Abstract
Bone strength – and, hence, fracture risk – reflects the structural and material properties of the skeleton, which changes with bone turnover during aging and following effective pharmacotherapy. A variety of powerful new techniques (quantitative computed tomography, as well as peripheral quantitative computed tomography and high-resolution peripheral quantitative computed tomography) provide precise images of bone structure and can be used to model the response of specific bones to different types of mechanical load. This review explores the various components of bone strength and the clinical significance of measures, such as bone mineral density, bone turnover markers, and modern imaging data, with regard to fracture risk in women with postmenopausal osteoporosis, before and after initiating antiresorptive therapy. These imaging and related techniques offer an ever-clearer picture of the changes in bone structure and bone mineral metabolism during normal aging and in osteoporosis, as well as in response to treatment. However, because the newer techniques are not yet available in routine practice, validated tools for absolute fracture risk assessment remain essential for clinical decision making. These tools, which are tailored to patient risk data in individual countries, are based on bone mineral density and other readily available clinical data. In addition, bone turnover marker measurements can be useful in assessing risk and guiding treatment decisions for women with postmenopausal osteoporosis. Such tests may be used before starting a patient on antiresorptive therapy and for ongoing monitoring of treatment effectiveness.
Introduction
The human skeleton is well designed to resist physical insults, but bone, like other materials, will break under a sufficiently great load. Bone strength is therefore defined as resistance to fracture, and fracture provides the most clinically meaningful indicator of bone strength in primary care. Fragility fractures – those that occur with minimal trauma, such as in falls from standing height – clearly demonstrate decreased bone strength. Indeed, a history of fragility fractures in peri- and postmenopausal women is strongly associated with a risk of future fractures.Citation1
What is bone strength?
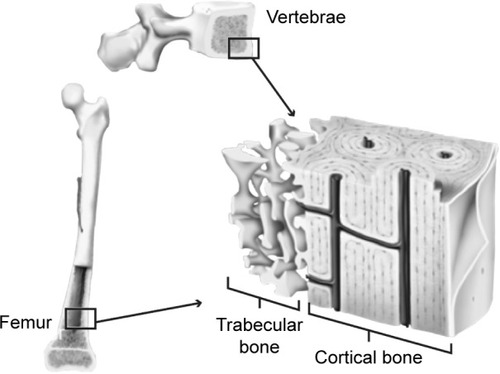
Bones are hard because they consist of hydroxyapatite crystals, set in a matrix of collagen and other connective tissue. They owe their strength not just to their composition (bone mineral density [BMD]), but also to their structure – their overall size and shape, along with their microarchitecture.Citation2 Viewed up close, every bone has a complex microarchitecture, with pores and channels running through the apparently solid cortex (). Inside the cortex is the medullary cavity, where bone tissue is not solid, but spongy in appearance, consisting of an extensive cross-linked mesh-work of projections (trabeculae).
The distribution of trabecular and cortical tissue varies across anatomical sites, with the vertebrae containing >75% trabecular bone and long bones, such as the femur, containing >75% cortical bone.Citation3 At the microarchitectural level, features also differ. Trabecular tissue varies with regard to the number of trabeculae, as well as their length, thickness, and degree of cross-linking. Cortical bone is of variable thickness and porosity (the number and size of pores). Both trabecular and cortical bone may also show microcracks as a result of mechanical loading.
These features, which greatly influence the overall mechanical strength of the bone, are all in a state of flux: bone is constantly being turned over and replaced by resident osteoclasts and osteoblasts.Citation2,Citation4 Because loss of bone strength leads to increased risk of fracture, antiresorptive agents and other interventions that reduce bone turnover can improve bone strength and reduce fracture risk in women with postmenopausal osteoporosis (PMO).Citation5,Citation6
Patterns of age-related bone loss
Women commonly experience some degree of bone loss as they age, particularly as their estrogen levels decline during and after menopause.Citation7 Along with this loss of BMD, bone microarchitecture changes in several ways, including: loss and thinning of trabeculae, which reduce cross-linking between neighboring trabeculae; expansion of the medullary cavity (cortical thinning) as the inner endosteal surface is eroded by an imbalance in bone turnover;Citation8 and increased porosity within the cortex itself.Citation9 Each of these changes will ultimately lead to loss of bone strength and increased fracture risk.
