Abstract
Vulvar lichen sclerosus (LS) is a chronic, inflammatory dermatosis that may lead to scarring of the vulva and sexual dysfunction. LS affects women of all ages and often goes unrecognized and underreported. Uncertainty continues to exist around its pathogenesis, histologic diagnosis, and treatment. However, there have been great advances in our understanding of autoimmunogenic targets in disease formation and progression. In addition, there has been recent investigation of potential non-steroid-based treatments, including platelet-rich plasma therapy and energy-based modalities such as the fractional CO2 laser, photodynamic therapy, and high intensity focused ultrasound. Refinement of surgical techniques for restoring vulvar anatomy and treating clitoral phimosis, introital stenosis, and vulvar granuloma fissuratum is leading to improved patient outcomes. This review summarizes current perspectives on the pathogenesis, symptomatology, diagnosis, and treatment for vulvar lichen sclerosus.
Introduction
Lichen sclerosus (LS) is a chronic, inflammatory, cutaneous disorder that can lead to scarring, impaired sexual function, and malignancy. While LS can affect any area of the body of both males and females, it has a predilection for female anogenital epithelium. There is a reported bimodal peak incidence in premenarchal girls and in menopausal women. However, up to 40% of women with LS will display onset of symptoms and cutaneous changes of vulvar LS during their reproductive years.Citation1,Citation2 It has been questioned if the bi-modal peak incidence is, in fact, just detection bias. In a cohort of women with LS which included 46% premenopausal women, up to 39% of women were asymptomatic in the setting of advanced disease.Citation3 Pruritis is often described in hypoestrogenic states and it is not known if women of reproductive age with LS may experience less pruritis, possibly contributing to the known delay in diagnosis.Citation1–Citation4
Although the exact prevalence is unknown, LS has been found to affect one in 70 women presenting to a general gynecology practice with practitioners experienced in diagnosing this condition.Citation3 The time from reported onset of symptoms until diagnosis may range from 5 to 15 years.Citation1,Citation2 This suggests that this condition is commonly unrecognized and misdiagnosed for several years.Citation2,Citation5 The incidence of LS in the general population is largely unknown. A recent report from the Netherlands estimated that the incidence of histology-proven LS in women rose from 7.4 to 14.6 per 100,000 woman-years between 1991 and 2011. The authors propose that this rise in incidence reflects an increased awareness of the condition leading to higher rates of biopsy and diagnosis.Citation6 However, these incidences are almost certainly underestimated, as women with the clinical diagnosis of LS (without biopsy) or with non-definitive pathology were excluded in this study.
While this condition was first described over a century ago, uncertainty continues to exist around its pathogenesis, histologic diagnosis, and treatment. However, there have been great advances in our understanding of autoimmunogenic targets in disease formation and progression, as well as investigation of new and promising treatment modalities. The purpose of this review is to discuss our current understanding of the pathogenesis, diagnostic challenges, and emerging treatments for vulvar lichen sclerosus.
Pathogenesis
Although the etiology of LS is still unclear, evidence suggests that LS is an autoimmune disorder with a genetic component. Familial studies indicate a positive family history of LS, with 12% of over 1000 women with vulvar LS reporting a first-degree female relative with the same condition.Citation7 Case studies also describe vulvar LS in monozygotic twins.Citation8,Citation9 Providing support for genetic susceptibility, studies indicate a significant association of LS with genes regulating human leukocyte antigen (HLA) class II antigens, which are involved in humoral immunity.Citation10–Citation12 Women with LS have an increased prevalence of HLA-DQ7, –DQ8, –DQ9, and –DR12 compared with controls, with 50% of adult females and 66% of prepubertal females expressing HLA-DQ7.Citation10,Citation11,Citation13 In contrast, HLA-DR17 shows a negative association with LS, inferring protective qualities.Citation13 These specific HLA antigens and their associated haplotypes may play a role in susceptibility and protection from LS.Citation12,Citation13
In women, LS is hypothesized to be an autoimmune disorder. LS displays characteristics consistent with other autoimmune conditions, including a higher prevalence in women and association with other female autoimmune conditions.Citation2,Citation14–Citation16 The most frequent autoimmune diseases associated with LS in women include autoimmune thyroid diseases (Hashimoto thyroiditis and Graves’ disease), alopecia areata, vitiligo, and pernicious anemia. Autoimmune thyroiditis is much more common among women with LS, comprising 12–16% of two studied cohorts.Citation16,Citation17 This strong association raises the question if women diagnosed with LS should undergo screening for other immune diseases, in particular thyroid disease, although current guidelines recommend only clinical evaluation.Citation12,Citation18
Although the strong association with autoimmune disease and familial occurrence of LS has been recognized, the exact etiology of the disease remains unknown. Increased understanding of immune and genetic targets implicated in LS pathology involve autoimmunogenic activation, sclerotic tissue formation, and oxidative stress.Citation12 An absence of the suppressive function of regulatory T cells likely plays a role in inducing autoimmunity.Citation19 Gene expression profiles support LS as an inflammatory disease, mediated by upregulation of T-helper type I (Th1) cytokines.Citation20 There is an established association between Th1 responses and autoimmune diseases.Citation12,Citation21 MicroRNA-155 (miR-155) is involved in promoting Th1 differentiation.Citation20 When overexpressed, miR-155 can disrupt suppression mediated by T regulatory (Treg) cells, triggering a loss of self-tolerance and promoting inflammation, and thereby inducing autoimmunity.Citation12 Dysregulation due to overexpression of miR-155 is also associated with increased collagen synthesis, leading to sclerotic tissue formation. In addition, miR-155 inhibits tumor suppressor genes FOXO3 and CDKN1B, leading to even more collagen synthesis.Citation22
Autoantigen disruption involving extracellular matrix protein 1 (ECM1), a scaffolding glycoprotein which acts as a “biological glue” at the dermal-epidermis junction, was one of the first targets implicated in the development of LS.Citation12,Citation23 However, more recent studies indicate that autoimmunity to ECM1 alone is not sufficient in explaining the pathogenesis of LS.Citation12,Citation24 Autoantibodies to ECM1, which are found in 74% of females with LS, affect the regulatory binding of ECM1 to matrix metallopeptidase 9 (MMP9), leading to overactive collagen synthesis, especially type V collagen, and disrupting the focal basement membrane through degradation and thickening.Citation24,Citation25 Sclerotic tissue formation is also facilitated by dysregulation involving a keratinocyte protein regulated by p53 called galectin-7, which inhibits fibroblast growth and increases collagen synthesis.Citation26
The inflammation of the Th1 cytokine environment leads to the release of reactive oxygen species (ROS), promoting autoimmunity and oxidative stress.Citation12 Oxidative stress contributes to inactivation of tumor suppressor genes involving p53 and CDKN2A, leading to cell proliferation and transformation to malignancy.Citation12,Citation27
An increased understanding of the differences between the genomic and proteomic profiles between LS and normal skin may aid in the identification of potential biomarkers to be used for early diagnosis, treatment, and even prevention of the disease. There is an ongoing clinical trial [NCT03561428] that aims to identify and validate genes, protein, or glycoproteins that serve as biomarkers for LS.Citation28 Identification of specific biomarkers will facilitate the development of assays that may be incorporated in minimally invasive tests or screening tools for early detection of LS, as well as more specific tests for biopsy-based tissue diagnosis.
