Abstract
Many women experience bothersome vasomotor and vaginal symptoms during the menopausal transition. Decreasing levels of estrogens during menopause are also associated with reduced bone density and an increased risk of osteoporosis. Combined estrogen/progestin therapy (hormone therapy) effectively treats menopausal symptoms and prevents bone loss, but has been associated with some safety and tolerability concerns. A novel menopausal therapy is the tissue selective estrogen complex, which pairs a selective estrogen receptor modulator with one or more estrogens. In preclinical studies, the tissue selective estrogen complex partnering bazedoxifene (BZA) with conjugated estrogens (CE) antagonized stimulation of breast and endometrial tissue, reduced vasomotor instability, and preserved bone mass in rat and mouse models. The specific attributes seen with BZA/CE were different from those observed with other selective estrogen receptor modulator/estrogen pairings. BZA/CE has undergone clinical evaluation in the Phase III Selective estrogens, Menopause, And Response to Therapy (SMART) trials in postmenopausal women with an intact uterus. Of the various doses of BZA/CE evaluated, BZA 20 mg/CE 0.45 mg and 0.625 mg were associated with a low incidence of endometrial hyperplasia (<1%) similar to placebo, and showed significant improvements in hot flushes and vulvar/vaginal symptoms and increases in bone mineral density. BZA 20 mg/CE 0.45 mg and 0.625 mg were associated with a low incidence of breast-related adverse events and demonstrated no difference from placebo in age-related changes in mammographic breast density. Both BZA/ CE doses showed a favorable tolerability profile, with no increases in uterine bleeding or breast tenderness, and had positive effects on metabolic parameters and quality of life. BZA/CE may be a promising alternative to hormone therapy for the treatment of menopausal symptoms and prevention of osteoporosis in nonhysterectomized postmenopausal women.
Introduction
Postmenopausal women may experience a variety of symptoms associated with a decline in the level of estrogens.Citation1 Vasomotor symptoms (VMS), such as hot flushes, are reported by 60%–85% of women during menopause.Citation2 Vulvar/vaginal atrophy (VVA; symptoms of which may include vaginal dryness, irritation, soreness, or dyspareunia, and increases in urinary frequency, urgency, or incontinence) is reported by 50% of postmenopausal women.Citation3 The menopausal transition may also accelerate the rate of bone loss, leading to susceptibility to postmenopausal osteoporosis and associated fractures.Citation4–Citation6 In addition to the burden of the reduced quality of life (QoL) imposed by menopausal symptoms, the financial costs of menopausal treatments, lost productivity, and increased health care expenses present a significant economic burden.Citation7
Many menopausal therapies are designed to treat individual symptoms.Citation5 Topical estrogens or estradiol preparations such as creams, pessaries, tablets, and vaginal rings have been shown to be effective for the treatment of VVA.Citation8 Tibolone has been shown to effectively treat VMS and VVA.Citation9 Bisphosphonates, selective estrogen receptor modulators (SERMs), parathyroid hormone, estrogens, and calcitonin are approved pharmacologic agents for the prevention and/ or treatment of postmenopausal osteoporosis.Citation10
The most common treatments for postmenopausal women who experience moderate-to-severe VMS along with other menopausal symptoms are estrogens and combined estrogen/ progestin or estradiol/progestin therapy (hormone therapy [HT]).Citation11 HT options include conjugated estrogens (CE)/ medroxyprogesterone acetate (MPA), sequential estrogens/ trimegestone, drospirenone/estradiol, estradiol/norethindrone, and transdermal estradiol/norethindrone acetate.Citation5 In addition to effectively treating VMS and VVA, estrogen monotherapy and HT have also shown efficacy in preventing postmenopausal osteoporosis and are considered comprehensive treatment options.Citation11 Although the addition of progestins to estrogens provides protection against endometrial stimulation for postmenopausal women with a uterus,Citation12,Citation13 HT has been associated with some safety and tolerability concerns, including cardiovascular risk, breast stimulation, and irregular vaginal bleeding.Citation11,Citation14,Citation15
A novel therapy currently under investigation for the treatment of menopausal symptoms and the prevention of osteoporosis is the tissue selective estrogen complex (TSEC), which pairs a SERM with one or more estrogens.Citation16 SERMs have estrogen receptor (ER) agonist or antagonist activities depending on the target tissue.Citation17,Citation18 The goal of TSEC is to combine the established efficacy of estrogens on VMS, VVA, and bone with the protective effects of a SERM on the uterus and breast.Citation16 The first TSEC in clinical development partners bazedoxifene (BZA) with CE. BZA is a SERM that has demonstrated efficacy in the treatment and prevention of osteoporosis with a favorable endometrial/breast safety profile in postmenopausal women. This review provides an overview of the key preclinical findings for BZA/CE and describes the results from the Phase III clinical trials of BZA/CE in postmenopausal women with an intact uterus. The emerging role of TSEC in the current treatment paradigm for postmenopausal women is also discussed.
