Abstract
Background:
Elevated triglycerides (TGs) are a common lipid disorder in the US and are associated with comorbidities such as pancreatitis, obesity, type 2 diabetes, and metabolic syndrome. TGs are generally elevated in postmenopausal women compared with premenopausal women. Meta-analysis has shown that elevated TGs are associated with an increased risk of coronary heart disease (CHD).
Objective:
This article provides a general overview of TG metabolism and reviews data on the epidemiology and risk of elevated TGs in women, as pregnancy and menopause, in particular, have been associated with unfavorable changes in the lipoprotein profile, including elevations in TGs. In addition, this review seeks to explain the recommended TG goals and treatment options for hypertriglyceridemia with an emphasis on severe hypertriglyceridemia (TGs ≥ 500 mg/dL) and its respective treatment with prescription omega-3-acid ethyl esters (P-OM3).
Methods:
MedLine was searched for articles published through August 2009 using the terms “hypertriglyceridemia” and “dyslipidemia”, with subheadings for “prevalence”, “women”, “treatment”, “guidelines”, “risk”, and “omega-3 fatty acids”. Publications discussing the epidemiology of hypertriglyceridemia, CHD risk, treatment guidelines for lipid management, or clinical trials involving P-OM3 were selected for review. The reference lists of relevant articles were also examined for additional citations.
Results:
Hypertriglyceridemia is associated with increased CHD risk. Women, especially those with polycystic ovarian syndrome, type 2 diabetes, or who are postmenopausal, should be monitored regularly for the impact of hypertriglyceridemia on their lipid profile. Cardiovascular risk of TGs can be indirectly assessed by monitoring non-high-density lipoprotein cholesterol (non-HDL-C) levels. There are multiple sets of guidelines providing recommendations for desirable low-density lipoprotein cholesterol, TG, and non-HDL-C levels. Treatment of hypertriglyceridemia includes lifestyle interventions and, if needed, pharmacologic therapy. In patients with severe hypertriglyceridemia, P-OM3 can reduce TGs by up to 45%.
Conclusion:
Physicians should regularly monitor the lipid profile of their female patients. Any lipid abnormality should be managed promptly according to established guidelines. P-OM3 provide a well-tolerated option for the treatment of severe hypertriglyceridemia.
Introduction
Lipid abnormalities, including elevated cholesterol or triglyceride (TG) concentrations, are a common finding among adult patients in the US. Prior to 1996, abnormal TGs were an underappreciated lipid disorder in the US.Citation1 However, over the past decade, TGs have gained prominence, both as an individual lipid measurement and for their effect on non-high-density lipoprotein cholesterol (non-HDL-C) levels.Citation1,Citation2 Although low-density lipoprotein cholesterol (LDL-C) is often used to assess dyslipidemia, it is now becoming clear that it is important to monitor and treat abnormal TG levels as well.Citation3,Citation4 Indeed, as an indirect measurement of apolipoprotein B (apo B)-containing TG-rich lipoproteins, the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) added non-HDL-C as a secondary goal of therapy in 2001.Citation5 This represents an emerging challenge for all health care practitioners treating female patients, including gynecologists, an increasingly popular choice as the primary care provider for many women. The increasingly fragmented approach to women’s primary health care (ie, relegating some primary care by generalists and other primary care by gynecologists) adds complexity to overall women’s health care, as it is unlikely that at any stage of life women receive comprehensive and systematic health care from only one provider.Citation6
This article reviews the impact of elevated TGs in women and the importance of monitoring TG levels and other atherogenic surrogates in women. Guidelines for patient management are reviewed, as well as the evidence supporting the use of prescription omega-3-acid ethyl esters (P-OM3) as monotherapy for severe hypertriglyceridemia (TGs ≥ 500 mg/dL) and in combination with statins in patients with persistently high TGs (200–499 mg/dL). Other treatment strategies, including diet and lifestyle modifications, are also discussed or mentioned.
Methods
MedLine was searched for articles published through August 2009 using the terms “hypertriglyceridemia” and “dyslipidemia”, with subheadings for “prevalence”, “women”, “treatment”, “guidelines”, “risk”, and “omega-3 fatty acids”. Publications discussing the epidemiology of hypertriglyceridemia, coronary heart disease (CHD) risk, treatment guidelines for lipid management, or clinical trials involving P-OM3, were selected for review. The reference lists of relevant articles were also examined for additional citations.