Clinical Presentation
While some patients with LS are asymptomatic, most report a history of pruritus, dyspareunia, or vulvar pain.Citation3,Citation15 Multiple studies have shown a high rate of sexual dysfunction in women with LS.Citation29–Citation32 Women with LS are less likely to be sexually active (vaginal intercourse, oral intercourse, and masturbation) than control groups.Citation29,Citation32 Furthermore, 79% of women with LS report chronic vulvar pain.Citation30 Progressive scarring may lead to clitoral phimosis and narrowing of the vaginal introitus. With loss of tissue elasticity, tearing at the base of the fourchette may occur with intercourse.Citation15 Of all quality of life domains, sexual function was found to be most impacted in women with vulvar LS. Women with LS reported significantly lower sexual desire, arousal, lubrication, orgasm, and satisfaction, even after adequate treatment.Citation32 A recent small cohort study found that women with vulvar LS scored significantly lower on a validated scale rating genital self-image, which was found to correlate with sexual function.Citation33 Because treatment with corticosteroids does not reverse existing vulvar scarring, this may explain why treating active disease may not improve sexual function related to poor genital self-image.
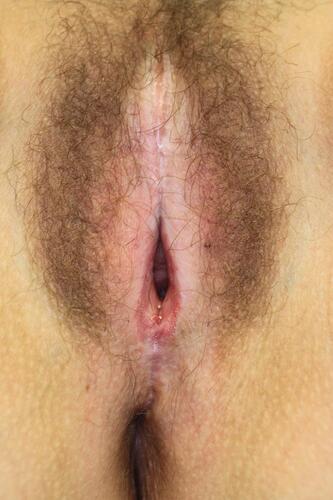
Physical examination reveals ivory white atrophic plaques with a waxy texture or epidermal wrinkling (“cigarette paper” appearance), depigmentation or hyperpigmentation, ecchymoses, resorption of the labia, narrowing of the introitus, and distortion of the vulvar architecture (). LS may involve the labia minora and inner portion of the labia majora, interlabial sulcus, clitoris, vestibule, perineum, and the perianal region. Unlike lichen planus, LS rarely involves the vaginal mucosa; however, case reports indicate vaginal disease may be more common than once thought and may be underdiagnosed.Citation34,Citation35 Scarring of the clitoral prepuce may cause clitoral phimosis, which in turn can lead to formation of a smegmatic pseudocyst abscess between the prepuce and clitoris.Citation15
Vulvar LS is associated with a 4–6.7% risk of squamous cell carcinoma (SCC) of the vulva.Citation1,Citation6 Unlike the more common HPV-associated vulvar high-grade squamous intraepithelial lesion (HSIL) and basaloid SCC, the LS-mediated pathway leads to vulvar intraepithelial neoplasia (VIN), differentiated type resulting in keratinizing SCC.Citation36,Citation37 Differentiated VIN, which accounts for less than 5% of VIN, often occurs in older women with inadequately treated LS or lichen planus. Findings have indicated that women compliant with topical corticosteroid treatment demonstrate lower rates of vulvar SCC compared to women who were inconsistent with this treatment.Citation1,Citation38–Citation41 However, these studies were not sufficiently powered to determine if treatment prevents progression of vulvar LS to SCC.Citation19 More recently, Lee and colleagues (2015) conducted a prospective longitudinal cohort study of over 500 women with vulvar LS spanning almost 6 years. None of the 357 women who were compliant with topical corticosteroid treatment developed SSC. Alternatively, seven (4.7%) of the women who were only partially compliant with treatment and follow-up developed biopsy-proven SCC.Citation42 These findings suggest that optimal treatment modifies the course of the disease, and may prevent malignant transformation.
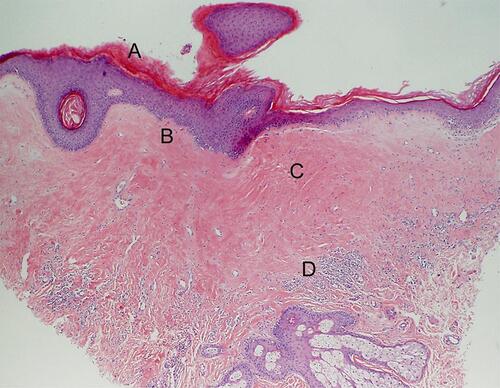
A biopsy to confirm LS prior to treatment is not necessary in typical presentations.Citation42–Citation44 However, with atypical features, uncertainty of diagnosis, concern for malignancy, or in failed response to treatment, obtaining a histological specimen is advised.Citation15,Citation42,Citation44 Biopsies should ideally be collected without prior application of corticosteroids, as treatment can resolve the pathognomonic histopathologic changes of LS. Characteristic pathologic findings generally include hyperkeratosis of the epidermis, epidermal atrophy with loss of rete ridges, homogenization of the collagen in the upper dermis, and a lichenoid (band-like) inflammatory infiltrate in the dermis (). There are inconsistencies in the description and reliability of histologic samples in the diagnosis of LS.Citation12 In a retrospective review of almost 70 cases of patients with clinically diagnosed LS who had a vulvar biopsy performed within 5 years, one-third of biopsies did not meet histological criteria for diagnosis of LS.Citation45 Early LS may be histologically misdiagnosed as eczema or “non-specific vulvitis.”Citation46,Citation47 While classic histologic findings may confirm the diagnosis, a nonspecific biopsy should not rule out clinically suspected LS.Citation45,Citation48
Treatment
Although there is no cure for LS, there are a number of treatment options that have been explored to achieve remission and prevent progression of disease. The goals of treatment are to alleviate symptoms of itching and pain, prevent anatomic changes due to scarring, and possibly prevent malignant transformation. Scarring of the vulva may cause clitoral phimosis, introital stenosis, and recurrent mechanical fissure of the posterior fourchette, leading to decreased sensation and dyspareunia. This section will discuss medical and energy-based treatment modalities to prevent disease progression, as well as surgical techniques to restore vulvar anatomy and function.