The timing of increased fracture risk varies among different women, reflecting both the peak bone mass they reached in their youth and the rate at which they lose BMD during the years around menopause.Citation10 In addition, the relative timing of mineral loss from the cortical and trabecular compartments can vary among different bones, due to differences in bone architecture and load.Citation11
In general, trabecular bone loss occurs first, largely affecting the spine and resulting in compression fractures. Although often unnoticed clinically, these fractures can lead to height loss.Citation12 Trabecular bone loss begins by age 40, when women still produce substantial levels of estrogen; approximately a third of a woman’s lifetime loss of trabecular bone occurs in the decades before menopause. Trabecular bone loss accelerates with estrogen deficiency for the first 4–8 years of menopause, after which it continues at a lower rate.Citation13
Because the vertebrae contain a large proportion of trabecular bone, it is expected that vertebral fracture risk begins to rise relatively early in life, as trabecular BMD declines.Citation14 However, the large majority (80%) of osteoporotic fractures are nonvertebral, occurring in bones, such as the wrist and the hip, where cortical thickness is a key predictor of bone strength and stiffness.Citation15 In Canada and the US, the majority of these nonvertebral fractures occur in the wrist.Citation16,Citation17
As postmenopausal women age, BMD loss moves from trabecular bone to the inner cortex, where loss of mechanical strength appears to drive the growing risk of nonvertebral fractures.Citation14,Citation18,Citation19 Changes in the cortex occur by cortical thinning and increased cortical porosity – that is, erosion of the bone tissue on the inner (endosteal) surface and also within the cortical compartment. Endosteal resorption produces porous structures resembling trabeculae.Citation7,Citation18 This process is observed in the earlier years of menopause and accelerates after age 65,Citation18 and continues at a lower rate even into extreme old age.Citation18,Citation20 Likewise, cortical porosity appears to increase in a woman during her 40s to 50s in different bones and continues indefinitely ().Citation9,Citation19 Cortical bone loss is strongly related to estrogen deficiencyCitation7 and accelerates after menopause,Citation18 playing a major role in nonvertebral fractures later in a woman’s life.
Figure 2 Postmenopausal changes in bone architecture.
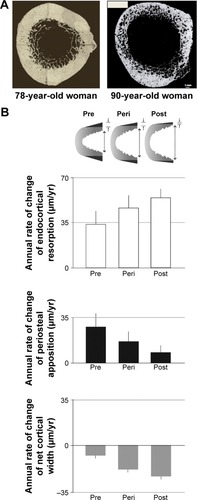
Aging is also associated with an adaptative expansion of the outer diameter of bones (periosteal apposition),Citation20–Citation22 which occurs throughout adult life. As a result of appositional growth on the outer (periosteal) surface, along with erosion on the inner (endosteal) surface, the cortex is gradually displaced outward. At the same time, the cortex thins, because periosteal growth does not keep up with endosteal resorption (). Following menopause, cortical loss accelerates and periosteal appositional growth declines. In long bones, these changes lead to substantial loss of bending strength.Citation13,Citation23
Measures of bone strength
Bone strength assessment in clinical practice generally relies on standard radiological (eg, dual-energy X-ray absorptiometry [DXA]) and laboratory tests (eg, serum bone turnover markers [BTMs]). Validated prognostic tools incorporate patient history and clinical observations, along with DXA findings, to estimate an individual’s absolute risk of fracture over the following 10 years (eg, the Canadian Association of Radiologists and Osteoporosis Canada Tool [CAROC] and the WHO Fracture Risk Assessment Tool [FRAX]). However, some newer mechanical modeling approaches that use high-resolution imaging data have had impressive success predicting whether and how a bone will fracture in a fall. Insights from these studies are important for primary care and fracture prevention, because they confirm that available treatments improve bone strength and reduce fracture risk by reversing some of the microarchitectural changes associated with aging bone.
Here, we discuss a variety of tools and measurements used to assess bone strength and fracture risk. We consider how these tools demonstrate different components of bone strength and the effects of pharmacotherapy on fracture risk in women with PMO.
Measuring bone strength: tools of the trade and what they tell us
Clinical measures of bone strength provide clues to a patient’s risk of suffering a fracture. As shown in , not all of these tools are widely used in clinical practice. However, all offer insights into the loss of BMD with aging, as well as the material and structural changes to the bone that occur during effective pharmacotherapy.
Table 1 Clinical and experimental evaluation of bone strength: some common approaches
DXA
DXA is used to assess the “areal” BMD, meaning the mass of bone mineral per unit area when this mass is projected onto a 2D surface, as in an X-ray image. By definition, a reduction in BMD indicates the extent of mineral loss from the bone, but it measures the mineral mass of cortical and trabecular bone collectively and does not identify the compartment where mineral is lost.
Uses and limitations of DXA
While DXA correlates with incident fracture rate, in the postmenopausal years 60% of nonvertebral fractures occur in women who have nonosteoporotic BMD,Citation24 highlighting a limitation of using this measure on its own as an indicator of fracture risk.Citation2 DXA scans can also be used to calculate a Trabecular Bone Score (TBS). This approach, which uses grayscale variations in the scanning data to quantify trabecular bone density, is broadly predictive of incident fractures in postmenopausal women. In some at-risk populations, such as individuals with diabetes or primary hyperparathyroidism or those receiving long-term glucocorticoid therapy, it has been suggested that the TBS can improve fracture prediction, relative to DXA alone. Conversely, in postmenopausal women, TBS so far has not proved superior to standard DXA measurements of the hip (reviewed in Bousson et alCitation25).