Triglyceride metabolism
By facilitating trafficking of fatty acids (FAs), TGs play an important role in metabolism, serving as a molecular source of energy for muscles and other cells in the body. Excess FAs not needed for energy are reassembled into TGs and stored in adipose tissue. TGs, also known as triacylglycerols, comprise glycerol esterified with three FAs (acyl groups). TGs, along with other plasma-insoluble lipids such as cholesterol, cholesteryl ester, and phospholipids, are trafficked via protein-enwrapped particles called lipoproteins.Citation7,Citation8 TGs can be derived from two pathways, the exogenous (intestinal) pathway and the endogenous (hepatic-derived) pathway.Citation7 Dietary consumption and absorption of FAs and glucose influence intestinal and hepatic metabolism of TGs.Citation9
In the exogenous pathway, lipases hydrolyze dietary TGs throughout the upper gastrointestinal tract. Once absorbed into enterocytes, FAs are reassembled into TGs and incorporated into large, apoB48-enwrapped, TG-rich lipoproteins called chylomicrons. Chylomicrons enter the lymphatic system and ultimately the circulation, where they are exposed in muscular and adipocyte tissues to lipoprotein lipase (LPL), which hydrolyzes their core TGs, causing the release of FAs and surface phospholipids. The liver actively removes the remaining chylomicron remnants (TG-poor, cholesterol-rich particles) from circulation.Citation10
In the endogenous pathway, hepatic TGs synthesized from FAs, and carbohydrate-derived glycerol and the other lipids, are packaged into apoB100-enwrapped very low-density lipoprotein (VLDL) particles.Citation7 VLDL transports TGs from the liver to peripheral tissues, such as muscle and adipocytes.Citation8 TG-rich particles shrink during lipolysis, and phospholipids, as well as certain apolipoproteins (apo C-I and -III and apo E), are exchanged with high density lipoproteins (HDL) particles. The remaining, now smaller, VLDL particles are termed remnants, or intermediate-density lipoproteins (IDLs), most of which are then removed from circulation by the liver LDL receptors.Citation10 The remaining smaller VLDLs and IDLs undergo further lipolysis via hepatic lipase and are converted to LDL particles.Citation7,Citation8 Because of its long half-life of 2–3 days, the vast majority of apo B lipoproteins are LDLs.Citation11,Citation12 Increased numbers of apo B or LDL particles not cleared by hepatic LDL receptors have increased plasma residence time and may enter the arterial intima.Citation13,Citation14 Cholesterol is also trafficked in multiple directions to and from tissues via HDL.Citation10,Citation15
In patients with elevated TGs, VLDL particles are increased in number and size and contain more lipids, especially TGs.Citation16 In insulin-resistant patients, these larger TG-rich VLDL particles have delayed lipolysis, which may be due to a lower affinity for lipase activity and tissue or hepatic receptors that promote the degradation and clearance of VLDL particles.Citation17 These TG-rich VLDL particles with increased plasma residence time transfer some of their core TG to HDL and LDL via cholesteryl ester transfer protein (CETP), in exchange for cholesteryl ester (CE).Citation13,Citation17 CETP-mediated activity decreases the core CE content but increases the core TG content of HDL and LDL particles. Subsequent exposure of TG-rich, CE-poor HDL particles and LDL particles to hepatic lipase leads to the formation of small, dense LDL particles and HDL particles.Citation18–Citation20 Small, dense HDL particles are more likely to be degraded with their surface apo A-I excreted in the urine, reducing the capacity for HDL-mediated cholesterol clearance.Citation15 Small, dense LDL particles are not readily cleared by LDL receptors and therefore accumulate, which leads to increased LDL particle (apo B) numbers.Citation8,Citation20
In patients with normal hepatic TG pools, VLDL particles are smaller in size and number, and CETP activity is decreased, lessening the transfer of TGs to LDL and HDL particles, resulting in greater LDL particle core cholesterol composition, which may increase LDL-C levels.Citation7
Triglycerides in women
Epidemiology
The classification for assessing CVD risk related to TG levels according to the NCEP ATP III defines fasting TGs < 150 mg/dL as normal, TG levels 150–199 mg/dL as moderately high risk, TG levels 200–499 mg/dL as high risk, and TG levels ≥500 mg/dL (severe hypertriglyceridemia, also referred to as very high TGs) as very high risk.Citation21 According to the National Health and Nutrition Examination Survey (NHANES) 1999–2004, about 29.6% of US women (age 20 years and older) have hypertriglyceridemia (TGs > 150 mg/dL). In the US, severe hypertriglyceridemia (TGs ≥ 500 mg/dL) occurs in about 1.7% (3.8 million) of diagnosed patients, and in about 0.8% (1.2 million) of all US females.Citation1 An analysis of the Framingham Heart Study showed that the 61% of women who had incident CHD had TGs > 200 mg/dL.Citation22 Severe hypertriglyceridemia arises from multiple causes, including rare genetic mutations that reduce the expression of LPL and/or apo C-II (Fredrickson type I and V hyperlipidemia), as well as very poorly controlled or acute onset of diabetes, nephrosis, or certain drugs.Citation21 More modest increases in TGs in women are seen with insulin resistance, type 2 diabetes (affects about 19 million people in the US), pregnancy, obesity, high-carbohydrate diets, alcohol use, hypothyroidism, and metabolic disorders (especially the polycystic ovarian syndrome), as well as the use of some prescription drugs, including antipsychotics (clozapine, olanzapine), β-blockers (atenolol, metropolol), and anti-inflammatories, among others.Citation23,Citation24
Changes in triglyceride levels in menopausal women
Metabolic changes associated with menopause and aging can be a major cause of abnormal lipid profiles in women.Citation25 An analysis of subjects in the NHANES 1999–2002 cohort reveals that although women have lower TGs than men throughout most of their life, a reversal happens in women above 60 years of age ().Citation26,Citation27 Postmenopausal women tend to have higher levels of TGs compared with premenopausal women.Citation28 TGs can be significantly influenced by menopausal status and follicle-stimulating hormone levels.Citation29 Aging has also been shown to increase TG levels.Citation29 These TG increases are likely associated with insulin resistance related to age, menopausal status, weight increase, and obesity.Citation29,Citation30 The transition through menopause is associated with selective deposition of visceral fat.Citation31 Abdominal visceral fat deposition best predicts changes in lipid parameters and insulin sensitivity.Citation32 Increased LPL activity is observed after the withdrawal of estrogens,Citation32 which leads to the elevation of the FAs locally and accumulation of abdominal fat.Citation25