Ultrapotent Topical Corticosteroids
The gold standard treatment for LS is ultrapotent topical corticosteroids (TCSs), most commonly clobetasol propionate ointment. According to the British Association of Dermatologists (BAD) Guidelines for the management of LS, which were updated in 2018, recommended treatment for anogenital LS in women is with clobetasol propionate 0.05% ointment.Citation44,Citation49 This is based upon evidence from randomized control trials that found that clobetasol propionate ointment 0.05% is more effective in the treatment of vulvar LS compared to topical tacrolimus 0.1%, topical testosterone 2%, and phototherapy, and equally effective as mometasone furoate 0.1%.Citation50–Citation53 The recommended dose is a half-fingertip unit (approximately 0.5 g) applied to the affected area once daily for 1 month, then every other day for 1 month, and then twice weekly for a third month.Citation44 The 2015 European Guidelines recommend daily application for the first 3 months.Citation18
There is variation in practice regarding maintenance therapy after the initial 3 months of treatment.Citation54 According to the 2018 BAD Guidelines, once symptoms are controlled, topical steroid may be applied as needed for recurrent symptoms.Citation44 The 2015 European Guidelines recommend individualized maintenance ranging from 1–2 times per month to 2–3 times per week.Citation18 However, other experts contend that maintenance therapy in the form of once to twice weekly application of clobetasol propionate ointment 0.05% is necessary. In a survey to determine current expert opinion in the treatment of vulvar LS, Selk found that 64% of physicians continue maintenance therapy in all patients.Citation54 In addition, dermatologists and physicians practicing in the United States are more likely to treat with maintenance therapy compared to gynecologists and physicians practicing in Europe.Citation54
Based on the findings of Lee et al (2015), women who were compliant with long-term topical corticosteroid treatment decreased their risk of malignant transformation and progression of vulvar scarring as compared to women who were partially compliant with treatment.Citation42 Of note, this study included treatment regimens using different topical corticosteroid agents, including betamethasone dipropionate ointment 0.05% (64% of patients), methylprednisolone aceponate ointment 0.1% (31% of patients), clobetasol propionate ointment 0.05% (3% of patients), or hydrocortisone ointment 1% (2% of patients). This study indicates that only optimal treatment of vulvar LS and appropriate follow-up may lower the risk of malignant transformation. Findings such as this also provide support for maintenance therapy following treatment of active disease, as well as initiating treatment in asymptomatic patients with clinically active LS. The authors of this paper support optimal treatment of vulvar LS with clobetasol propionate ointment 0.05% with regular follow-up to ensure resolution of active disease, followed by maintenance therapy twice weekly to be continued indefinitely.
Topical Calcineurin Inhibitors
The topical calcineurin inhibitors (TCIs), tacrolimus, and pimecrolimus, which block the release of inflammatory cytokines from T lymphocytes, have been studied as alternatives to corticosteroids for the treatment of LS.Citation50,Citation55 The potential advantage of these newer medications is that they do not inhibit collagen synthesis so they do not cause dermal atrophy, which may be especially useful in pediatric LS patients.Citation19 However, randomized trials comparing clobetasol to both pimecrolimus and tacrolimus have shown that while both calcineurin inhibitors effectively treat LS, clobetasol is superior in its ability to decrease underlying inflammation.Citation56 As such, these newer agents are considered second-line treatments for vulvar LS.
Platelet-Rich Plasma
Decreasing inflammation is the primary goal for treatment of vulvar LS. Platelet-rich plasma (PRP) therapy promotes the healing process of tissue by stimulating the release of cytokines and growth factors. The effectiveness of PRP is based upon the high level of growth factors such as PDGF, TGF-B, and EGF, which modulate mesenchymal cell proliferation and extracellular matrix synthesis.Citation57 PRP has shown to be effective at propagating new healthy tissue growth in a wide range of medical conditions, such as venous ulcers, diabetic foot ulcers, and tendinopathy.Citation58–Citation62
Outside of one-patient case reports, there have been few studies investigating the use of PRP in patients with biopsy-proven LS.Citation63–Citation65 A 2010 study examined injections of a combination of PRP and fat-derived mesenchymal cells in 15 women with vulvar LS. The authors reported that all 15 patients had complete resolution of pain and symptoms.Citation66 However, a significant limitation of the study was that two concurrent interventions were performed, PRP and fat-derived mesenchymal cells, which limits the ability to determine which intervention was efficacious. In addition, the study did not provide an objective measurement of efficacy. Another pilot study evaluated the efficacy and safety of autologous PRP injections in 15 patients with biopsy proven vulvar LS.Citation62 Each patient received two treatments of PRP injections separated by 5 weeks, with an initial-screening biopsy and a repeat biopsy 6 weeks after the second treatment. The objective efficacy variable was the change in inflammation between the pre- and post-treatment biopsies as measured by two blinded dermatopathologists. Secondary efficacy variables included changes from baseline in vulvar pruritus and burning using visual analogue scales (VAS) and change in Investigator’s Global Assessment (IGA) of the severity of disease (0–3 scale). Of the 12 patients that completed the study, seven had decreased inflammation, three had no change, and two had a “minimal” increase in inflammation on post-treatment biopsy, which was statistically significant (F(1,11)=6.81, P=0.024). There was also a statistically significant difference in the pre- and post-treatment IGA scores. However, the changes in VAS scores for pruritus and burning were unchanged.Citation62 Limitations of this study include the small sample size and lack of placebo control.