For practical purposes, estimates of 10-year absolute fracture risk (AFR) overcome some of the limitations of DXA by incorporating other patient data, including age, medical history, and prior fracture.Citation26,Citation27 Comorbidities that affect bone strength without necessarily changing BMD in a predictable way, such as glucocorticoid-induced osteoporosis,Citation28,Citation29 are included in these AFR tools. Canadian guidelines recommend AFR estimated by the Canadian FRAXCitation30 or CAROC tools (which are ~90% concordant) be used in preference to DXA alone to identify patients who will most benefit from pharmacotherapy.Citation26,Citation31 Recent updates of the FRAX tool for some jurisdictions now allow the user to include the TBS, if available, as a component of AFR evaluation.Citation32
Use of BMD in treatment monitoring
At the population level, antiresorptive treatment clearly reduces vertebral and nonvertebral fracture risk in PMO.Citation5,Citation6 To monitor the effectiveness of therapy, DXA scans are usually repeated every 1–3 years, with a decrease in testing once the patient appears to have responded.Citation26,Citation31
In clinical practice, maintenance or increase in BMD is traditionally taken as evidence of decreased fracture risk in patients treated with antiresorptives, such as oral bisphosphonates. However, the optimal frequency of testing and the most useful target BMD have not been established.Citation33–Citation35 Moreover, it is common in many areas to restrict follow-up DXA to a single scan unless the patient’s risk factors for fracture change.
Ongoing DXA monitoring would be justified by clear evidence showing that BMD predicts incident fracture in women on therapy, as it does in treatment-naïve women. Indeed, one study of women on antiresorptives showed that a DXA T-score that remains in the osteoporotic range (≤−2.5) predicts continued high risk of incident fracture.Citation36 In addition, prospective, placebo-controlled trials using potent antiresorptive agents have shown good correlations between BMD change and incident fracture rate.Citation5,Citation6
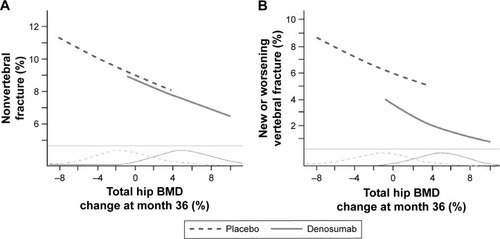
The clearest evidence of such link in women with PMO comes from a study of denosumab, an inhibitor of the receptor activator of nuclear factor kappa B ligand, which is essential for the formation, function, and survival of osteoclasts. Austin et al reported strong correlations between changes in total hip BMD and the incidences of vertebral and nonvertebral fracture over 3 years.Citation37 As shown in , total hip BMD increased in most subjects on denosumab, while declining or remaining unchanged in most placebo-treated subjects. For every 1% increase in total hip BMD, there was a 3% decrease in nonvertebral fractures () and a 4.9% decrease in new vertebral fractures (). This relationship between the increase in total hip BMD and reduced fractures persists out to 6 years of treatment.Citation38
Figure 3 Improvement in total hip BMD with 3 years of denosumab treatment predicts incident of nonvertebral (A) and vertebral (B) fractures in women.
Abbreviation: BMD, bone mineral density.
Another study, which followed women on zoledronic acid in a prospective manner over 3 years, found a similar relationship between change in BMD and fracture risk.Citation39 Based on these two studies, it appears that BMD increase can serve as a surrogate for improved bone strength in the treatment setting, at least in patients receiving relatively potent antiresorptive drugs. Thus, ongoing monitoring with DXA may provide helpful guidance in deciding whether to maintain or switch therapy.
QCT, pQCT, and HR-pQCT
Quantitative computed tomography (QCT) and related techniques use X-ray tomography to generate an average BMD for a whole bone or a specified volume within the bone.Citation40,Citation41 This volumetric BMD measurement differs from the standard (areal) BMD reported by DXA scanning, because it calculates the mass of mineral within a volume of the bone tissue, rather than a 2D projection of the bone.
Structural changes affecting bone strength can be seen using each of these imaging technologies.Citation40,Citation42–Citation44 QCT and peripheral QCT (pQCT) can show internal bone structure with a resolution of 300–500 μm.Citation40,Citation41 These techniques separate a bone’s trabecular and cortical compartments, allowing the average BMD to be determined separately for each compartment. With high-resolution pQCT (HR-pQCT), the resolution is generally ~60–82 μm.Citation45,Citation46 Thus, while pQCT is generally not able to resolve internal structures within the compartments,Citation47 HR-pQCT shows this structure with considerable detail.Citation48 Using HR-pQCT, researchers can observe and quantify specific microarchitecture changes, such as thinning of the bone cortex and the specific location (eg, the inner cortex), an increase in the size or number of cortical pores, or the loss of trabecular cross-links.Citation43
Uses and limitations of QCT, pQCT, and HR-pQCT
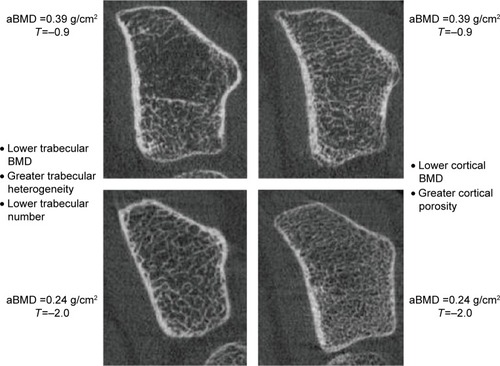
As shown in , different classes of bone mineral loss (endosteal cortical thinning, increased cortical porosity, and loss of trabeculae) may have essentially identical effects on BMD but different effects on bone strength.Citation40,Citation49,Citation50 Using HR-pQCT imaging, it is possible to improve estimates of fracture risk, particularly in women who are not in the “osteoporotic” range by DXA but have low BMD (T-scores between −1 and −2.5).Citation51
Figure 4 Identical T-scores in bones with different structural features.