Figure 1 Influence of aging on TG levels of men and women in three cultures. All TG values between the ethnic groups are significant (P < 0/001) except between Sweden and the US (P = 0.65). In Iranians, the combination of high TG was associated with elevated apo B, suggesting that increased hepatic fatty acid flux may be an important driver of the increased apo B. The increased numbers of TG-rich VLDL result in relative enrichment of LDL and HDL in TG and depletion in cholesterol ester and therefore higher apo B and apo A–I than LDL-C and HDL-C, respectively. Copyright © 2009. Elsevier. Reprinted with permission from Solhpour A, Parkhideh S, Sarrafzadegan N, et al. Levels of lipids and apolipoproteins in three cultures. Atherosclerosis. 2009;207(1):200–207.Citation26
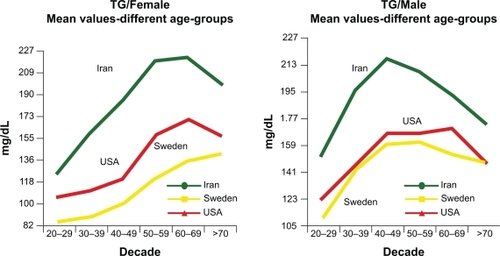
Clinical impact
TGs are a significant risk factor for CHD irrespective of LDL-C levels and other established risk factors.Citation3,Citation33 In addition, the combination of enlarged waist circumference (≥88 cm or 35 inches) and elevated TGs (≥128 mg/dL) can also be a useful marker of CVD risk in postmenopausal women.Citation34 Similar to elevated levels of fasting TGs, very high levels of nonfasting TGs may also indicate the presence of increased numbers of atherogenic apo B particles, including remnant lipoproteins and LDL, which can increase CVD risk.Citation35 Hypertriglyceridemia is also related to low HDL-C levels, another independent CVD risk factor, and both of these lipid abnormalities are components of the metabolic syndrome.Citation3,Citation36,Citation37
Hypertriglyceridemia is the third leading cause of acute pancreatitis after gallstone disease and alcohol. Severe TG levels ≥1000 mg/dL are associated with approximately 10% of all acute pancreatitis episodes and half of all cases of gestational pancreatitis. It has been suggested that high levels of circulating TG-rich lipoproteins are hydrolyzed by pancreatic lipase into FAs. The elevation in serum FAs may induce the formation of FAs, phospholipid micelles, and subsequent inflammation due to the disruption of platelets and the vascular endothelium. Hyperviscosity due to elevated serum FAs may also aggravate this condition.Citation38
Reducing triglyceride levels in women
Goals of therapy
Plasma cholesterol measurements are strong predictors of atherogenesis.Citation21 However, the measurement of apo B provides an estimate of the total burden of particles considered most atherogenic, and the use of apo B or LDL particle concentrations has been shown to be a stronger predictor of CVD risk than any other lipid concentration.Citation39 LDL-C, VLDL-C (calculated as TG/5), and, in particular, non-HDL-C lipid concentrations serve as surrogates of apo B concentration.Citation40 However, non-HDL-C is a better surrogate of all atherogenic particles than LDL-C, as it includes cholesterol within all apo B-containing particles.Citation21 The non-HDL-C value can be calculated easily by subtracting the HDL-C value from the total cholesterol (TC) value and can be assessed in the nonfasting patient.Citation2 Data from the Framingham Heart Study indicate that non-HDL-C is a better predictor of CVD risk than LDL-C, regardless of TG levels.Citation40
According to NCEP ATP III guidelines (), in patients with hypertriglyceridemia (TGs 200–499 mg/dL), LDL-C is the primary target for therapy, with non-HDL-C as a secondary treatment goal.Citation21 LDL-C goals range from <70 mg/dL in very high-risk patients to <100 mg/dL for patients with CHD or CHD equivalents and to 160 mg/dL for those without CHD risk factors.Citation41 Non-HDL-C treatment goals are 30 mg/dL (which represents a normal VLDL-C level) higher than LDL-C goals stratified by CHD risk.Citation21 In patients with severe hypertriglyceridemia (defined in guidelines as very high TGs [TGs ≥500 mg/dL]), lowering TGs is the first priority, and reducing CHD risk (LDL-C and non-HDL-C levels) is a secondary goal, once TG levels are reduced to <500 mg/dL.Citation4,Citation21
Table 1 NCEP ATP III goals for LDL-C and non-HDL-C in patients with elevated TGs, stratified by CHD risk levelCitation20,Citation41
The American Heart Association (AHA) guidelines to prevent CVD in women () state that non-HDL-C of <130 mg/dL is desirable.Citation42 In a patient who has reached her LDL-C goal but still has a high level of non-HDL-C, indicating a high level of apo B particles, residual risk remains.Citation39,Citation44 For patients with cardiometabolic risk factors, including diabetes, hypertriglyceridemia, and low HDL-C, the American Diabetes Association (ADA) and American College of Cardiology have established LDL-C, non-HDL-C, and apo B treatment goals () and state that in patients with cardiometabolic risk, pharmacological decisions should be made with consideration of their effect on apo B levels.Citation39
Table 2 Selected statements from American Heart Association guidelines for the prevention of coronary heart disease in women
Table 3 Summary of Lipid and Lipoprotein treatment recommendations from ADA/ACCFCitation39 and AACC.Citation72
Current management practices
During patient visits, physicians must be diligent in educating women regarding cardiovascular health, along with informing women that regular lipid assessments and proper management of lipid abnormalities are an important part of their overall health care. A recent national telephone-based survey revealed that confusion generated by the media (ie, inconsistent efforts by the media to faciliatate cardiovascular health awareness campaigns) is a major barrier to cardiovascular health in women (49% of respondents). In addition, 36% of respondents do not perceive themselves to be at risk for CHD, and 25% of respondents reported that their health care provider did not discuss the importance of heart health. Approximately 20% of respondents also reported that health care providers did not clearly explain how they could improve their risk status.Citation45 It is important that health care providers clearly explain cardiovascular risks to their female patients and also discuss how to reduce these risks through appropriate diet and lifestyle changes.Citation46
Although reducing LDL-C, a surrogate of apo B, has been a focus of managing lipid-related risk, it may not address the apo B particles related to TG elevation, such as remnants, small LDL particles, and TG-rich LDL. Overlooking the rest of the lipid profile can lead to the underdiagnoses and undertreatment of atherogenic apo B particles, particularly in women.Citation22,Citation47 In the US, only about one-third of physicians accept and use non-HDL-C measurements.Citation47 Increased efforts may be necessary to educate physicians about the importance of monitoring non-HDL-C in women. It is likely that the NCEP ATP IV, due for release in 2011, will elevate the importance of non-HDL-C.