In 2019, there was a follow-up randomized, placebo-controlled, double-blinded clinical trial comprised of 30 patients with biopsy-proven LS treated with PRP.Citation67 A blinded pathologist with an expertise in vulvar pathology evaluated inflammatory infiltration on the pre- and post-treatment biopsies. The secondary endpoint was the change in the “Clinical Scoring System for Vulvar Lichen Sclerosus” (CSS), a validated instrument that assesses the severity of LS based on patient’s symptoms and investigator’s impression. The objective inflammatory results between placebo and PRP groups were not statistically significant. The difference in CSS mean score pre-and post-treatment for the PRP arm was −7.74 and −9.44 for the placebo arm. The results of this study showed that PRP therapy is not an optimal treatment for vulvar LS. The main limitation of this study was the small sample size.Citation67
Given the limited number of randomized, placebo-controlled PRP studies and the results of the studies that have been completed, PRP cannot be a recommended treatment for lichen sclerosus at this time. Additional studies must be conducted with a larger sample size to determine if PRP can be used as a treatment for LS.
Energy-Based Modalities
Three energy based modalities have recently been studied for the treatment of LS: photodynamic therapy (PDT), High-Intensity Focused Ultrasound (HIFU), and fractional CO2 lasers (FxCO2).
Photodynamic Therapy
Photodynamic therapy (PDT) relies on the interaction among three components: photosensitizing agents, appropriate wavelengths of light, and oxygen. The photodynamic process creates intracellular reactive oxygen radicals from uptake of the exogenous-photosensitizing agent or via endogenous production.Citation68 Photosensitizers target cells that play a role in inflammation and fibrosis, with limited damage to healthy tissue. A recent systematic review examined 11 studies using photodynamic therapy for the treatment of LS with a total of 337 women. PDT led to significant improvement in symptoms related to LS, but changes in histopathologic inflammation were inconsistent.Citation69 Although PDT seems promising, additional research is needed to determine efficacy of this treatment modality.
High-Intensity Focused Ultrasound
High-Intensity Focused Ultrasound (HIFU) stimulates cell proliferation, protein synthesis, and revascularization, thereby accelerating tissue reconstruction. In a study of 41 cases of LS treated with HIFU, 90% of patients showed symptom improvement or resolution within 6 months of treatment. Pre and post-treatment biopsies demonstrated decreased signs of inflammation. However, almost 10% of patients reported adverse side effects, most commonly skin burns and blistering.Citation70 A more rigorous study of over 380 women with non-neoplastic epithelial disorders of the vulva (NNEDV) including 68 women with vulvar LS, 51% of patients treated with HIFU reported complete resolution of symptoms and an additional 47% noted improvement in symptoms. Approximately 6.5% of the patients treated (for all vulvar conditions studied) developed blistering that resolved with anti-inflammatory medication and no residual scarring.Citation71
A larger retrospective study of 950 women with LS or vulvar squamous hyperplasia found that following treatment with HIFU, 42% of patients had complete resolution of signs and symptoms of disease and an additional 56% of patients noted improvement. Disease recurrence was found to be almost 10%, which was significantly higher in women with LS. There were no severe adverse effects reported.Citation72 A recent multi-center, randomized control trial of 62 patients with NNEDV (>30% diagnosed as LS) compared histology specimens (pre and post treatment) of HIFU therapy versus 3-month treatment with a high-potency topical corticosteroid. The study found that treatment with HIFU led to “curative effects” on histology compared to the topical corticosteroid group.Citation73 Additional research is needed to support adoption of this promising treatment modality.
Fractional CO2 Laser Therapy
Fractional CO2 laser (FxCO2) therapy has shown positive results in the treatment of vaginal atrophy, and has been proposed for the management of LS. This type of laser has a wavelength of 10,600 nm that allows a superficial microablative effect in soft tissues and a pulsed beam that protects the tissues from possible overheating damage. The laser beam is delivered to the tissue in a fractional manner, creating small spots alternating parts of tissue treated and not treated.Citation74 In a case series, four patients with vulvar LS demonstrated significant improvement in symptoms and visual appearance of disease.Citation74 In a subsequent study, a larger cohort of 27 women with symptomatic LS underwent three to four treatments at 4–6-week intervals with the fractional CO2 laser set at 20 Watts. Twenty-four women (89%) reported resolution of their itching and pain symptoms.Citation75 The major limitations of these two studies are the lack of objective measures of disease improvement, lack of sham control, and treatment with clobetasol prior to FxCO2 laser therapy.
There is currently one active randomized, double-blinded, sham-controlled trial investigating the efficacy of FxCO2 laser therapy in 40 women with biopsy-proven lichen sclerosus [NCT02573883]. The primary endpoint for this study is improvement in histologic inflammation determined by a blinded dermatopathologist. Results for this trial are pending.Citation76 In addition, there is also an ongoing trial comparing the safety and efficacy of FxCO2 laser therapy to topical clobetasol treatment of vulvar LS [NCT02573883].Citation77
Other types of laser treatment modalities have also been explored. There has been one case report of two women with anogenital LS refractory to topical corticosteroid treatment whose symptoms improved with Erbium YAG (Er:YAG) fractionally ablative laser therapy.Citation78 Until there is objective data from more methodologically robust studies, laser therapy cannot be recommended as the primary treatment modality for women with LS.