Abbreviations: BMD, bone mineral density; vBMD, volumetric bone mineral density; aBMD, areal bone mineral density.
Complementing the material and structural data from HR-pQCT and pQCT measurements are several mathematical models for estimating bone strength. These analytic tools, such as finite element analysis (FEA, which models bone strength and the bone’s ability to resist fracture under different kinds of mechanical load) and polar moment of inertia (PMI, reflecting the bone’s ability to resist torsion), are adapted from engineering, where they are used to analyze the mechanical strength of various structures.
FEA has been used successfully even with low-resolution structural data from DXA scans, leading to improved prediction of hip fractures, relative to DXA alone.Citation49 However, FEA and PMI are most powerful when applied to data from higher-resolution QCT and related methods that capture cortical and trabecular structure.Citation40,Citation41,Citation43,Citation52 Using FEA to model the strength of the imaged bone, researchers can model the outcomes of specific kinds of impact, such as a femoral neck fracture resulting from an unprotected sideways fall, and the changes in these parameters when patients are treated for osteoporosis.Citation52–Citation54
In a study of older adults (average age 75 years), femoral neck BMD proved to be a good surrogate for hip strength measured by QCT FEA. This finding is significant for clinical practice because it helps confirm the value in fracture risk assessment of the femoral neck BMD T-score, which is central to the CAROC and FRAX tools.Citation55
Limitations of HR-pQCT and pQCT include motion artifacts (particularly for the distal forearm with HR-pQCT), and the inability to image clinically important sites, such as spine and hip. HR-pQCT is generally used for just two sites in the peripheral skeleton, the distal tibia and distal radiusCitation43 while pQCT can image mid-shaft tibia and occasionally mid-femur. Conversely, QCT can be used for imaging central skeletal sites, such as the spine and hip, although with its limited resolution, it provides less precise information about changes in bone microarchitecture. Another limitation is that these techniques cannot inform us regarding the nonmineral aspects of the bone matrix, such as cells and collagen, which provide toughness to the bone. Turnover of these nonmineral components is not evaluated by imaging in the clinical setting but may be assessed biochemically.
BTMs
Complementing the insights of medical imaging, BTMs are biochemical markers that can be assayed in the serum or urine to follow changes in bone remodeling. BTMs measure either bone resorption by osteoclasts (eg, the cross-linked collagen telopeptides CTX and NTX) or bone formation by osteoblasts (eg, serum procollagen type 1 N-terminal propeptide; bone-specific alkaline phosphatase; and serum osteocalcin).
Markers of bone resorption and formation tend to rise or fall together; most commonly, both are high, as in PMO; more rarely, both are low in certain “low-turnover” states, such as adynamic bone disease.Citation56,Citation57 This is because bone resorption and bone formation are tightly coupled, with osteoclasts and osteoblasts acting together on the bone surface.Citation58 However, coupling does not mean that resorption and formation always remain in balance. On the contrary, in postmenopausal women, increased bone remodeling is associated with excess bone resorption, and high BTM levels (resorption or formation markers) correlate loosely with lower BMD and reduced bone strength.Citation5,Citation59
Uses and limitations of BTMs
BTMs offer a rapid and inexpensive test for changes in bone remodeling, commonly responding within days to weeks of the initial treatment with an osteoporosis agent,Citation35,Citation58,Citation60–Citation62 much sooner than BMD changes can be measured. For this reason, they offer an early indication that the patient is responding to therapy as expected.Citation35
This reassurance may be helpful for managing patients with high AFR and high BTMs at baseline. For instance, one study followed outcomes in high-risk women on bisphosphonates (alendronate or risedronate) and found that about a fourth experienced an inadequate treatment response, defined as multiple incident fractures and/or significant BMD loss over 3 years. This outcome was significantly associated with elevated levels of the BTM bone-specific alkaline phosphatase, both at baseline and the end of the study period.Citation63