Lifestyle and diet modification
Changes in lifestyle habits are first-line therapy for all lipid disorders, including elevated TGs, and include body weight control, regular physical activity, smoking cessation, restriction of alcohol use (in selected persons), and avoidance of high-carbohydrate diets.Citation21 The AHA recommends that women should accumulate at least 30 minutes of moderately intense physical activity (eg, brisk walking) preferably daily, and more exercise to lose weight.Citation42
The NCEP ATP III dietary guidelines call for caloric intake that meets daily needs without exceeding them, limited consumption of saturated and monounsaturated fats and carbohydrates, and increased consumption of fiber and plant stanols/sterols.Citation21 However, more current evidence has questioned dietary supplementation with plant sterols.Citation48 As part of adopting a healthy diet, multiple health associations, including the AHA,Citation49 the ADA,Citation50 and the US Departments of Agriculture and Health and Human Services,Citation51 recommend regular consumption of fatty fish (salmon, tuna, herring, sardines, mackerel, and trout) that provides the omega-3 FAs docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). Eating 8 oz of fatty fish in a week provides an average of ∼500 mg/d DHA and EPA.Citation52 In 1992, the AHA further recommended 2–4 g/d DHA and EPA (under a physician’s care) for patients trying to lower TGs.Citation53 However, newer data show that P-OM3 have a threshold effect on lowering TGs, and a dose of 4 g/d is required. Like other TG-modulating drugs, greater reductions are seen in those with high TGs concentrations of >500 mg/dL.Citation54 Obtaining 4 g/d of DHA and EPA through diet alone would require the consumption of ∼32–64 oz of fatty fish in a week,Citation54 which may be an impractical solution for many patients. In addition, due to concerns regarding mercury and other environmental contaminants in dietary fish, supplementation may be a more reasonable way to provide fish oil to patients.Citation55 OM3-FAs are also available in a variety of nonregulated dietary supplements. The use of purified and concentrated P-OM3 (four capsules/day, 1 g each) for patients with severe hypertriglyceridemia may improve compliance and reliability compared with dietary supplements containing fish oil, which may contain as little as 300 mg of DHA and EPA.Citation55,Citation56
Pharmacologic treatment options
In spite of dietary and lifestyle modifications, some patients require the use of medication to achieve non-HDL-C and TG treatment goals. In patients with multiple lipid abnormalities and TGs < 500 mg/dL, statins, at the dose required to achieve risk-adjusted goals, are recommended as first-line therapy after lifestyle interventions have been offered.Citation21 As an alternative option, lower-dose statins in combination with fibrates or niacin can be used. Adding ezetimibe to statins can also help achieve goals,Citation58 and bile acid sequestrants can be added if the TGs are <200 mg/dL.Citation21 Statin-treated patients who have not achieved non-HDL-C goals should be offered additional lifestyle modifications or a higher statin dose.Citation21 However, it should be noted that patients treated with statin monotherapy often meet LDL-C goals (61%) but may be less likely to meet both LDL-C and non-HDL-C goals (39%).Citation57 The addition of a fibrate, niacin, ezetimibe, or P-OM3 might be considered to help patients treated with a statin achieve non-HDL-C goals.Citation21,Citation58,Citation59
For patients with severe hypertriglyceridemia (TGs ≥ 500 mg/dL), therapeutic options recommended to lower TGs include 4 g/d P-OM3, fibrates, high doses of niacin, and, if needed, high doses of statins (both niacin and fibrates have cautionary regulatory labeling relative to their use with statins).Citation21 Although elevated TGs should be treated promptly, no evidence from well-controlled, blinded studies has shown that decreasing TGs per se with P-OM3 4 g/d or any other medication reduces CVD risk.Citation21,Citation39
Prescription omega-3-acid ethyl esters for the treatment of severe hypertriglyceridemia
Monotherapy
For treatment of severe hypertriglyceridemia, only one prescription formulation of P-OM3 (Lovaza®, a registered trademark of Reliant Pharmaceuticals, Inc., a member of the GlaxoSmithKline group of companies, Research Triangle Park, NC, USA) is US Food and Drug Administration (FDA) approved. Each 1 g capsule contains at least 900 mg P-OM3 (of which DHA comprises ∼375 mg and EPA ∼465 mg).Citation60 P-OM3 monotherapy is indicated as an adjunct to diet for adults only with severe hypertriglyceridemia (TGs ≥ 500 mg/dL). The FDA-approved 4 g/d dose significantly lowers TG and non-HDL-C levels, which appears to be due primarily to VLDL-C reduction, in patients with severe hypertriglyceridemia.Citation61–Citation63
The effects of P-OM3 4 g/d monotherapy for lowering TGs in adult patients (N = 84; 42 on P-OM3 and 42 on placebo) with severe hypertriglyceridemia were evaluated in two randomized, double-blind, placebo-controlled, parallel-group studies of 6 and 16 weeks’ duration.Citation61,Citation62 In a pooled analysis of both monotherapy studies, baseline TG levels ranged from 500 mg/dL to 2000 mg/dL with a median TG concentration of 792 mg/dL. Baseline median HDL-C and calculated LDL-C levels in these patients were 23 mg/dL and 100 mg/dL, respectively.Citation61 Treatment with 4 g/d P-OM3 resulted in median reductions of TGs, VLDL-C, and non-HDL-C of 44.9%, 41.7%, and 13.8%, respectively, and increases in LDL-C and HDL-C of 44.5% and 9.1%, respectively, from baseline levels ().Citation61 Patients treated with placebo had a median TG increase of 6.7% and reductions in VLDL-C, non-HDL-C, LDL-C, and HDL-C of 0.9%, 3.6%, 4.8%, and 0.0%, respectively, from baseline levels ().Citation61
Figure 2 Effect of P-OM3 vs placebo on lipid parameters in patients with severe hypertriglyceridemia. P values for lipid changes were TGs (P < 0.00001), VLDL-C (P < 0.0001), HDL-C (P = 0.014), and LDL-C (P = 0.0014). Copyright© 2009. GlaxoSmithKline group of companies. Adapted with permission from Copyright© 2009. GlaxoSmithKline group of companies. Adapted with permission from LOVAZA [prescribing information]. Research Triangle Park, NC: GlaxoSmithKline; 2009.Citation60