Lysis of Vulvar Adhesions and Perineoplasty
Vulvar lichen sclerosus can lead to significant vulvar scarring, resorption of the labia minora, clitoral phimosis, introital stenosis, and recurrent mechanical fissure of the posterior fourchette, termed vulvar granuloma fissuratum.Citation48,Citation79–Citation82 Vulvar scarring, secondary to LS, can cause significant distortion of the vulvar architecture, and therefore, can cause significant emotional trauma and perceived diminution of sexuality and femininity. Clitoral phimosis, scarring of the prepuce and/or labia majora to the glans clitoris, is a complication of vulvar lichen sclerosus that can cause significant morbidities including loss of clitoral sensitivity leading to secondary anorgasmia.Citation79–Citation84 In addition, a smegmatic pseudocyst can develop in the space between the prepuce and clitoris which can lead to chronic inflammation and/or infection causing the need for lysis of clitoral adhesions.Citation79,Citation83
Clitoral phimosis may be treated successfully through surgical intervention. A minimally invasive surgical procedure can treat clitoral phimosis and improve clitoral sensitivity and ability to achieve orgasm.Citation29,Citation79,Citation83 The surgical procedure for clitoral phimosis described by Goldstein and Burrows (2007) includes bluntly lysing adhesions by insertion of a lacrimal duct probe between the prepuce and clitoris. For additional lysis, a 5 mm dorsal incision is made along the prepuce using Iris scissors.Citation83 If necessary, the edges of the prepuce may be trimmed with a scalpel to prevent recurrent adhesions.Citation83 Pressure, silver nitrate, electrocautery, or ferric sulfate solution can be used to obtain hemostasis.Citation79–Citation83 To prevent post-operative Koebnerization, patients apply clobetasol 0.05% ointment daily to the surgical site until well healed.Citation80,Citation82,Citation83 In a study examining the surgical outcomes of eight patients with clitoral phimosis, 88% reported that they were “very satisfied” with the results of their surgery, and one patient reported she was “satisfied.” All eight patients reported that they would recommend surgery to other women experiencing negative effects due to clitoral phimosis. Of the four women who reported decreased clitoral sensitivity prior to surgery, 100% experienced increased clitoral sensitivity and orgasm post-operatively.Citation84
An additional complication many women with vulvar lichen sclerosus develop is introital stenosis.Citation48 For many women, narrowing of the introitus causes significant dyspareunia, sexual dysfunction, and vulvar granuloma fissuratum. Conservative treatment for introital stenosis consists of topical ultrapotent corticosteroids and aggressive manual dilation with graduated vaginal dilators. If conservative treatment fails, it may be necessary to perform a superficial perineoplasty with vaginal advancement, to correct the introital narrowing. The scarred endothelium of the posterior fourchette and the scarred epithelium of the perineum are excised, and a vaginal advancement flap is used to close the defect.Citation80,Citation82 Rouzier and colleagues reported the functional outcomes following perineoplasty for introital stenosis in a cohort of 64 women over a 10-year period. Post-operatively, 92% reported relief of introital dyspareunia, 86% reported improvement in the quality of sexual intercourse, and only 5 of 64 patients had recurrent introital dyspareunia. Of the five patients, three had recurrent tearing of the perineum and two experienced dehiscence of the vaginal advancement flap.Citation85 Surgery for women with LS should be performed by an experienced surgeon who is familiar with this disease and the potential complications.
Even more recently, less invasive procedures have also been shown to successfully improve introital dyspareunia, clitoral sensitivity, and ability to achieve orgasm in women with introital stenosis, VGF, and clitoral phimosis secondary to LS. In a 2015 case series of 28 women, Flynn et al reported that sharp dissection of the introital scar tissue combined with post-operative clobetasol use and aggressive post-operative retraction of the incisions (without perineoplasty), improved dyspareunia in 85% of women who experienced pain with intercourse prior to surgery and clitoral sensitivity in 75% of women who experienced decreased clitoral sensitivity prior to surgery.Citation84 In addition, 84% of women who underwent surgery would recommend surgery to women with similar symptoms.Citation84 These studies demonstrate that women with clitoral phimosis, introital stenosis, and VGF secondary to LS can be successfully treated with minimally invasive surgical intervention.
Conclusion
Advances in disease formation and progression hold promise for improved diagnosis of LS, bringing us one step closer to minimally invasive testing or screening options that could facilitate detection of early disease. Emerging treatments, including energy-based modalities such as the fractional CO2 laser and HIFU, are currently being studied to find more effective treatments for vulvar LS other than ultrapotent topical corticosteroids. However, additional studies are required to determine the efficacy and safety of these emerging treatments. The current gold standard for treating vulvar LS and preventing associated scarring and malignancy is topical clobetasol 0.05% ointment daily for 4 weeks to 12 weeks, after 4 weeks treatment frequency may be tapered to every other day for 4 weeks, then maintenance therapy at two times per week. Surgical techniques for restoring vulvar anatomy and treating clitoral phimosis, introital stenosis, and vulvar granuloma fissuratum lead to improved sexual dysfunction and satisfactory outcomes.
Disclosure
Dr Andrew T Goldstein reports grants from Elen, Cellular Medicine Association, Gynecologic Cancers Research Foundation, during the conduct of the study; personal fees from Amag, grants, personal fees from Ipsen, grants from endoceutics, grants, personal fees from SST, outside the submitted work; and Dr Goldstein is the founder and president of the Gynecologic Cancers Research Foundation, a 501c3. The authors report no other conflicts of interest in this work.
References
- Cooper SM, Gao XH, Powell JJ, et al. Does treatment of vulvar lichen sclerosus influence its prognosis? Arch Dermatol. 2004;140:702–706. doi:10.1001/archderm.140.6.702
- Schlosser BJ, Mirowski GW. Lichen sclerosus and lichen planus in women and girls. Clin Obstet Gynecol. 2015;58(1):125–142. doi:10.1097/GRF.0000000000000090
- Goldstein AT, Marinoff SC, Christopher K, et al. Prevalence of vulvar lichen sclerosus in a general gynecology practice. J Reprod Med. 2005;50:477–480.