Preclinical studies with BZA/CE
In vitro studies
BZA/CE was developed with the goal of achieving an optimal balance of ER agonist and antagonist activities. Berrodin evaluated BZA/CE, raloxifene (RLX)/CE, and lasofoxifene (LAS)/CE in a multiplex biochemical assay.Citation19 In this assay, BZA interacted with some cofactor peptides and not others, while LAS and RLX inhibited all of the peptides to which they were exposed. These results suggest that when combined with CE, BZA is more likely to exhibit tissue-selective effects than LAS or RLX.Citation19 In a cell-based system of Michigan Cancer Foundation-7 (MCF-7) breast cancer cells, the global gene expression pattern associated with BZA/CE differed from that with RLX/CE or LAS/CE. The expression profile of BZA/CE was more similar to that with CE alone; in contrast, RLX/CE and LAS/CE had profiles that were similar to the SERM alone.Citation19 In another study, the proliferation of MCF-7 cells was not induced by BZA/CE, although some proliferation was observed with RLX/CE and LAS/CE.Citation20 These findings support a distinct activity profile for BZA/CE among other TSEC pairings in displaying agonist activity in some tissues and antagonist activity in others.Citation19
In vivo studies
The effects of BZA, RLX, and LAS (all 3 mg/kg) combined with estradiol (1 μg/kg) on breast and uterine tissues have been evaluated in ovariectomized (OVX) mice.Citation21 In this model, BZA, RLX, and LAS prevented estradiol-induced increases in uterine wet weight (P < 0.05 vs estradiol alone). BZA was a more effective antagonist of estradiol-induced increases in uterine wet weight than RLX or LAS (P < 0.05 vs estradiol/RLX or estradiol/LAS). In addition, treatment with BZA and RLX, but not LAS, reduced the estradiol-induced mammary gland end bud proliferation and all three SERMs significantly reduced the estradiol-induced expression of GPR105 and INDO mRNA markers of uterine and breast stimulation, respectively (P < 0.05 vs estradiol alone for both markers).Citation21
In a study of mature OVX rats treated with BZA/CE daily for 6 weeks,Citation16 the combination of BZA 3.0 mg/kg with CE 0.5, 1.0, or 2.5 mg/kg prevented CE-induced increases in uterine wet weight. Treatment with BZA 0.1, 0.3, 1.0, or 3.0 mg/kg/CE 2.5 mg/kg and BZA 3.0 mg/kg/CE 0.5, 1.0, 2.5, or 5.0 mg/kg resulted in higher proximal tibia total bone mineral density (BMD) than with vehicle control (P < 0.01). CE 10.0 mg/kg significantly reduced tail skin temperature versus vehicle control in the morphine-addicted rat model of vasomotor instability. Addition of BZA 0.3, 3.0, or 10.0 mg/kg to CE 10.0 mg/kg maintained this response (P < 0.05 vs vehicle control). BZA 3.0 mg/kg/CE 0.5, 1.0, 2.5, or 5.0 mg/kg was also associated with significant decreases in serum total cholesterol compared with vehicle control (P < 0.01).Citation16
The endometrial and breast effects of BZA/CE were further evaluated in OVX sexually immature mice.Citation22 The minimal doses of BZA, RLX, and LAS that prevented CE-induced increases in uterine wet weight were determined to be BZA 2 mg/kg, RLX 10 mg/kg, and LAS 2 mg/kg. At these doses, the combinations of BZA and RLX with CE 3 mg/kg were more effective than LAS 2 mg/kg/CE 3 mg/kg at preventing CE-induced increases in uterine wet weight (P < 0.05). BZA was a better antagonist of CE-induced breast stimulation than RLX or LAS (P < 0.05), as measured by mammary gland amphiregulin mRNA expression. In an analysis of mammary gland whole mounts using these same SERM/CE doses, BZA/CE reduced the number of ductal branch points, a measure of ductal tree complexity, to a level similar to that with vehicle control and significantly lower than RLX/CE or LAS/CE (P < 0.05).Citation22