![Figure 2 Effect of P-OM3 vs placebo on lipid parameters in patients with severe hypertriglyceridemia. P values for lipid changes were TGs (P < 0.00001), VLDL-C (P < 0.0001), HDL-C (P = 0.014), and LDL-C (P = 0.0014). Copyright© 2009. GlaxoSmithKline group of companies. Adapted with permission from Copyright© 2009. GlaxoSmithKline group of companies. Adapted with permission from LOVAZA [prescribing information]. Research Triangle Park, NC: GlaxoSmithKline; 2009.Citation60](/cms/asset/0c7af8b7-a80d-4cb0-98c0-d3f1615ad733/djwh_a_16702_f0002_c.jpg)
Patients included in this pooled analysis had type IV or V hypertriglyceridemia, characterized by elevated cholesterol and TG levels associated with increased concentration of VLDL, and decreased HDL-C and LDL-C.Citation61,Citation62 Significant TG reductions in patients with hypertriglyceridemia are often accompanied by similar reductions in VLDL-C. LDL-C may increase in these patients, presumably due to more effective and rapid lipolysis and clearing of VLDL and IDL.Citation63 Reduction in VLDL-TGs reduces CETP activity, resulting in larger and more normally composed (more CE-rich, TG-poor) LDLs and HDLs, which also helps explain the rise in LDL-C and HDL-C and decrease in VLDL-C. This can also result in a net reduction in the aggregate levels of non-HDL-C. The largest increases in LDL-C can be expected to occur in patients with the highest baseline TG levels prior to P-OM3 therapy.Citation63 A similar LDL-C increase of 45% has been observed when fenofibrate was used to treat patients with type IV and V hypertriglyceridemia.Citation64 Despite the reduction in VLDL-C, there is little change in apo B levels with P-OM3 therapy.Citation59,Citation65 As with any lipid-regulating product, LDL-C levels should be monitored periodically during therapy with P-OM3.Citation60
Combination therapy with statins
Patients with elevated TGs typically have an increase in atherogenic VLDL remnants. Therefore, the residual risk in people with high TG levels cannot be accounted for by LDL-C measurements alone and should be monitored by measuring non-HDL-C (VLDL-C+LDL-C).Citation2,Citation21 In patients with hypertriglyceridemia (TGs 200–499 mg/dL), NCEP ATP III guidelines state that LDL-C is the primary goal, with non-HDL-C as a secondary target for therapy.Citation21 When LDL-C is not significantly elevated, the goal for non-HDL-C with TG-lowering agents can be obtained more easily.Citation21
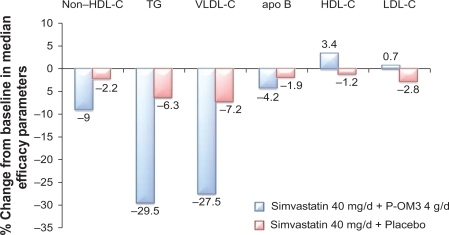
Studies have also demonstrated that the addition of P-OM3 to statin therapy leads to further reductions in non-HDL-C. In a randomized, placebo-controlled study, COMBOS (Combination of Prescription Omega-3 Plus Simvastatin), patients (N = 254) who had attained a mean LDL-C level below or within 10% of the patient’s NCEP goal with persistently high TGs (200–499 mg/dL) while taking simvastatin 40 mg/dL for 8 weeks were randomized to add P-OM3 4 g/d or placebo to their ongoing simvastatin therapy for an additional 8 weeks.Citation59 At baseline, in patients treated with P-OM3, median TG, LDL-C, and non-HDL-C levels were 268 mg/dL, 91 mg/dL, and 137 mg/dL, respectively. At the end of therapy, adding P-OM3 to the simvastatin treatment regimen reduced median non-HDL-C by 9% (vs 2.2% with placebo; P < 0.001; ).Citation59 Subjects treated with P-OM3 also had a greater decrease in median apo B concentrations beyond that induced by the statin alone (4.2% vs 1.9%; P = 0.023).Citation59 An open-label extension study in this persistently high TG (200–499 mg/dL) cohort was conducted to analyze the long-term safety and efficacy of simvastatin 40 mg/dL and P-OM3 4 g/d combination therapy for up to 24 months.Citation66 Eligible patients included those who completed the multicenter, randomized, placebo-controlled, double-blind, parallel-group study.Citation66 “Switchers” were patients who received placebo+simvastatin who were switched to P-OM3+simvastatin (n = 100). “Nonswitchers” were patients who were assigned P-OM3+simvastatin in COMBOS who remained on their original therapy (n = 88).Citation66 The difference between switchers and nonswitchers in median percentage change in non-HDL-C from COMBOS end of treatment to month 4 of the extension study was the primary endpoint of this study.Citation66 There was a greater response among switchers at month 4. Median percentage change in non-HDL-C from end of treatment was −9.4% versus 0.9% for switchers and nonswitchers (P < 0.001), respectively.Citation66 Median percentage change for switchers and nonswitchers combined at months 4 and 12 was −8.3% and 7.3%, respectively. At month 24, the median percent change in non-HDL-C for the combined groups was −8.9%.Citation67 Data indicate that sustained reductions in non-HDL-C occurred with longer-term treatment with P-OM3+simvastatin.Citation66
Figure 3 Response to the addition of P-OM3 4 g/d to ongoing simvastatin 40 mg/d therapy in patients with hypertriglyceridemia (TGs ≥ 200 mg/dL and ≤499 mg/dL). Values for differences of non-HDL-C, HDL-C, TGs, and VLDL-C between POM3 and placebo were all significant at P < 0.001 and for apo B P = 0.023. The LDL-C differences were not significant. Copyright © 2007. Elsevier. Adapted with permission from Davidson MH, Stein EA, Bays HE, et al. Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: an 8-week, randomized, double-blind, placebo-controlled study. Clin Ther. 2007;29(7):1354–1367.Citation59
An additional analysis of subjects from the original trial, examining the effects of P-OM3 4 g/d cotherapy with simvastatin 40 mg/d on lipoprotein particle concentrations, revealed that the addition of P-OM3 significantly reduced mean VLDL particle size and increased LDL particle size compared with placebo (P < 0.006 for both). HDL particle size was not altered in these subjects. The total concentrations of VLDL particles or LDL particles relative to placebo were not significantly changed; however, large VLDL particle and IDL particle concentrations were lowered (P < 0.01 for both). Total LDL-P was not affected, but the large LDL particle concentration (LDL-P) was increased and small LDL-P was reduced compared with placebo (P < 0.0001).Citation67 Remnant-like particle cholesterol, apo C-III, and Lp-PLA2 concentrations (factors associated with increased atherogenesis and CVD risk) were reduced compared with placebo (all P < 0.003),Citation67 the effects of which are consistent with those typically seen in hypertriglyceridemic subjects.Citation68 It is unknown which of these effects, if any, represent the direct effect of P-OM3.