- Günthert AR, Faber M, Knappe G, Hellriegel S, Emons G. Early onset vulvar lichen sclerosus in premenopausal women and oral contraceptives. Eur J Obstet Gynecol Reprod Biol. 2008;137(1):56–60. doi:10.1016/j.ejogrb.2007.10.005
- Nair PA. Vulvar Lichen Sclerosus et Atrophicus. J Midlife Health. 2017;8(2):55–62. doi:10.4103/jmh.JMH_13_17
- Bleeker MC, Visser PJ, Overbeek LI, Beurden M, Berkhof J. Lichen sclerosus: incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2016;25:1224–1230. doi:10.1158/1055-9965.EPI-16-0019
- Sherman V, McPherson T, Baldo M, Salim A, Gao XH, Wojnarowska F. The high rate of familial lichen sclerosus suggests a genetic contribution: an observational cohort study. J Eur Acad Dermatol Venereol. 2010;24(9):1031–1034. doi:10.1111/j.1468-3083.2010.03572.x
- Doulaveri G, Armira K, Kouris A, Karypidis D, Potouridou I. Genital vulvar lichen sclerosus in monozygotic twin women: a case report and review of the literature. Case Rep Dermatol. 2013;5:321–325. doi:10.1159/000356775
- Lis-Święty A, Mierzwińska K, Wodok-Wieczorek K, Widuchowska M, Brzezińska-Wcisło L. Co-existence of lichen sclerosus and localized scleroderma in female monozygotic twins. J Pediatr Adolesc Gynecol. 2014;27:e133–136. doi:10.1016/j.jpag.2013.11.010
- Marren P, Yell J, Charnock FM, Bunce M, Welsh K, Wojnarowska F. The association between lichen sclerosus and antigens of the HLA system. Br J Dermatol. 1995;132(2):197–203. doi:10.1111/j.1365-2133.1995.tb05013.x
- Powell J, Wojnarowska F, Winsey S, Marren P, Welsh K. Lichen sclerosus premenarche: autoimmunity and immunogenetics. Br J Dermatol. 2000;142(3):481–484. doi:10.1046/j.1365-2133.2000.03360.x
- Tran DA, Xiaohui T, Macri CJ, Goldstein AT, Fu SW. Lichen sclerosus: an autoimmunopathologenic and genomic enigma with emerging genetic and immune targets. Int J Biol Sci. 2019;15(7):1429–1439. doi:10.7150/ijbs.34613
- Gao X-H, Barnardo MCMN, Winsey S, et al. The association between HLA DR, DQ antigens, and vulval lichen sclerosus in the UK: HLA DRB1*12 and its associated DRB1*12/DQB1*0301/04/09/010 haplotype confers susceptibility to vulval lichen sclerosus, and HLA DRB1*0301/04 and its associated DRB1*0301/04/DQB1*0201/02/03 haplotype protects from vulval lichen sclerosus. J Invest Dermatol. 2005;125:895–899. doi:10.1111/j.0022-202X.2005.23905.x
- Meyrick Thomas RH, Ridley CM, McGibbon DH, et al. Lichen sclerosus et atrophicus and autoimmunity: a study of 350 women. Br J Dermatol. 1988;118:41–46. doi:10.1111/bjd.1988.118.issue-1
- Krapf JM, Goldstein AT. Vulvar lichen sclerosus. The Vulva. 2017;27.
- Kreuter A, Kryvosheyeva Y, Terras S, et al. Association of autoimmune diseases with lichen sclerosus in 532 male and female patients. Acta Derm Venereol. 2013;93:238–241. doi:10.2340/00015555-1512
- Cooper SM, Ali I, Baldo M, Wojnarowska F. The association of lichen sclerosus and erosive lichen planus of the vulva with autoimmune disease: a case-control study. Arch Dermatol. 2008;144(11):1432–1435. doi:10.1001/archderm.144.11.1432
- Kirtschig G, Becker K, Günthert A, et al. Evidence‐based (S3) Guideline on (anogenital) Lichen sclerosus. J Eur Acad Dermatol Venereol. 2015;29(10):e1–e43.
- Brodrick B, Belkin ZR, Goldstein AT. Influence of treatments on prognosis for vulvar lichen sclerosus: facts and controversies. Clin Dermatol. 2013;31(6):780–786. doi:10.1016/j.clindermatol.2013.05.017
- Terlou A, Santegoets LAM, van der Meijden WI, et al. An autoimmune phenotype in vulvar lichen sclerosus and lichen planus: a Th1 response and high levels of MicroRNA-155. J Invest Dermatol. 2012;132:658–666. doi:10.1038/jid.2011.369
- Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi:10.1146/annurev.immunol.21.120601.140942
- Ren L, Zhao Y, Huo X, Wu X. MiR-155-5p promotes fibroblast cell proliferation and inhibits FOXO signaling pathway in vulvar lichen sclerosis by targeting FOXO3 and CDKN1B. Gene. 2018;653:43–50. doi:10.1016/j.gene.2018.01.049
- Chan I. The role of extracellular matrix protein 1 in human skin. Clin Exp Dermatol. 2004;29:52–56. doi:10.1111/ced.2004.29.issue-1
- Oyama N, Chan I, Neill SM, et al. Autoantibodies to extracellular matrix protein 1 in lichen sclerosus. The Lancet. 2003;362(9378):118–123. doi:10.1016/S0140-6736(03)13863-9
- Godoy CAP, Teodoro WR, Velosa APP, et al. Unusual remodeling of the hyalinization band in vulval lichen sclerosus by type V collagen and ECM 1 protein. Clinics. 2015;70:356–362. doi:10.6061/clinics
- Zhao Y, Zhao S, Li H, Qin X, Wu X. Expression of galectin-7 in vulvar lichen sclerosus and its effect on dermal fibroblasts. Oncol Lett. 2018;16:2559–2564. doi:10.3892/ol.2018.8897
- Soufir N, Queille S, Liboutet M, et al. Inactivation of the CDKN2A and the p53 tumour suppressor genes in external genital carcinomas and their precursors. Br J Dermatol. 2007;156:448–453. doi:10.1111/bjd.2007.156.issue-3
- Goldstein AT Biomarkers of lichen sclerosus NCT03561428. Clinicaltrials.gov. June 2019. Available from: https://clinicaltrials.gov/ct2/show/NCT03561428. Accessed January 3, 2020.
- Dalziel KL. Effect of lichen sclerosus on sexual function and parturition. J Reprod Med. 1995;40(5):351–354.
- Gagne H, Dalton V, Haefner H, et al. Vulvar pain and sexual function in patients with lichen sclerosus. J Reprod Med. 2007;52:121–122.