The skeletal effects of BZA/CE were evaluated in OVX rats treated daily for 1 year with BZA 0.3 mg/kg, CE 2.5 mg/kg, or BZA 0.1, 0.3, or 1.0 mg/kg/CE 2.5 mg/kg.Citation23 BMD of the lumbar spine and right proximal femur was significantly increased at 1 year compared with OVX control for all doses of BZA/CE (P < 0.05). Similarly, trabecular BMD of the proximal tibia metaphysis was significantly increased at 1 year with all doses of BZA/CE compared with OVX control (P < 0.05). Histomorphometry evaluation showed that all doses of BZA/CE prevented the OVX-induced changes in static and dynamic parameters of the cortical compartment of the tibia and cancellous compartment of the L1 and L2 vertebrae. BZA 0.1, 0.3, and 1.0 mg/kg/CE 2.5 mg/kg reduced uterine wet weights in a dose-dependent manner. BZA 0.1, 0.3, and 1.0 mg/kg/CE 2.5 mg/kg were associated with significant decreases in serum total cholesterol compared with OVX control (P < 0.05).
Overall, preclinical studies have shown that BZA/CE effectively prevents stimulation of uterine and breast tissue in a variety of in vitro and in vivo models. BZA/CE has also been shown to preserve bone mass, reduce vasomotor effects, and reduce serum total cholesterol in OVX rats. These findings in preclinical models suggest that BZA/CE has the potential to effectively treat menopausal symptoms, prevent bone loss, and have favorable effects on the lipid profile while maintaining breast and endometrial safety in nonhysterectomized postmenopausal women. BZA/CE has been subsequently evaluated in a series of Phase III clinical trials.
Clinical studies with BZA/CE
The efficacy and safety of BZA/CE have been evaluated in multicenter, randomized, double-blind, placebo- and active-controlled, Phase III studies referred to as the Selective estrogens, Menopause, And Response to Therapy (SMART) trials (). These studies were conducted in postmenopausal women with an intact uterus, and assessed the efficacy, safety, and tolerability of BZA/CE in treating menopausal symptoms and preventing osteoporosis. Published reports of the Phase III studies of BZA/CE are described in this review.
Table 1 Study designs for Phase III clinical trials of BZA/CE
The 2-year SMART-1 trial (n = 3544) was conducted to evaluate the incidence of endometrial hyperplasia at 1 year in generally healthy women aged 40 to 75 years.Citation24–Citation27 Other endpoints included the effects of BZA/CE on BMD, bone turnover markers, VMS, VVA, safety, metabolic parameters, uterine bleeding, and QoL. The treatments evaluated in this trial were BZA 10, 20, and 40 mg/CE 0.45 and 0.625 mg, RLX 60 mg, and placebo.
The SMART-2 trial (n = 332)Citation28 evaluated the change from baseline in frequency and severity of hot flushes at 12 weeks in women aged 40 to 65 years who reported at least seven moderate-to-severe hot flushes per day (or ≥50 per week) at baseline. The SMART-3 trial (n = 664)Citation29 evaluated changes in four measures of VVA (the proportion of vaginal superficial cells, the proportion of parabasal cells, vaginal pH, and severity of the most bothersome VVA symptom) in women aged 40 to 65 years who had at least one moderate-to-severe symptom of VVA at baseline. Secondary endpoints included effects on sleep (SMART-2 only), QoL, and safety. The treatments evaluated in these trials were BZA 20 mg/CE 0.45 and 0.625 mg, BZA 20 mg (SMART-3 only), and placebo.