Simultaneous initiation of simvastatin and P-OM3 treatment can also lead to a greater reduction in non-HDL-C than treatment with simvastatin alone. A randomized, placebo-controlled trial was conducted in which 39 patients with persistently high TGs (200–600 mg/dL) were given simvastatin 20 mg/d plus P-OM3 4 g/d or placebo and then crossed over to the other treatment after 6 weeks.Citation69 Cotherapy with simvastatin plus P-OM3, when compared with simvastatin plus placebo, produced greater median reductions in TGs (43.6% vs 28.7%; P < 0.001), TC (31% vs 26.4%; P < 0.01), and non-HDL-C (40% vs 34.3%; P < 0.001), whereas P-OM3 did not provide additional benefits for LDL-C (37.2% vs 38.4%).Citation69 There was no difference in median apo B reduction between simvastatin and P-OM3 treatment when compared with simvastatin plus placebo.Citation69
In an open-label extension of this study, 14 participants were treated with P-OM3 4 g/d plus simvastatin 80 mg/d for another 6 weeks.Citation70 The increase in statin dosage led to a further reduction in non-HDL-C (51.0% with P-OM3 plus simvastatin 80 mg/d vs 40.8% with P-OM3 plus simvastatin 20 mg/d; P < 0.05) without affecting TGs (58.6% with P-OM3 plus simvastatin 80 mg/d vs 54.7% with P-OM3 plus simvastatin 20 mg/d).Citation70
In addition, the effects of adding P-OM3 or placebo to escalating doses of atorvastatin (10–40 mg/d) on non-HDL-C and TG levels were assessed in 243 patients with baseline non-HDL-C > 160 mg/dL and TGs 250–599 mg/dL.Citation71 Patients received escalating doses of open-label atorvastatin according to the following dosing schedule: weeks 0–8 = 10 mg/d, weeks 9–12 = 20 mg/d, and weeks 13–16 = 40 mg/d. Patients were blinded with respect to receiving P-OM3 4 g/d or placebo for 16 weeks. P-OM3 plus atorvastatin 10, 20, and 40 mg/day led to 6.5% (P = 0.0002), 7.9% (P < 0.0001), and 4.1% (P = 0.0007) greater reductions in median non-HDL-C when compared with placebo plus the same doses of atorvastatin at the end of 8, 12, and 16 weeks, respectively. In addition, P-OM3 plus atorvastatin reduced median TC, TGs, and VLDL-C and increased HDL-C levels to a significantly greater extent than placebo plus atorvastatin. Non-HDL-C efficacy was also noninferior to doubling the statin dose in patients receiving P-OM3 plus atorvastatin.Citation71 The results to date demonstrate that treatment with P-OM3 is efficacious and well tolerated as monotherapy or as part of a combination therapy to reduce TG levels in patients with hypertriglyceridemia.
Conclusion
Hypertriglyceridemia is a common lipid abnormality in the US that becomes more prevalent in women after menopause. Additional metabolic influences, including aging, central visceral obesity, and insulin resistance, can also negatively impact a woman’s lipid profile. Treatment of severe hyper-triglyceridemia (TGs ≥ 500 mg/dL) should be a priority for all affected patients. Dietary and lifestyle interventions, including weight control, regular physical activity, smoking cessation, restricted alcohol use, and low-carbohydrate diets with increased intake of oily fish, represent the first-line option for therapy. In many cases, patients with severe hypertriglyceridemia require pharmacologic intervention. P-OM3 and fibrates are both FDA-approved options for the treatment of severe hypertriglyceridemia in adult patients. As discussed in the previous paragraphs, P-OM3 has been shown to be well tolerated and effective as monotherapy for reducing TG levels in patients with severe hypertriglyceridemia. Primary care physicians and obstetricians/gynecologists assuming a primary care role should discuss regular lipid panel testing with their patients. The NCEP recommends monitoring lipid profiles every 5 years unless the profile is abnormal, then more frequent monitoring is called for, which may include lipoprotein testing in patients with cardiometabolic risk. Regular evaluations of female patients’ lipid parameters, including TGs and non-HDL-C, as well as apo B and LDL particle concentrations, will allow for more comprehensive evaluation and treatment of lipid disorders in this population, which has historically been undertreated.
Acknowledgements
The listed author meets the criteria for authorship set forth by the International Committee for Medical Journal Editors. The author wishes to acknowledge the following individuals for their critical review during the development of this manuscript: Doug Wicks MPH, Susan Johnson MD, Rosemary Schroyer MS, and Amy Meadowcroft PharmD, all of whom are employees of GlaxoSmithKline. Editorial support in the form of development of the draft outline and the first draft of the manuscript, editorial suggestions to draft versions of this paper, assembling tables and figures, collating author comments, copyediting, fact checking, and referencing was provided by Marcus Lynch PhD and Amanda McGeary MS, at AlphaBioCom, LLC, and was funded by GlaxoSmithKline.