- Van de Nieuwenhof H, Meeuwis K, Nieboer T, et al. The effect of vulvar lichen sclerosus on quality of life and sexual functioning. J Psychosom Obstet Gynaecol. 2010;31(4):279–284. doi:10.3109/0167482X.2010.507890
- Burrows L, Creasey A, Goldstein A. The treatment of vulvar lichen sclerosus and female sexual dysfunction. J Sex Med. 2011;8(1):219–222. doi:10.1111/j.1743-6109.2010.02077.x
- Hodges KR, Wiener CE, Vyas AS, Turrentine MA. The female genital self-image scale in adult women with vulvar lichen sclerosus. J Low Genit Tract Dis. 2019;23(3):210–213. doi:10.1097/LGT.0000000000000481
- Zendell K, Edwards L. Lichen sclerosus with vaginal involvement: report of 2 cases and review of the literature. JAMA Dermatol. 2013;149(10):1199–1202. doi:10.1001/jamadermatol.2013.4885
- Longinotti M, Schieffer YM, Kaufman RH. Lichen sclerosus involving the vagina. Obstet Gynecol. 2005;106(5):1217–1219. doi:10.1097/01.AOG.0000161058.02449.e8
- Ueda Y, Enomoto T, Kimura T, et al. Two distinct pathways to development of squamous cell carcinoma of the vulva. J Skin Cancer. 2011;2011:1–7. doi:10.1155/2011/951250
- Bornstein J, Bogliatto F, Haefner HK, et al., ISSVD Terminology Committee. The 2015 International Society for the Study of Vulvovaginal Disease (ISSVD) terminology of vulvar squamous intraepithelial lesions. Obstet Gynecol. 2016;127(2):264–268. doi:10.1097/AOG.0000000000001285
- Vilmer C, Cavelier-Balloy B, Nogues C, Trassard M, Le Doussal V. Analysis of alterations adjacent to invasive vulvar carcinoma and their relationship with the associated carcinoma: a study of 67 cases. Eur J Gynaecol Oncol. 1997;19(1):25–31.
- Bornstein J, Heifetz S, Kellner Y, Stolar Z, Abramovici H. Clobetasol dipropionate 0.05% versus testosterone propionate 2% topical application for severe vulvar lichen sclerosus. Am J Obstet Gyn. 1998;178(1):80–84. doi:10.1016/S0002-9378(98)70631-3
- Renaud-Vilmer C, Cavelier-Balloy B, Porcher R, et al. Vulvar lichen sclerosus: effect of long-term topical application of a potent steroid on the course of the disease. Arch Dermatol. 2004;140:709–712. doi:10.1001/archderm.140.6.709
- Bradford J, Fischer G. Long-term management of vulvar lichen sclerosus in adult women. Aust N Zeal J Obstet Gynaecol. 2010;50(2):148–152. doi:10.1111/j.1479-828X.2010.01142.x
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151(10):1061–1067. doi:10.1001/jamadermatol.2015.0643
- Neill S, Lewis F, Tatnall F, et al. British association of dermatologists’ guidelines for the management of lichen sclerosus 2010. Br J Dermatol. 2010;163:672–682. doi:10.1111/j.1365-2133.2010.09997.x
- Lewis FM, Tatnall FM, Velangi SS, et al. British association of dermatologists guidelines for the management of lichen sclerosus 2018. Br J Dermatol. 2018;178:823–824.
- McCarthy S, MacEoin N, O’Driscoll M, O’Connor R, Heffron CC, Murphy M. Should we always biopsy in clinically evident lichen sclerosus? J Low Genit Tract Dis. 2019;23(2):182–183. doi:10.1097/LGT.0000000000000457
- Niamh L, Naveen S, Hazel B. Diagnosis of vulval inflammatory dermatoses: a pathological study with clinical correlation. Int J Gynecol Pathol. 2009;28:554–558. doi:10.1097/PGP.0b013e3181a9fb0d
- Regauer S, Liegl B, Reich O. Early vulvar lichen sclerosus: a histopathological challenge. Histopathology. 2005;47:340–347. doi:10.1111/j.1365-2559.2005.02209.x
- Murphy R. Lichen sclerosus. Dermatol Clin. 2010;28:707–715. doi:10.1016/j.det.2010.07.006
- Akel R, Fuller C. Updates in lichen sclerosis: British Association of Dermatologists guidelines for the management of lichen sclerosus 2018. Br J Dermatol. 2018;178(4):823–824. doi:10.1111/bjd.16445
- Funaro D, Lovett A, Leroux N, et al. A double- blind, randomized prospective study evaluating topical clobetasol propionate 0.05% versus topical tacrolimus 0.1% in patients with vulvar lichen sclerosus. J Am Acad Dermatol. 2014;71:84–91. doi:10.1016/j.jaad.2014.02.019
- Ayhan A, Guven S, Guvendag Guven ES, Sakinci M, Gultekin M, Kucukali T. Topical testosterone versus clobetasol for vulvar lichen sclerosus. Int J Gynecol Obstet. 2007;96(2):117–121. doi:10.1016/j.ijgo.2006.09.018
- Terras S, Gambichler T, Moritz RK, et al. UV-A1 phototherapy vs clobetasol propionate, 0.05%, in the treatment of vulvar lichen sclerosus: a randomized clinical trial. JAMA Dermatol. 2014;150:621–627. doi:10.1001/jamadermatol.2013.7733
- Virgili A, Gorghi A, Toni G, Minghetti S, Corazza M. First randomized trial on clobetasol propionate and mometasone furoate in the treatment of vulvar lichen sclerosus: results of efficacy and tolerability. Br J Dermatol. 2014;171(2):388–396. doi:10.1111/bjd.12910
- Selk A. A survey of experts regarding the treatment of adult vulvar lichen sclerosus. J Low Genit Tract Dis. 2015;19(3):244–247. doi:10.1097/LGT.0000000000000106
- Andreassi M, Bilenchi R. Topical pimecrolimus in the treatment of genital lichen sclerosus. Expert Rev Derm. 2013;8(5):443–450. doi:10.1586/17469872.2013.835923
- Goldstein A, Creasey A, Pfau R, et al. A double blind, randomized controlled trial of clobetasol versus pimecrolimus in patients with vulvar lichen sclerosus. J Am Acad Dermatol. 2011;64(6):e99–e104. doi:10.1016/j.jaad.2010.06.011
- Lacci KM, Dardik A. Platelet-rich plasma: support for its use in wound healing. Yale J Biol Med. 2010;83(1):1.