Efficacy on menopausal symptoms
Vasomotor effects
The efficacy of BZA/CE on VMS was evaluated by measuring the frequency and severity of hot flushes in response to treatment. In the SMART-1 trial, vasomotor effects were assessed in a subset of women from the overall study population who had at least seven moderate-to-severe hot flushes per day (or ≥50 per week) at baseline (n = 216).Citation24 At Week 12, treatment with all doses of BZA/CE significantly reduced the mean daily number of moderate and severe hot flushes from baseline (range, −51.7% to −85.7%) compared with placebo (−17.1%; P < 0.05). The mean change in hot flush severity from baseline to Week 12 was reduced for all doses of BZA/CE compared with placebo. These reductions were significant (P < 0.001) for BZA 10 and 20 mg/CE 0.45 and 0.625 mg. The significant treatment effects on hot flush frequency and severity with BZA 10 and 20 mg/CE 0.45 and 0.625 mg were maintained through 2 years.Citation24
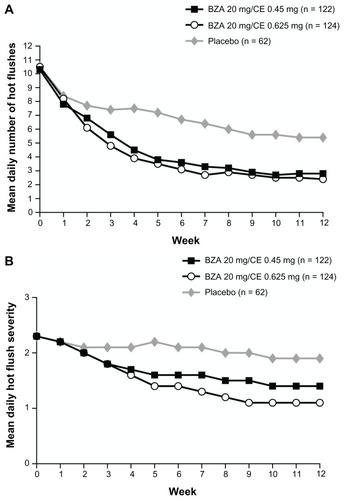
The SMART-2 trial evaluated the efficacy of VMS on a larger population of symptomatic women (n = 332). At Week 12,Citation28 treatment with BZA 20 mg/CE 0.45 and 0.625 mg significantly reduced the number of hot flushes from baseline by 74% and 80%, respectively, compared to 51% with placebo (P < 0.001; ). The mean daily hot flush severity was also significantly reduced for BZA 20 mg/CE 0.45 and 0.625 mg compared with placebo at Week 12 (P < 0.001). With BZA 20 mg/CE 0.45 and 0.625 mg, 61% and 73% of women, respectively, had a 75% or greater decrease in the mean number of hot flushes than those treated with placebo (27%; P < 0.001) at Week 12.Citation28
Figure 1 The mean daily number (A) and severity (B) of moderate-to-severe hot flushes over 12 weeks in the SMART-2 trial.
Copyright © 2009, Wolters Kluwer Health. Reprinted with permission from Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause. 2009;16(6):1116–1124.
Effects on VV A
The efficacy of BZA/CE in treating VVA symptoms was evaluated by changes in the degree of maturation of the vaginal epithelium, measured by the proportion of superficial, intermediate, and parabasal cells on a vaginal smear.Citation24 In the SMART-1 trial, VVA was assessed in a subset of women from the overall study population who had no more than 5% superficial cells at baseline (n = 1867).Citation24 At Month 24, BZA 10 and 20 mg/CE 0.45 and 0.625 mg were associated with a significant increase in superficial cells compared with placebo (P < 0.001 for BZA 10 mg/CE 0.45 and 0.625 mg; P < 0.01 for BZA 20 mg/CE 0.625 mg). There was a significant increase in intermediate cells and a significant decrease in parabasal cells with BZA 10 and 20 mg/CE 0.45 and 0.625 mg compared with placebo (P < 0.001 for all). BZA 10 mg/CE 0.625 mg and BZA 20 mg/CE 0.45 and 0.625 mg were also associated with a lower incidence of dyspareunia (defined as pain during sexual intercourse) than placebo (P < 0.05) during Weeks 9 to 12.Citation24