BTM responses seem to be maintained over the course of treatment, at least in clinical trials. At the population level, elevated BTMs are associated with increased fracture rates, an effect that seems to be independent of BMD in older women.Citation14,Citation35,Citation58,Citation62 In principle, these findings suggest that BTM testing could be incorporated into routine fracture risk assessment. However, validated risk assessment tools, such as FRAX, so far have not included BTMs. This is due in part to a lack of standardization of BTM assays, and in part to the substantial intra- and inter-individual variability seen with these markers, including dietary and circadian effects.Citation35,Citation58,Citation62
BTMs in treatment monitoring
BTM changes associated with treatment vary among different antiresorptive therapies, due to differences in their mechanisms and duration of action and potency.Citation26,Citation64,Citation65 No standardized set of BTM tests has been validated in women undergoing treatment for PMO.Citation33,Citation58,Citation66 Thus, it has been suggested that the primary benefit of BTM monitoring is to encourage treatment adherence.Citation59
For physicians wishing to use BTMs to monitor response to therapy, Canadian guidance suggests that serum CTX be examined before and 3–6 months after the onset of antiresorptive treatment; patients who show a <35% decrease in serum CTX should be asked about their adherence and any side effects that might be limiting their use of the therapeutic agent. Conversely, those who respond may be maintained on therapy, with regular but infrequent monitoring of serum CTX to detect changes in bone remodeling.Citation66
Impact of antiresorptive therapy on bone strength
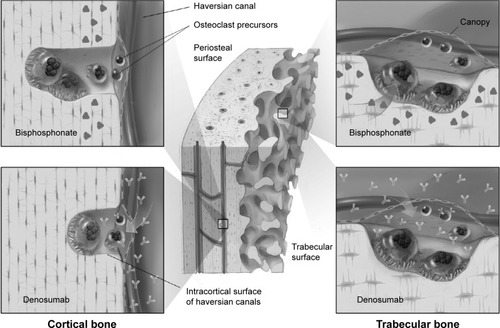
Although both denosumab and bisphosphonates are antiresorptive medications, they differ with regard to compartmental access in the bone. Bisphosphonates are preferentially adsorbed into trabecular bone, which has a high surface area per volume. Once incorporated into the bone mineral matrix exposed during the initial phase of the resorption lacunae, bisphosphonates are taken up by osteoclasts, causing apoptosis and, subsequently, reduced bone resorption. In contrast, because denosumab acts in the extracellular milieu to inhibit osteoclast formation, function, and survival, it inhibits resorption in both trabecular and cortical bone ().Citation67 The Osteoporosis Canada Clinical Practice Guidelines recommend denosumab and bisphosphonates (alendronate, risedronate, and zoledronic acid) as first-line antiresorptive therapies to significantly decrease vertebral, hip, and nonvertebral fracture risk.Citation26
Figure 5 In trabecular bone, osteoclasts engulf matrix containing alendronate, whereas denosumab accesses osteoclasts via the extracellular fluid.
Head-to-head fracture trials comparing fracture outcomes of these agents have not been conducted. However, there have been several head-to-head studies investigating the impact of denosumab compared to bisphosphonates on indices of bone strength.Citation60,Citation68–Citation71 Denosumab significantly increased BMD (by DXA) at all cortical and trabecular sites measured, relative to bisphosphonates, in subjects who were either treatment-naïveCitation60 or transitioning from a prior bisphosphonate treatment.Citation68,Citation69 At the distal 1/3 radius (a highly cortical site),Citation72,Citation73 a novel analysis of images obtained after in an HR-pQCT study showed that 1 year of denosumab treatment resulted in a more rapid and profound reduction in bone resorption, as well as a 1.5–2.0-fold greater reduction in cortical porosity, relative to alendronate ().Citation71 These findings complement data from the original 1-year study, which showed that denosumab significantly increased cortical volumetric BMD and calculated bone strength or PMI in the distal radius and distal tibia compared to alendronate.Citation70
Figure 6 Restoration of bone mass over 12 months of antiresorptive therapy in the distal radius of women with PMO.
Abbreviations: PMO, postmenopausal osteoporosis; BV/TV, bone volume over total volume.
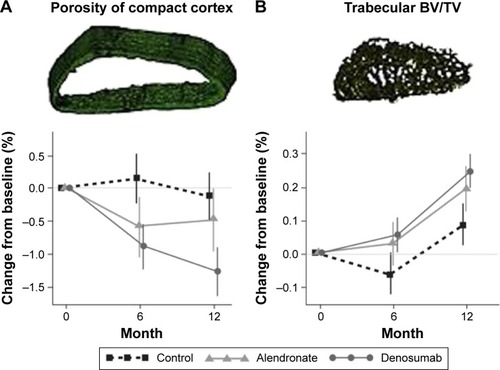
Recent observational data suggest that continued use of denosumab beyond 3 years of treatment resulted in a further significant reduction in nonvertebral fracture risk,Citation74 presumably reflecting the decreased cortical porosity and the improvement in calculated bone strength associated with this treatment.