Disclosure
The author reports the following association with GSK: Advisor and Speaker’s Bureau
References
- FordESLiCZhaoGHypertriglyceridemia and its pharmacologic treatment among US adultsArch Int Med2009169657257819307519
- BlahaMBlumenthalRBrintonEJacobsonTThe importance of non-HDL cholesterol reporting in lipid managementJ Clin Lipidol20082426727321291742
- CastelliWPEpidemiology of triglycerides: a view from FraminghamAm J Cardiol199270193H9H
- KnoppRHParamsothyPAtkinsonBDowdyAComprehensive lipid management versus aggressive low-density lipoprotein lowering to reduce cardiovascular riskAm J Cardiol20081018A48B57B
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III)Executive summary of the third report of the National Cholesterol Education Program (NCEP)JAMA2001285192486249711368702
- HendersonJTWeismanCSWomen’s patterns of provider use across the lifespan and satisfaction with primary care coordination and comprehensivenessMed Care200543882683316034297
- PackardCJDemantTStewartJPApolipoprotein B metabolism and the distribution of VLDL and LDL subfractionsJ Lipid Res20004130531710681415
- BaysHRationale for prescription omega-3-acid ethyl ester therapy for hypertriglyceridemia: a primer for cliniciansDrugs Today (Barc)200844320524618536782
- LichtensteinAHThematic review series: patient-oriented research. Dietary fat, carbohydrate, and protein: effects on plasma lipoprotein patternsJ Lipid Res20064781661166716738356
- RaderDHobbsHChapter 350. Disorders of lipoprotein metabolismFauciABraunwaldEKasperD, eds. Harrison’s Principles of Internal Medicine http://www.accessmedicine.com/content.aspx?aID=2882429. Accessed January 12, 2011.
- BrewerHBJrHypertriglyceridemia: changes in the plasma lipoproteins associated with an increased risk of cardiovascular diseaseAm J Cardiol1999839B3F12F
- LangerTStroberWLevyRIThe metabolism of low density lipoprotein in familial type II hyperlipoproteinemiaJ Clin Invest1972516152815364336943
- CaslakeMJPackardCJPhenotypes, genotypes and response to statin therapyCurr Opin Lipidol200415438739215243210
- El HarchaouiKvan der SteegWStroesEValue of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Prospective Population StudyJ Am Coll Cardiol200749554755317276177
- DayspringTHigh-density lipoproteins: emerging knowledgeCardiometab Syndr2007215962
- GeorgievaAMvan GreevenbroekMMKraussRMSubclasses of low-density lipoprotein and very low-density lipoprotein in familial combined hyperlipidemia: relationship to multiple lipoprotein phenotypeArterioscler Thromb Vasc Biol200424474474914751815
- GrundySMHypertriglyceridemia, insulin resistance, and the metabolic syndromeAm J Cardiol1999839B25F29F
- GuerinMLe GoffWLasselTSAtherogenic role of elevated CE transfer from HDL to VLDL(1) and dense LDL in type 2 diabetes: impact of the degree of triglyceridemiaArterioscler Thromb Vasc Biol200121228228811156866
- GrundySMHypertriglyceridemia, atherogenic dyslipidemia, and the metabolic syndromeAm J Cardiol1998814A18B25B
- TothPPDayspringTDPokrywkaGSDrug therapy for hypertriglyceridemia: fibrates and omega-3 fatty acidsCurr Atheroscler Rep2009111717919080732
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III)Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final reportCirculation2002106253143342112485966
- Lloyd-JonesDMO’DonnellCJD’AgostinoRBApplicability of cholesterol-lowering primary prevention trials to a general population: the Framingham Heart StudyArch Int Med2001161794995411295957
- MoscaLJOptimal management of cholesterol levels and the prevention of coronary heart disease in womenAm Fam Phys2002652217226
- American Diabetes AssociationWinning at work: diabetes facts http://www.diabetes.org/communityprograms-and-localevents/waw-diabetes-facts.jsp. Accessed January 2, 2011.
- KolovouGDBilianouHGInfluence of aging and menopause on lipids and lipoproteins in womenAngiology200859Suppl 254S57S18515273
- SolhpourAParkhidehSSarrafzadeganNLevels of lipids and apolipoproteins in three culturesAtherosclerosis2009207120020719766218
- CarrollMDLacherDASorliePDTrends in serum lipids and lipoproteins of adults, 1960–2002JAMA2005294141773178116219880
- DerbyCACrawfordSLPasternakRCLipid changes during the menopause transition in relation to age and weight: the Study of Women’s Health Across the NationAm J Epidemiol2009169111352136119357323
- CarrMThe emergence of the metabolic syndrome with menopauseJ Clin Endocrinol Metab20038862404241112788835
- SowersMZhengHTomeyKChanges in body composition in women over six years at midlife: ovarian and chronological agingJ Clin Endocrinol Metab200792389590117192296
- PicheMELapointeAWeisnagelSJRegional body fat distribution and metabolic profile in postmenopausal womenMetab Clin Exp20085781101110718640388
- TchernofADesmeulesARichardCOvarian hormone status and abdominal visceral adipose tissue metabolismJ Clin Endocrinol Metab20048973425343015240626
- SarwarNDaneshJEiriksdottirGTriglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studiesCirculation2007115445045817190864
- TankoLBBaggerYZQinGEnlarged waist combined with elevated triglycerides is a strong predictor of accelerated atherogenesis and related cardiovascular mortality in postmenopausal womenCirculation2005111151883189015837940
- NordestgaardBGBennMSchnohrPTybjaerg-HansenANonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and womenJAMA2007298329930817635890
- FordESGilesWHDietzWHPrevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination SurveyJAMA2002287335635911790215
- AssmannGSchulteHFunkeHvon EckardsteinAThe emergence of triglycerides as a significant independent risk factor in coronary artery diseaseEur Heart J199819Suppl MM8M149821011
- EwaldNHardtPDKloerHUSevere hypertriglyceridemia and pancreatitis: presentation and managementCurr Opin Lipidol200920649750419770656