- Sarvajnamurthy S, Suryanarayan S, Budamankuntala L, et al. Autologous platelet rich plasma in chronic venous ulcers: study of 17 cases. J Cutan Aesthet Surg. 2013;6:97–99. doi:10.4103/0974-2077.112671
- Yotsu RR, Hagiwara S, Okochi H, et al. Case series of patients with chronic foot ulcers treated with autologous platelet-rich plasma. J Dermatol. 2015;42:288–295. doi:10.1111/1346-8138.12777
- Wesner M, Defreitas T, Bredy H, et al. A pilot study evaluating the effectiveness of platelet-rich plasma therapy for treating degenerative tendinopathies: a randomized control trial with synchronous observational cohort. PLoS ONE. 2016;11:e0147842. doi:10.1371/journal.pone.0147842
- Wu PI-K, Diaz R, Borg-Stein J. Platelet-rich plasma. Phys Med Rehabil Clin N Am. 2016;27(4):825–853. doi:10.1016/j.pmr.2016.06.002
- Goldstein AT, King M, Runels C, Gloth M, Pfau R. Intradermal injection of autologous platelet-rich plasma for the treatment of vulvar lichen sclerosus. J Am Acad Dermatol. 2017;76(1):158–160. doi:10.1016/j.jaad.2016.07.037
- Kim SH, Park ES, Kim TH. Rejuvenation using platelet-rich plasma and lipofilling for vaginal atrophy and lichen sclerosus. J Menopausal Med. 2017;23(1):63–68. doi:10.6118/jmm.2017.23.1.63
- Franic D, Iternicka Z, Franic-Ivanisevic M. Platelet-rich plasma (PRP) for the treatment of vulvar lichen sclerosus in premenopausal women: a case report. Case Rep Women’s Health. 2018;16:e00062. doi:10.1016/j.crwh.2018.e00062
- Tedesco M, Pranteda G, Chichierchia G, et al. The use of PRP (platelet-rich plasma) in patients affected by genital lichen sclerosus: clinical analysis and results. J Eur Acad Dermatol Venereol. 2019;33(2):e58–e59. doi:10.1111/jdv.2019.33.issue-2
- Casabona F, Priano V, Vallerino V, et al. New surgical approach to lichen sclerosus of the vulva: the role of adipose-derived mesenchymal cells and platelet-rich plasma in tissue regeneration. Plast Reconstr Surg. 2010;126:210e–211e. doi:10.1097/PRS.0b013e3181ea9386
- Goldstein AT, Mitchell L, Govind V, Heller D. A randomized double-blind placebo-controlled trial of autologous platelet-rich plasma intradermal injections for the treatment of vulvar lichen sclerosus. J Am Acad Dermatol. 2019;80(6):1788–1789. doi:10.1016/j.jaad.2018.12.060
- Ozog DM, Rkein AM, Fabi SG, et al. Photodynamic therapy: a clinical consensus guide. Dermatol Surg. 2016;42(7):804–827.
- Prodromidou A, Chatziioannou E, Daskalakis G, Stergios K, Pergialiotis V. Photodynamic therapy for vulvar lichen sclerosus—a systematic review. J Low Genit Tract Dis. 2018;22(1):58–65. doi:10.1097/LGT.0000000000000362
- Ruan L, Xie Z, Wang H, et al. High-intensity focused ultrasound treatment for non-neoplastic epithelial disorders of the vulva. Int J Gyn Obstet. 2010;109(2):167–70.\\. doi:10.1016/j.ijgo.2009.12.014
- Sun X, Xue M, Deng X, Wan Y. Clinical factors analysis of curative effect of focused ultrasound treatment for non neoplastic epithelial disorders of the vulva. J Cent Sout Univ. 2010;35(9):933–939.
- Ye M, Deng X, Mao S, Xue M. High intensity focused ultrasound treatment for non-neoplastic epithelial disorders of the vulva: factors affecting effectiveness and recurrence. Int J Hyperthermia. 2015;31(7):771–776. doi:10.3109/02656736.2015.1053101
- Zhou W, Zhu L, Zhou H, et al. The efficacy of high-intensity, focused ultrasound treatment for non-neoplastic epithelial disorders of the vulva. Cell Mol Biol (Noisy-Le-Grand). 2016;62(4):111–115.
- Lee A, Lim A, Fischer G. Fractional carbon dioxide laser in recalcitrant vulval lichen sclerosus. Australas J Dermatol. 2016;57(1):39–43. doi:10.1111/ajd.12305.
- Baggish MS. Fractional CO2 laser treatment for vaginal atrophy and vulvar lichen sclerosus. J Gynecol Surg. 2016;32(6):309–317. doi:10.1089/gyn.2016.0099
- Center for Vulvovaginal Disorders. MonaLisa touch laser for the treatment of vulvar lichen sclerosus NCT03665584. Clinicaltrials.gov [Internet]. November 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT03665584?cond=lichen+sclerosus&rank=2. Accessed January 3, 2020.
- Medstar Health Research Institute. Clobetasol proprionate versus fractionated carbon dioxide laser for the treatment of lichen sclerosus (CuRLS) NCT02573883. Clinicaltrials.gov [Internet]. August 2019. Available from: https://clinicaltrials.gov/ct2/show/NCT02573883. Accessed January 3, 2020.
- Hobson JG, Ibrahim SF, Mercurio MG. Recalcitrant vulvar lichen sclerosus treated with erbium YAG laser. JAMA Dermatol. 2019;155(2):254–256. doi:10.1001/jamadermatol.2018.4461
- Goldstein I. Dorsal slit surgery for clitoral phimosis. Sex Med. 2008;5(11):2485–2488. doi:10.1111/j.1743-6109.2008.01019.x
- Goldstein A. Perineoplasty and vaginal advancement flap for vulvar granuloma fissuratum. J Sex Med. 2011;8(11):2984–2987. doi:10.1111/j.1743-6109.2011.02528.x
- Kennedy CM, Dewdney S, Galask RP. Vulvar granuloma fissuratum: a description of fissuring of the posterior fourchette and the repair. Obstet Gynecol. 2005;105(5):1018–1023. doi:10.1097/01.AOG.0000158863.70819.53
- King M, Rubin R, Goldstein AT. Current uses of surgery in the treatment of genital pain. Current Sexual Health Rep. 2014;6(4):252–258. doi:10.1007/s11930-014-0032-8
- Goldstein AT, Burrows LJ. Surgical treatment of clitoral phimosis caused by lichen sclerosus. Am J Obstet Gynecol. 2007;196(2):126.e1–4. doi:10.1016/j.ajog.2006.08.023
- Flynn A, King M, Rieff M, Krapf J, Goldstein AT. Patient satisfaction of surgical treatment of clitoral phimosis and labial adhesions caused by lichen sclerosus. Sexual Med. 2015;3(4):251. doi:10.1002/sm2.90
- Rouzier R, Haddad B, Deyrolle C, et al. Perineoplasty for the treatment of introital stenosis related to vulvar lichen sclerosus. Am J Obstet Gynecol. 2002;186:49–52. doi:10.1067/mob.2002.119186