Abstract
Recurrent molar pregnancy is very rare. In this case report, we highlight a case of a patient who experienced five recurrent molar pregnancies without an intervening normal pregnancy. A 22-year-old patient was admitted to our labour room with a fifth consecutive molar pregnancy. The patient underwent suction and evacuation and was followed up with serial serum human chorionic gonadotropin (beta-hCG) estimation. The patient did not require chemotherapy. Karyotype of the patient and her husband was normal. Nonetheless, the couple was counselled for adoption.
Introduction
Hydatidiform mole (HM) is the most common form of gestational trophoblastic neoplasia, but its recurrence is very rare. Here, we highlight the case of a patient who experienced five consecutive molar pregnancies without even a single viable gestation in between them.
Case Report
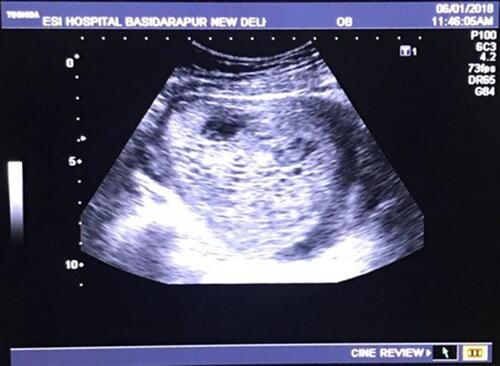
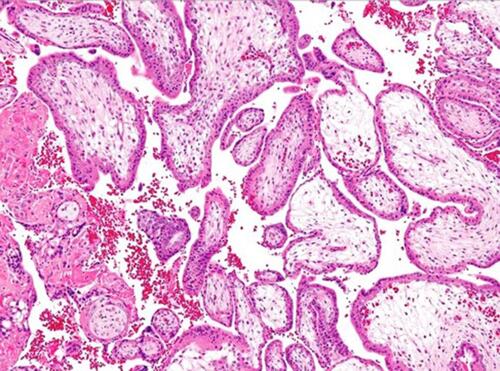
A 22-year-old patient, married for four years, fifth gravida, previous four abortions presented at 11th week of gestation and, was diagnosed with a complete molar pregnancy on ultrasonography (as shown in ). She gave a history of four previous histologically confirmed molar pregnancies, the last evacuation being 10 months prior to the present pregnancy. Her family had no history of recurrent molar pregnancy. Her blood group was A positive; and her complete blood count, renal function tests, liver function tests and chest X- ray were normal. Serum TSH was 0.02 micro international units/mL and her beta HCG was more than 100,000 milli–international units/mL. Karyotyping of the patient and her husband was normal. After informed consent and arrangement for adequate blood, the patient underwent an uneventful suction and evacuation procedure. The histopathology picture of the products showed characteristic hydropic changes in villi, as shown in . Post procedure, her serum human chorionic gonadotropin (beta-hCG) levels was measured weekly until undetectable and then monthly for 6 months. The patient was discharged on oral contraceptive pills and counselled for adoption.
Discussion
Hydatidiform mole (HM) is characterised by hydropic swelling of the placental villi, hyperplasia of villous trophoblast, and absent or abnormal foetal development. Hydatidiform moles occur as two types, complete and partial. Complete hydatidiform moles are most likely to result from the fertilisation of an empty egg, (ie one from which the nuclear material is either lost or inactivated), by a single haploid (23x chromosomes) sperm. This haploid set of chromosomes then duplicates to 46xx, so that the complete mole is homozygous and paternal in origin.Citation1 Less frequently, the empty egg is fertilised by two separate sperms, resulting in either a 46xx or a 46xy heterozygous chromosomal constitution.Citation2 In a partial hydatidiform mole, maternal chromosomes are present, along with 2 sets of paternal chromosome, leading to triploidy.Citation3 Both these variants can turn malignant, however complete moles are more commonly involved in the cancerous process.
