKD-EPI-cystatin C equation (P=0.523).
Figure 1 Bland–Altman plots of the different estimated GFR equations in comparison with 24-hour urine creatinine clearance or standard GFR.
Abbreviations: GFR, glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; SD, standard deviation; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration.
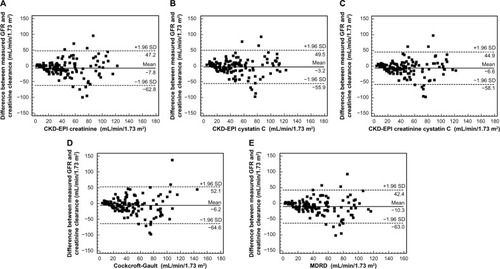
Table 3 Comparison of estimation of the different GFR equations to the standard GFR (24-hour urine creatinine clearance 51.59±32.24 mL/min/1.73 m2)
Classification of all patients according to CKD staging is summarized in . The CKD-EPI-creatinine, CKD-EPI-cystatin C, CKD-EPI-creatinine-cystatin C, Cockcroft-Gault and MDRD equation classified 33% (Cohen’s k=0.192), 43% (Cohen’s k=0.300), 50% (Cohen’s k=0.345), 38% (Cohen’s k=0.195), and 38% (Cohen’s k=0.205) of the CGN patients correctly, respectively. CKD-EPI-creatinine-cystatin C equation classified most patients correctly. It underestimated GFR in 32% and overestimated GFR in 18% of the patients.
Table 4 Comparison of classification of patients in stages of CKD according to different GFR equations with the standard GFR
Discussion
The validity of estimated GFR equations based on serum creatinine and/or cystatin C was evaluated in our cross-sectional study of CGN patients. Most of the study population included diabetic nephropathy, lupus nephropathy, and IgA nephropathy with persistent proteinuria, and received corticosteroid therapy, confirming the need for validating GFR equations in this setting. An agreement was established between standard GFR versus all equations, whereas overall mean estimated GFR equations were underestimated. This study, conducted in a clinical CGN setting, also showed that the smallest absolute bias and the highest accuracy was present in the CKD-EPI-creatinine-cystatin C equation. Our finding showed that the CKD-EPI-creatinine-cystatin C equation improved accuracy and agreement after classification in subgroups of CKD.
The CKD-EPI creatinine and/or cystatin equation has been developed and proposed to estimate GFR in CKD populations.Citation11,Citation12 Recently, an equation combining filtration markers of serum creatinine and serum cystatin C provided greater accuracy and may be useful.Citation13 The estimation of GFR in CGN remains challenging in daily practice. Initially, serum cystatin C appears to have high sensitivity for a screening test for renal injury in patients with nephrotic syndrome.Citation14 Corticosteroid therapy in glomerulonephritis influences serum and urine cystatin C levels in patients with nephrotic syndrome.Citation15 In addition, systemic inflammatory response may alter creatinine production and increase serum cystatin C levels, which could influence both the CKD-EPI creatinine and/or cystatin equation.Citation16 Our results confirmed that CKD-EPI-creatinine-cystatin C and CKD-EPI-cystatin C indicated the best option for evaluating GFR in a CGN population with 47% receiving corticosteroid treatment. In addition, the CKD-EPI-creatinine-cystatin C equation had a higher performance than the CKD-EPI-creatinine equation. This agrees with the results of Ma et al who reported that using combined equations based on serum creatinine and serum cystatin C in an Asian CKD population significantly improved GFR estimation.Citation17 Using these equations in children, young adults, the elderly, and people with cirrhosis and HIV also confirmed the high diagnostic performance.Citation18–Citation21 With our results, we can probably conclude that the CKD-EPI-creatinine-cystatin C can be used reliably in CGN patients.
The accuracy within 30% of the estimated gold standard values demonstrated the superiority of CKD-EPI-creatinine-cystatin C compared with CKD-EPI-creatinine, Cockcroft-Gault and MDRD equation. Moreover, stage misclassification was reduced by the equation based on CKD-EPI-creatinine-cystatin C. The misclassification of CKD by the combined equation was decreased from 67% to 50% compared with CKD-EPI-creatinine, and it also decreased from 62% to 50% compared with Cockcroft-Gault and MDRD equation. The results were similar in the paper published by Bevc et al, evaluating the cystatin C-based equations in comparison with 51Cr-EDTA clearance in adult patients with diabetic kidney disease.Citation22 This will help physicians to diagnose CKD more correctly, and treat CKD properly. However, the performance of CKD-EPI-creatinine-cystatin C was limited and was only slightly superior to all equations. All equations could not completely replace the “24-hour urine creatinine clearance” to estimate GFR in a population of CGN patients. These results demonstrated that the accuracy of estimated GFR formulas might not be as precise as a GFR marker in CGN with inflammatory condition and treatment with systemic corticosteroids.