Similar to the subset within the SMART-1 trial, the SMART-3 trial enrolled women with at least one moderate-to- severe VVA symptom. BZA 20 mg/CE 0.45 and 0.625 mg significantly increased superficial cells (P ≤ 0.001), decreased parabasal cells (P ≤ 0.001), and increased intermediate cells (P < 0.01 for BZA 20 mg/CE 0.45 mg; P ≤ 0.001 for BZA 20 mg/CE 0.625 mg) compared with placebo at 12 weeks.Citation29 Mean vaginal pH significantly decreased from baseline to Week 12 for both doses of BZA/CE (P < 0.001). With BZA 20 mg/CE 0.625 mg, but not BZA 20 mg/CE 0.45 mg, severity scores for the vaginal symptom originally identified by each woman as the most bothersome were significantly improved at Week 12 compared with placebo (P = 0.048). At Week 12, 81% of women treated with BZA 20 mg/CE 0.625 mg and 78% of women treated with BZA 20 mg/CE 0.45 mg had responded to treatment (defined as vaginal superficial cells >5%, vaginal pH <5, and/or improvement from baseline in most bothersome vaginal symptom) compared with 66% of women given placebo (P = 0.005 and P = 0.027, respectively).Citation29
Bone effects
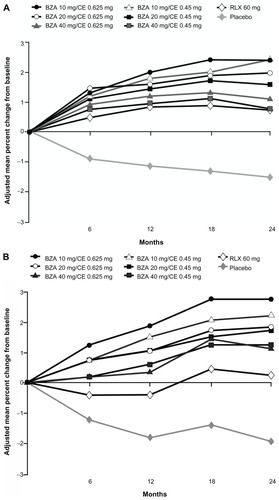
The efficacy of BZA/CE in preventing bone loss was evaluated by changes in BMD as measured by dual-energy X-ray absorptiometry; changes in bone turnover markers (surrogate markers of bone formation and bone resorption) were also assessed. Although there has been some variability associated with bone turnover marker measurements, these markers are widely used to evaluate bone metabolism and treatment response to bone-active therapies.Citation30 Effects on BMD were evaluated in two substudies within the SMART-1 trial, in women who were between 1 and 5 years since menopause (YSM) and in those who were greater than 5 YSM. All doses of BZA/CE significantly increased lumbar spine and total hip BMD compared with placebo at 2 years in the SMART-1 trial (P < 0.001 for lumbar spine for all women, P < 0.01 for total hip in women 1–5 YSM, and P < 0.001 for total hip in women >5 YSM; ).Citation26
Figure 2 Adjusted mean percent change in BMD from baseline over 2 years in the SMART-1 trial.
Copyright © 2009, Elsevier. Reprinted with permission from Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045–1052.
Abbreviations: BMD, bone mineral density; SMART, Selective estrogens, Menopause And Response to Therapy; YSM, years since menopause; BZA, bazedoxifene; CE, conjugated estrogens; RLX, raloxifene.
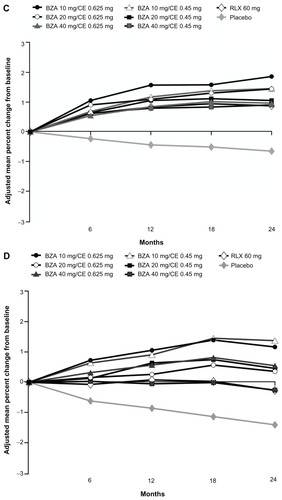
Changes from baseline in C-telopeptide and osteocalcin were measured in women who were between 1 and 5 YSM. At 2 years, the median percent change in levels of C-telopeptide (range, −42.72% to −53.40%) and osteocalcin (range, −19.78% to −28.05%) with all doses of BZA/CE were significantly reduced compared with placebo (−13.81% and 3.08%, respectively; P < 0.001 for all).Citation26
Safety
Endometrial safety
In the SMART-1 trial, endometrial safety was assessed by the incidence of endometrial hyperplasia, which has been used as a surrogate marker for endometrial cancer.Citation27 In the SMART-1 trial, endometrial biopsy results showed one case of endometrial hyperplasia (0.32%) with BZA 20 mg/CE 0.625 mg and no cases with BZA 20 mg/CE 0.45 mg or BZA 40 mg/CE 0.45 or 0.625 mg at 1 year. These hyperplasia rates were not significantly different from placebo or RLX 60 mg. With BZA 10 mg/CE 0.45 and 0.625 mg, there were three cases (0.94%) and 13 cases (3.81%) of endometrial hyperplasia, respectively. The 3.81% rate of endometrial hyperplasia with BZA 10 mg/CE 0.625 mg was determined to be unacceptable per study criteria. Citation27 As a result, further investigations of BZA/CE have focused on doses of BZA 20 mg/CE 0.45 and 0.625 mg.