Conclusion: goals of therapy and implications for everyday practice
The goal of osteoporosis therapy is to decrease fracture risk by improving bone strength. The available clinical data confirm that bone strength can be improved with antiresorptive treatment and that both trabecular and cortical BMD must be maintained and restored to prevent fracture in older women. Long-term studies of 7 to 10 years’ duration indicate that gains in BMD are either maintainedCitation75–Citation77 or continue to increaseCitation78 at both cortical and trabecular sites with long-term antiresorptive treatment. Similarly, BTMs decline with the onset of treatment and generally remain suppressed over long-term treatment. As a consequence of improved bone strength, fracture incidence may be suppressed over the course of long-term therapy and may even continue to decline with time.Citation74
The newer imaging technologies complement BMD and may give physicians confidence that their therapies are working as intended. While these technologies offer an extraordinary view of the changes occurring with aging, osteoporosis, and antiresorptive therapy, approaches in common use today (DXA, BTMs, and validated fracture risk assessment tools [ie, CAROC, FRAX]) still suffice for routine clinical decision making. Baseline and postinitiation DXA and BTM measurements may provide reassurance that the patient has responded to therapy and that she maintains reasonable adherence to the intended dosing.
Acknowledgments
The authors gratefully acknowledge writing assistance by John Ashkenas PhD (SCRIPT, Toronto Ontario) and support from Amgen Canada throughout the development of this manuscript. All opinions are those of the authors.
Disclosure
Dr Cheung has received grants (to UHN) and honoraria from Amgen, Eli Lilly, and Merck. Dr Frame has served as a speaker or on advisory board for Amgen, Eli Lilly, Merck, Novartis, and Sanofi. Dr Ho has served as speaker for Merck and AstraZeneca and is on advisory board for Amgen, AstraZeneca, and Tribute. Dr Mackinnon is employed by Amgen Canada. Dr Brown has received research grants, consulting fees, or speakers’ bureau fees from Abbvie, Actavis, Amgen, Eli Lilly, and Takeda. The authors report no other conflicts of interest in this work.
References
- MorinSNLixLMLeslieWDThe importance of previous fracture site on osteoporosis diagnosis and incident fractures in womenJ Bone Miner Res20142971675168024535832
- CheungAMDetskyASOsteoporosis and fractures: missing the bridge?JAMA2008299121468147018364489
- DempsterDWAnatomy and functions of the adult skeletonFavusMJPrimer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism6th edAmes, IowaASBMR2006711
- SeemanEDelmasPDBone quality – the material and structural basis of bone strength and fragilityN Engl J Med2006354212250226116723616
- HochbergMCGreenspanSWasnichRDMillerPThompsonDERossPDChanges in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agentsJ Clin Endocrinol Metab20028741586159211932287
- WasnichRDMillerPDAntifracture efficacy of antiresorptive agents are related to changes in bone densityJ Clin Endocrinol Metab200085123123610634392
- CheungAMTileLCardewSBone density and structure in healthy postmenopausal women treated with exemestane for the primary prevention of breast cancer: a nested substudy of the MAP.3 randomised controlled trialLancet Oncol201213327528422318095
- MayhewPMThomasCDClementJGRelation between age, femoral neck cortical stability, and hip fracture riskLancet2005366948012913516005335
- MacdonaldHMNishiyamaKKKangJHanleyDABoydSKAge-related patterns of trabecular and cortical bone loss differ between sexes and skeletal sites: a population-based HR-pQCT studyJ Bone Miner Res2011261506220593413
- HansenMAOvergaardKRiisBJChristiansenCRole of peak bone mass and bone loss in postmenopausal osteoporosis: 12 year studyBMJ199130368089619641954420
- BurghardtAJKazakiaGJSodeMde PappAELinkTMMajumdarSA longitudinal HR-pQCT study of alendronate treatment in postmenopausal women with low bone density: Relations among density, cortical and trabecular microarchitecture, biomechanics, and bone turnoverJ Bone Miner Res201025122558257120564242
- XuWPereraSMedichDHeight loss, vertebral fractures, and the misclassification of osteoporosisBone201148230731120870048
- KhoslaSPacificiEstrogen deficiency, postmenopausal osteoporosis, and age-related bone lossMarcusRFeldmanDDempsterDWLuckeyMCauleyJAOsteoporosis4th edSan DiegoAcademic Press201311131136
- SambrookPCooperCOsteoporosisLancet200636795272010201816782492
- MacNeilJABoydSKLoad distribution and the predictive power of morphological indices in the distal radius and tibia by high resolution peripheral quantitative computed tomographyBone200741112913717442649
- BurgeRDawson-HughesBSolomonDHWongJBKingATostesonAIncidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025J Bone Miner Res200722346547517144789
- BessetteLSte-MarieLGJeanSThe care gap in diagnosis and treatment of women with a fragility fractureOsteoporos Int2008191798617641811
- ZebazeRMGhasem-ZadehABohteAIntracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional studyLancet201037597271729173620472174