The study had a few limitations. First, we should have standard GFR with insulin clearance or iohexol clearance. Endogenous creatinine clearance is correlated well with standard GFR, but creatinine is variably secreted by the proximal tubule. Therefore, endogenous creatinine clearance might overestimate true GFR, depending on the rate of tubular secretion of creatinine. Second, the stable renal function in CGN patients was considered by the nephrologists who took care of the patients, but only 78% of subjects had previous serum creatinine within 3 months of treatment. Third, our study only analyzed a Thai population, but all serum creatinine and cystatin C-based equations were developed from studies involving participants of all races. Finally, a relatively small number of patients were enrolled in the subgroups of CKD stage.
In conclusion, this study demonstrated a correlation of all estimated GFR equations in CGN patients. CKD-EPI-creatinine-cystatin C had high accuracy for estimated GFR, although the performance was close to that of CKD-EPI-cystatin C. These equations should help physicians in daily practice to assess renal function in their CGN patients.
Acknowledgments
This work was supported by a grant from the Phramongkutklao Hospital and College of Medicine, and the National Science and Technology Development Agency (NSTDA, P-13-00505), Bangkok, Thailand.
Disclosure
The authors have no conflicts of interest to declare.
References
- RatainJSPetriMHochbergMCHellmannDBAccuracy of creatinine clearance in measuring glomerular filtration rate in patients with systemic lupus erythematosus without clinical evidence of renal diseaseArthritis Rheum19903322772802306295
- LinYCBansalNVittinghoffEGoASHsuCYDeterminants of the creatinine clearance to glomerular filtration rate ratio in patients with chronic kidney disease: a cross-sectional studyBMC Nephrol20131426824305166
- SatirapojBSupasyndhOPatumanondJChoovichianPEstimating glomerular filtration rate in Asian patients with chronic kidney diseases from bioelectrical impedance analysisJ Med Assoc Thai200689101584159117128831
- InkerLASchmidCHTighiouartHEstimating glomerular filtration rate from serum creatinine and cystatin CN Engl J Med20123671202922762315
- LeveyASStevensLASchmidCHA new equation to estimate glomerular filtration rateAnn Intern Med2009150960461219414839
- LeveyASBeckerCInkerLAGlomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic reviewJAMA2015313883784625710660
- SatirapojBNastCCAdlerSGNovel insights into the relationship between glomerular pathology and progressive kidney diseaseAdv Chronic Kidney Dis20121929310022449346
- AndreevEKoopmanMAriszLA rise in plasma creatinine that is not a sign of renal failure: which drugs can be responsible?J Intern Med1999246324725210475992
- RischLHerklotzRBlumbergAHuberAREffects of glucocorticoid immunosuppression on serum cystatin C concentrations in renal transplant patientsClin Chem200147112055205911673383
- Du BoisDDu BoisEFA formula to estimate the approximate surface area if height and weight be known. 1916Nutrition1989553033112520314
- RuleADBergstralhEJSlezakJMBergertJLarsonTSGlomerular filtration rate estimated by cystatin C among different clinical presentationsKidney Int200669239940516408133
- HojsRBevcSEkartRGorenjakMPuklavecLSerum cystatin C-based equation compared to serum creatinine-based equations for estimation of glomerular filtration rate in patients with chronic kidney diseaseClin Nephrol2008701101718793543
- TidmanMSjostromPJonesIA Comparison of GFR estimating formulae based upon s-cystatin C and s-creatinine and a combination of the twoNephrol Dial Transplant200823115416017911090
- GheissariARezaiiZMerrikhiAMadihiYKelishadiRAssociation of neutrophil gelatinase associated lipocalin and cystatin-C with kidney function in children with nephrotic syndromeInt J Prev Med20134895696324049623
- TkaczykMNowickiMLukamowiczJIncreased cystatin C concentration in urine of nephrotic childrenPediatr Nephrol200419111278128015309601
- StevensLASchmidCHGreeneTFactors other than glomerular filtration rate affect serum cystatin C levelsKidney Int200975665266019119287
- MaYCZuoLChenJHImproved GFR estimation by combined creatinine and cystatin C measurementsKidney Int200772121535154217898698
- BouvetYBouissouFCoulaisYGFR is better estimated by considering both serum cystatin C and creatinine levelsPediatr Nephrol20062191299130616794818
- LopesMBAraujoLQPassosMTEstimation of glomerular filtration rate from serum creatinine and cystatin C in octogenarians and nonagenariansBMC Nephrol20131426524295505
- MindikogluALDowlingTCWeirMRSeligerSLChristensonRHMagderLSPerformance of chronic kidney disease epidemiology collaboration creatinine-cystatin C equation for estimating kidney function in cirrhosisHepatology20145941532154223744636
- Gagneux-BrunonADelanayePMaillardNPerformance of creatinine and cystatin C-based glomerular filtration rate estimating equations in a European HIV-positive cohortAIDS201327101573158123435293
- BevcSHojsREkartRZavrsnikMGorenjakMPuklavecLSimple cystatin C formula for estimation of glomerular filtration rate in overweight patients with diabetes mellitus type 2 and chronic kidney diseaseExp Diabetes Res2012201217984923008697