In India and the Middle East, the incidence of molar pregnancy is estimated at 1 in 160 pregnancies.Citation4 In the UK, the rate is lower, at 1 in 600.Citation14 After one molar pregnancy, the risk of a HM in a subsequent pregnancy increases only to ∼1–2%.Citation5 However, this risk depends on the variant of the mole. Following a complete molar pregnancy, the risk of HM in the following pregnancy has been reported as 0.91 percent. The risk, however, seems to be lower, at 0.28 percent, following a partial mole in the prior pregnancy according to the literature. The risk can be further lowered if a molar pregnancy is followed by an intervening normal viable gestation. After two consecutive molar pregnancies, the risk increases to 23 percent.Citation5 Approximately 80% of the second HMs are of the same histopathological type as the index mole.Citation6
Molar pregnancy is associated with an increased risk of persistent trophoblastic gestational disease (PTD); consequently, proper diagnosis and risk assessment is imperative. Notably, patients with a uterine size at 4 weeks that is larger than the size during the period of amenorrhea, and those with the presence of a thecal lutein cyst with a size >6cm, have a 50% risk of persistent disease. Patients with complete molar pregnancies have a 5 percent risk of PTD, whereas this risk declines to <1% in cases of partial molar pregnancies.Citation7 Recurrent complete moles have significant implications, including a risk of malignant transformation and a poor reproductive future for the woman.Citation12
Patients with recurrent hydatidiform moles can be divided into two groups: one with and other without a positive family history. Those patients with a positive family history of recurrent complete moles and consanguinity are usually biparental.Citation11 Patients with a personal history of recurrent moles but no positive family history of recurrent moles usually have androgenetic complete hydatidiform moles.Citation10
Familial recurrent HM (FRHM) are extremely rare, with their occurrence reported thus far in only 21 families in the medical literature. In these cases, the HM are diploid, but bi-parental, unlike the androgenetic origin of sporadic complete moles. These patients have an autosomal recessive condition that results in recurrent molar pregnancies with little chance of a successful pregnancy.
The precise incidence of FRHM is not known. At present, two genes, NLRP7Citation8 and KHDC3LCitation9 account for ∼75% and 5% of the affected cases, respectively. The mechanism by which these mutations lead to molar pregnancies still remains to be deciphered. The pathological features, however, are exactly the same as those of a sporadic complete mole that is paternal in origin. In women with three or more molar pregnancies, genotyping is warranted for the correct diagnosis of FRHM. Looking for the above-mentioned mutations in patients with bi-parental molars is also recommended.Citation6 This becomes important in the management of this condition, as women with androgenetic complete moles who undergo in-vitro fertilisation (IVF) and pre-gestational diagnosis may have subsequent normal pregnancies that reduce the risk of further complete moles. Conversely, women with FRHM might consider IVF with a donor egg to achieve a viable pregnancy. In our case, the woman had no family history of molar pregnancy and the karyotypes of her and her husband were both normal. Genotyping of the molar tissue could not be done due to lack of resources.
Adequate follow-up of patients with HM should be done by measuring serial β-hCG levels to detect persistent gestational trophoblastic disease (PTD), which can potentially undergo malignant changes. Prophylactic chemotherapy is required in selected cases. The factors to be taken into consideration for this are high levels of beta-hCG at 4 weeks post evacuation (serum>20,000 IU/L; urine>30,000 IU/24 hrs), ongoing bleeding per vaginum, progressive rises in serum beta hCG level at any time following evacuation, detectable hCG at 4–6 months post-evacuation, and evidence of brain, kidney, liver, gastrointestinal tract or lung metastases, irrespective of beta- hCG levels. In our case, beta-HCG was undetectable by 1 month following the evacuation, so the patient did not receive chemotherapy.
Unlike persistent disease, repetitive molar pregnancy does not always warrant chemotherapy.Citation13 However, counselling on future pregnancies poses a real obstetric dilemma. With the acceptability, available resources, risk of malignancy, and potential psychological trauma due to recurrent miscarriage in mind, the couple in our case was counselled for adoption.