- BrunzellJDDavidsonMFurbergCDLipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology FoundationDiabetes Care200831481182218375431
- LiuJSemposCTDonahueRPNon-high-density lipoprotein and very-low-density lipoprotein cholesterol and their risk predictive values in coronary heart diseaseAm J Cardiol200698101363136817134630
- GrundySMCleemanJIMerzCNImplications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelinesCirculation2004110222723915249516
- MoscaLBankaCLBenjaminEJEvidence-based guidelines for cardiovascular disease prevention in women: 2007 updateCirculation2007115111481150117309915
- DayspringTHelmboldAYou have a new job: monitor the lipid profileOBG Management20082012227239
- MoscaLMochariHChristianANational study of women’s awareness, preventive action, and barriers to cardiovascular healthCirculation2006113452553416449732
- CookeCEHammerashWJJrRetrospective review of sex differences in the management of dyslipidemia in coronary heart disease: an analysis of patient data from a Maryland-based health maintenance organizationClin Ther200628459159916750470
- SteinEASnidermanALaskarzewskiPAssessment of reaching goal in patients with combined hyperlipidemia: low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, or apolipoprotein BAm J Cardiol2005969A36K43K discussion34K35K
- WeingartnerOLutjohannDJiSVascular effects of diet supplementation with plant sterolsJ Am Coll Cardiol200851161553156118420097
- LichtensteinAHAppelLJBrandsMDiet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition CommitteeCirculation20061141829616785338
- BantleJPWylie-RosettJAlbrightALNutrition recommendations and interventions for diabetes: a position statement of the American Diabetes AssociationDiabetes Care200831Suppl 1S61S7818165339
- US Department of Health and Human Services and US Department of AgricultureDietary guidelines for Americans, 20056th edWashington, DCUS Government Printing Office2005
- Kris-EthertonPMInnisSAmerican Dietetic Association, Dietitians of Canada. Position of the American Dietetic Association and Dietitians of Canada: dietary fatty acidsJ Am Diet Assoc200710791599161117936958
- Kris-EthertonPMHarrisWSAppelLJFish consumption, fish oil, omega-3 fatty acids, and cardiovascular diseaseCirculation2002106212747275712438303
- JacobsonTARole of n-3 fatty acids in the treatment of hypertriglyceridemia and cariovascular diseaseAm J Clin Nutr20088761981S1990S18541599
- OhRCBeresfordSALaffertyWEThe fish in secondary prevention of heart disease (FISH) survey: primary care physicians and omega3 fatty acid prescribing behaviorsJ Am Board Fam Med200619545946716951295
- HarrisWSn-3 fatty acids and serum lipoproteins: human studiesAm J Clin Nutr199765Suppl 51645S1654S9129504
- DavidsonMHMakiKCPearsonTAResults of the National Cholesterol Education (NCEP) Program Evaluation ProjecT Utilizing Novel E-Technology (NEPTUNE) II survey and implications for treatment under the recent NCEP Writing Group recommendationsAm J Cardiol200596455656316098311
- DavidsonMHBallantyneCMKerznerBEfficacy and safety of ezetimibe coadministered with statins: randomised, placebo-controlled, blinded experience in 2382 patients with primary hypercholesterolemiaInt J Clin Pract200458874675515372846
- DavidsonMHSteinEABaysHEEfficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: an 8-week, randomized, double-blind, placebo-controlled studyClin Ther20072971354136717825687
- LOVAZA [prescribing information]Research Triangle Park, NCGlaxoSmithKline2009
- HarrisWSGinsbergHNArunakulNSafety and efficacy of Omacor in severe hypertriglyceridemiaJ Cardiovasc Risk199745–63853919865671
- PownallHJBrauchiDKilincCCorrelation of serum triglyceride and its reduction by omega-3 fatty acids with lipid transfer activity and the neutral lipid compositions of high-density and low-density lipoproteinsAtherosclerosis1999143228529710217357
- BaysHETigheAPSadovskyRDavidsonMHPrescription omega-3 fatty acids and their lipid effects: physiologic mechanisms of action and clinical implicationsExpert Rev Cardiovasc Ther20086339140918327998
- GoldbergACSchonfeldGFeldmanEBFenofibrate for the treatment of type IV and V hyperlipoproteinemias: a double-blind, placebo-controlled multicenter US studyClin Ther198911169832655907
- CalabresiLDonatiDPazzucconiFOmacor in familial combined hyperlipidemia: effects on lipids and low density lipoprotein subclassesAtherosclerosis2000148238739610657575
- BaysHMakiKMcKenneyJLong-term up to 24 month efficacy and safety of concomitant prescription omega-3-acid ethyl esters and simvastatin in hypertriglyceridemic patientsCurr Med Res Opin201026490791520156032
- DavidsonMMakiKBaysHEffects of prescription omega-3-acid ethyl esters on lipoprotein particle concentrations, apolipoproteins AI and CIII, and lipoprotein associated phospholipase A2 mass in statin-treated subjects with hypertriglyceridemiaJ Clin Lipidol20093533234021291831
- GinsbergHNZhangYLHernandez-OnoARegulation of plasma triglycerides in insulin resistance and diabetesArch Med Resear2005363232240
- MakiKCMcKenneyJMReevesMSEffects of adding prescription omega-3 acid ethyl esters to simvastatin (20 mg/day) on lipids and lipoprotein particles in men and women with mixed dyslipidemiaAm J Cardiol2008102442943318678300
- MakiKLubinBReevesMDicklinMPrescription omega-3 acid ethyl esters plus simvastatin 20 and 80 mg: effects in mixed dyslipidemiaJ Clin Lipidol200931333821291786
- BaysHMcKenneyJMakiKCPrescription omega-3-acid ethyl esters: effects on non-high-density lipoprotein cholesterol in combined hyperlipidemic patients when co-administered with escalating doses of atorvastatinMayo Clin Proc20108512212820118387
- ContoisJHMcConnellJPSethiAAApolipoprotein B and Cardiovascular Disease Risk: Position Statement from the AACC Lipoproteins and Vascular Diseases Division Working Group on Best PracticesClinical Chemistry200955340741919168552