- BurghardtAJKazakiaGJRamachandranSLinkTMMajumdarSAge- and gender-related differences in the geometric properties and biomechanical significance of intracortical porosity in the distal radius and tibiaJ Bone Miner Res201025598399319888900
- AhlborgHGJohnellOTurnerCHRannevikGKarlssonMKBone loss and bone size after menopauseN Engl J Med2003349432733412878739
- SeemanEPeriosteal bone formation – a neglected determinant of bone strengthN Engl J Med2003349432032312878736
- LauretaniFBandinelliSGriswoldMELongitudinal changes in BMD and bone geometry in a population-based studyJ Bone Miner Res200823340040817997708
- SzulcPSeemanEDuboeufFSornay-RenduEDelmasPDBone fragility: failure of periosteal apposition to compensate for increased endocortical resorption in postmenopausal womenJ Bone Miner Res200621121856186317002580
- CranneyAJamalSATsangJFJosseRGLeslieWDLow bone mineral density and fracture burden in postmenopausal womenCMAJ2007177657558017846439
- BoussonVBergotCSutterBTrabecular bone score: where are we now?Joint Bone Spine201582532032525921803
- PapaioannouAMorinSCheungAM2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summaryCMAJ2010182171864187320940232
- UnnanuntanaAGladnickBPDonnellyELaneJMThe assessment of fracture riskJ Bone Joint Surg Am201092374375320194335
- Van StaaTPLaanRFBartonIPCohenSReidDMCooperCBone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapyArthritis Rheum200348113224322914613287
- AminSGabrielSEAchenbachSJAtkinsonEJMeltonLJ3rdAre young women and men with rheumatoid arthritis at risk for fragility fractures? A population-based studyJ Rheumatol201340101669167623950189
- LeslieWDLixLMLangsetmoLConstruction of a FRAX(R) model for the assessment of fracture probability in Canada and implications for treatmentOsteoporos Int201122381782721161509
- PapaioannouAMorinSCheungAMClinical Practice Guidelines for the Diagnosis and Management of Osteoporosis in Canada: Background and Technical Report Available from: http://www.osteoporosis.ca/multimedia/pdf/Osteoporosis_Guidelines_2010_Background_And_Technical_Report.pdfAccessed July 2016
- FRAX fracture risk assessment tool output can now be modified by TBS [updated May 15, 2015]. Available from: https://www.iofbonehealth.org/news/frax-fracture-risk-assessment-tool-output-can-now-be-modified-tbsAccessed June 2016
- LewieckiEMCummingsSRCosmanFTreat-to-target for osteoporosis: is now the time?J Clin Endocrinol Metab201398394695323337726
- MuncieHLeblancLLMonitoring osteoporosis treatment: DXA should not be routinely repeatedAm Fam Phys2010827752754
- WheaterGElshahalyMTuckSPDattaHKvan LaarJMThe clinical utility of bone marker measurements in osteoporosisJ Transl Med20131120123984630
- KurodaTShirakiMShirakiYTanakaSThe importance of absolute bone mineral density in the assessment of antiresorptive agents used for the prevention of osteoporotic fracturesJ Clin Densitom201215439239822521539
- AustinMYangYCVittinghoffERelationship between bone mineral density changes with denosumab treatment and risk reduction for vertebral and nonvertebral fracturesJ Bone Miner Res201227368769322095631
- MillerPCummingsSReginsterJYRelationship between changes in BMD and incidence of Fracture with 6 years of denosumab treatmentPresented at: ASMBR Annual MeetingMinneapolis, MNOctober 14, 2012 Abstract 1099; Presentation
- JacquesRMBoonenSCosmanFRelationship of changes in total hip bone mineral density to vertebral and nonvertebral fracture risk in women with postmenopausal osteoporosis treated with once-yearly zoledronic acid 5 mg: the HORIZON-Pivotal Fracture Trial (PFT)J Bone Miner Res20122781627163422532515
- EngelkeKAssessment of bone quality and strength with new technologiesCurr Opin Endocrinol Diabetes Obes201219647448223076043
- EngelkeKAdamsJEArmbrechtGClinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults: the 2007 ISCD official positionsJ Clin Densitom200811112316218442757
- NishiyamaKKShaneEClinical imaging of bone microarchitecture with HR-pQCTCurr Osteoporos Rep201311214715523504496
- CheungAMAdachiJDHanleyDAHigh-resolution peripheral quantitative computed tomography for the assessment of bone strength and structure: a review by the Canadian Bone Strength Working GroupCurr Osteoporos Rep201311213614623525967
- DhainautAHoffMSyversenUHaugebergGCortical hand bone porosity and its association with distal radius fracture in middle aged and elderly womenPLoS One201387e6840523844197
- WongACaMos Bone Quality Studies: Plans & Pilot Studies2011 Available from: http://www.camris.ca/med/document/WongAKO-SaskatoonCaMosPilots-1-7.pdfAccessed September 2014
- SCANCO MedicalXtremeCT II Available from: http://www.scanco.ch/en/systems-solutions/clinical-microct/xtremect1.htmlAccessed September 2015
- LeeDCGilsanzVWrenTALimitations of peripheral quantitative computed tomography metaphyseal bone density measurementsJ Clin Endocrinol Metab200792114248425317684050
- BouxseinMLTechnology insight: noninvasive assessment of bone strength in osteoporosisNat Clin Pract Rheumatol20084631031818431371