Conclusion
Recurrent molar pregnancy, although rare, is associated with significant mental trauma to the couple and an increased incidence of malignancy. Keeping genetic inheritance in consideration, we recommend that patients who present with recurrent molar pregnancy should be provided with the option of genetic testing. This would help to provide a better understanding of the woman’s future prognosis, thereby aiding in more effective counselling of the patient.
Consent for Publication
Written informed consent was obtained from the patient for submitting the case report and using the pictures. Institutional approval was not required for publishing the case details. The institutional policy is to grant ethical clearance for a study or a trial. For case report our institute recommends consent letter from individual(s) only.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- Wolf NG, Lage JM. Genetic analysis of gestational trophoblastic disease; A review. Semin Oncol. 1995;22:113–118.
- Lawler SD, Fisher RA, Dent J. A prospective study of complete and partial hydatidiform moles. Am J Obstet Gynecol. 1991;164:1270–1277. doi:10.1016/0002-9378(91)90698-Q
- Lawler SD, Fisher RA, Pickthall VJ, Povey S, Evans MW. Genetic studies on hydatidiform moles I: the origin of partial moles. Cancer Genet Cytogenet. 1985;5:309–320. doi:10.1016/0165-4608(82)90096-6
- Daftary SN, Padubidri VG. Trophoblastic diseases. In: Padubidri VG, Daftary SN, editors. Shaw’s Textbook of Gynaecology. 13th ed. New Delhi: Elsevier India, Ltd.; 2004:248–259.
- Bagshawe KD, Dent J, Webb J. Hydatidiform mole in England and Wales 1973-83. Lancet. 1986;2:673–677. doi:10.1016/S0140-6736(86)90179-0
- Eagles N, Sebire NJ, Short D, Savage PM, Seckl MJ, Fisher RA. Risk of recurrent molar pregnancies following complete and partial hydatidiform moles. Hum Reprod. 2015;30:2055–2063. doi:10.1093/humrep/dev169
- Sebire NJ, Foskett RA, Fisher RA, Rees H, Seckl M, Newlands E. Risk of partial and complete hydatidiform molar pregnancy in relationship to age. BJOG. 2002;109:99–102. doi:10.1111/j.1471-0528.2002.t01-1-01037.x
- Murdoch S, Djuric U, Mazhar B, et al. Mutations in NALP7 cause recurrent hydatidiform moles and reproductive wastage in humans. Nat Genet. 2006;38:300–302. doi:10.1038/ng1740
- Parry DA, Logan CV, Hayward BE, et al. Mutations causing familial biparental hydatidiform mole implicate c6orf221 as a possible regulator of genomic imprinting in the human oocyte. Am J Hum Genet. 2011;89:451–458. doi:10.1016/j.ajhg.2011.08.002
- Van Der Smagt JJ, Scheenjes E, Kremer JA, Hennekam FA, Fisher RA. Heterogeneity in the origin of recurrent complete hydatidiform moles: not all women with multiple molar pregnancies have biparental moles. BJOG. 2006;113:725–728. doi:10.1111/bjo.2006.113.issue-6
- Al-hussaini TK, Abd El-Aal DM, Den Veyver IB. Recurrent pregnancy loss due to familial and non-familial habitual molar pregnancy. Int J Gynaecol Obstet. 2003;83:179–186. doi:10.1016/S0020-7292(03)00209-1
- Madhudas C, Khursheed F, Srichand P. Analysis of patients with recurrent molar pregnancy. Eur J Biol Sci. 2011;3:102–104.
- Marakani LR, Gundabattula SR. Recurrent molar pregnancy: an obstetric dilemma? Int J Infertility Fetal Med. 2012;3:63–64. doi:10.5005/jp-journals-10016-1043
- Savage P, Williams J, Wong SL, et al. The demographics of molar pregnancies in England and Wales from 2000–2009. J Reprod Med. 2010;55:341–345.