- NaylorKEMcCloskeyEVEastellRYangLUse of DXA-based finite element analysis of the proximal femur in a longitudinal study of hip fractureJ Bone Miner Res20132851014102123281096
- KazakiaGJBurghardtAJLinkTMMajumdarSVariations in morphological and biomechanical indices at the distal radius in subjects with identical BMDJ Biomech201144225726621071031
- BoutroySBouxseinMLMunozFDelmasPDIn vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomographyJ Clin Endocrinol Metab200590126508651516189253
- SheuYZmudaJMBoudreauRMBone strength measured by peripheral quantitative computed tomography and the risk of nonvertebral fractures: the osteoporotic fractures in men (MrOS) studyJ Bone Miner Res2011261637120593412
- GenantHKEngelkeKHanleyDADenosumab improves density and strength parameters as measured by QCT of the radius in postmenopausal women with low bone mineral densityBone201047113113920399288
- OrwollESMarshallLMNielsonCMFinite element analysis of the proximal femur and hip fracture risk in older menJ Bone Miner Res200924347548319049327
- KopperdahlDLAspelundTHoffmannPFAssessment of incident spine and hip fractures in women and men using finite element analysis of CT scansJ Bone Miner Res201429357058023956027
- WattsNBClinical utility of biochemical markers of bone remodelingClin Chem1999458 Pt 21359136810430819
- CoenGBallantiPBonucciEBone markers in the diagnosis of low turnover osteodystrophy in haemodialysis patientsNephrol Dial Transplant1998139229423029761512
- BurchJRiceSYangHSystematic review of the use of bone turnover markers for monitoring the response to osteoporosis treatment: the secondary prevention of fractures, and primary prevention of fractures in high-risk groupsHealth Technol Assess20141811118024534414
- Funck-BrentanoTBiverEChopinFClinical utility of serum bone turnover markers in postmenopausal osteoporosis therapy monitoring: a systematic reviewSemin Arthritis Rheum201141215716921507464
- BrownJPPrinceRLDealCComparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trialJ Bone Miner Res200924115316118767928
- DelmasPDMunozFBlackDMEffects of yearly zoledronic acid 5 mg on bone turnover markers and relation of PINP with fracture reduction in postmenopausal women with osteoporosisJ Bone Miner Res20092491544155119338427
- VasikaranSEastellRBruyereOMarkers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standardsOsteoporos Int201122239142021184054
- CairoliEEller-VainicherCUlivieriFMFactors associated with bisphosphonate treatment failure in postmenopausal women with primary osteoporosisOsteoporos Int20142541401141024510095
- HanleyDAAdachiJDBellABrownVDenosumab: mechanism of action and clinical outcomesInt J Clin Pract201266121139114622967310
- BoonenSFerrariSMillerPDPostmenopausal osteoporosis treatment with antiresorptives: effects of discontinuation or long-term continuation on bone turnover and fracture risk – a perspectiveJ Bone Miner Res201227596397422467094
- BrownJPAlbertCNassarBABone turnover markers in the management of postmenopausal osteoporosisClin Biochem20094210–1192994219362543
- BaronRFerrariSRussellRGDenosumab and bisphosphonates: different mechanisms of action and effectsBone201148467769221145999
- KendlerDLRouxCBenhamouCLEffects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapyJ Bone Miner Res2010251728119594293
- RouxCHofbauerLCHoPRDenosumab compared with risedronate in postmenopausal women suboptimally adherent to alendronate therapy: efficacy and safety results from a randomized open-label studyBone201458485424141036
- SeemanEDelmasPDHanleyDAMicroarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronateJ Bone Miner Res20102581886189420222106
- ZebazeRMLibanatiCAustinMDiffering effects of denosumab and alendronate on cortical and trabecular boneBone20145917317924275677
- FiroozabadiRMorshedSQualitative and quantitative assessment of bone fragility and fracture healing using conventional radiography and advanced imaging technologies – focus on wrist fractureJ Orthop Trauma2008228 SupplS83S9018753895
- WahnerHWEastellRRiggsBLBone mineral density of the radius: where do we stand?J Nucl Med19852611133913414056930
- FerrariSAdachiJDLippunerKFurther reductions in nonvertebral fracture rate with long-term denosumab treatment in the FREEDOM open-label extension and influence of hip bone mineral density after 3 yearsOsteoporos Int201526122763277126068295
- BlackDMReidIRBoonenSThe effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-pivotal fracture trial (PFT)J Bone Miner Res201227224325422161728
- BoneHGHoskingDDevogelaerJPTen years’ experience with alendronate for osteoporosis in postmenopausal womenN Engl J Med2004350121189119915028823
- MellstromDDSorensenOHGoemaereSRouxCJohnsonTDChinesAASeven years of treatment with risedronate in women with postmenopausal osteoporosisCalcif Tissue Int200475646246815455188
- PapapoulosSLippunerKRouxCThe effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension studyOsteoporos Int201526122773278326202488
- HuangAJChangCYThomasBJMacMahonPJPalmerWEUsing cone-beam CT as a low-dose 3D imaging technique for the extremities: initial experience in 50 subjectsSkeletal Radiol201544679780925652734