Breast safety
High mammographic breast density has been shown to be a moderate independent risk factor for breast cancer.Citation31,Citation32 The effects of BZA/CE on age-related changes in mammographic breast density were evaluated in an ancillary study of the SMART-1 trial. Findings showed that the change in baseline breast density was similar for BZA 20 mg/CE 0.45 mg (−0.39%), BZA 20 mg/CE 0.625 mg (−0.05%), RLX 60 mg (−0.23%), and placebo (−0.42%) over 2 years of treatment.Citation33 The incidence of breast cancer in the SMART-1 trial was low and similar for BZA 20 mg/CE 0.45 mg (n = 1), BZA 20 mg/CE 0.625 mg (n = 0), and placebo (n = 1) over 2 years.Citation34 There was no significant difference in the incidence of abnormal mammograms for BZA 20 mg/CE 0.45 mg (4.4%), BZA 20 mg/CE 0.625 mg (4.2%), and placebo (2.6%).Citation35,Citation34 In a pooled analysis of data from the SMART-1, SMART-2, and SMART-3 trials, the incidence of breast-related adverse events (AEs) was low (<0.3%) and similar for BZA 20 mg/CE 0.45 mg and placebo (BZA 20 mg/CE 0.625 mg was not included in this analysis).Citation35 There were no reports of fibrocystic breast disease or breast mass with BZA 20 mg/CE 0.45 mg or placebo in these three trials.Citation35 Together, these results suggest that BZA/CE has a neutral effect on the breast.
General safety and tolerability
Among all the SMART trials, the overall incidence of treatment-emergent AEs was similar among groups.Citation24,Citation28,Citation29 There were no significant differences between the BZA/CE and placebo groups in the percentage of women reporting breast pain in the SMART-1, SMART-2, and SMART-3 trials.Citation24,Citation28,Citation29 In the SMART-1 trial, BZA 20 mg/CE 0.45 and 0.625 mg were associated with low rates of bleeding/spotting and high rates of amenorrhea over consecutive 4-week cycles (>83% during Cycles 1–13 and >93% during Cycles 10–13 during Year 1), with no significant differences compared with placebo.Citation25
Venous thromboembolic events
In the SMART-1 trial, the incidence of venous thromboembolic events (VTEs) was 0.75 per 1000 woman-years for the combined BZA/CE treatment groups compared with 1.56 per 1000 woman-years for the placebo group (relative risk, 0.48; 95% confidence interval, 0.05–4.66). These VTEs included one report of deep vein thrombosis in each of the BZA 10 mg/CE 0.625 mg and BZA 40 mg/CE 0.625 mg groups and one case of deep vein thrombosis in the placebo group, as well as one case of pulmonary embolism with BZA 40 mg/CE 0.625 mg.Citation24 No VTEs were reported in the SMART-2 or SMART-3 trials, but these studies were of 12-week duration.Citation28,Citation29
Lipid and coagulation parameters
Lipids
Women may experience adverse changes to the metabolic profile during the menopausal transition, including increases in total cholesterol, triglycerides, low-density lipoprotein (LDL) cholesterol, and lipoprotein (a) and decreases in high-density lipoprotein (HDL) cholesterol.Citation36 The effects of BZA/CE on lipid parameters were evaluated in the SMART-1 trial. At Month 24, BZA 20 mg/CE 0.45 and 0.625 mg were associated with decreases from baseline in total cholesterol (P < 0.05 vs placebo for BZA 20 mg/ CE 0.45 mg) and LDL cholesterol (P < 0.01 vs placebo for both). HDL cholesterol increased from baseline (P < 0.01 vs placebo) with BZA 20 mg/CE 0.45 and 0.625 mg, as did triglyceride (P < 0.01), HDL-2 cholesterol (P < 0.001), and apolipoprotein A1 (P < 0.001) levels compared with placebo. BZA 20 mg/CE 0.45 and 0.625 also showed significant decreases from baseline in apolipoprotein B (P < 0.05) and lipoprotein (a) (P < 0.01) versus placebo.Citation24
At Week 12 of the SMART-2 trial, both doses of BZA/CE were associated with significant decreases from baseline compared with placebo in total and LDL cholesterol. HDL cholesterol decreased from baseline over 12 weeks with placebo, but was maintained at baseline levels with BZA/ CE. Triglyceride levels significantly increased from baseline with BZA 20 mg/CE 0.45 and 0.625 mg and with placebo. The increase in triglycerides was clinically important for more women in the placebo group than in either BZA/CE group (P = 0.04).Citation28
Both doses of BZA/CE were associated with significant decreases in LDL cholesterol (P < 0.01) at Week 12 in the SMART-3 trial. BZA 20 mg/CE 0.625 mg showed a small increase in triglyceride levels that was statistically significant compared with placebo (P < 0.05).Citation29
Coagulation parameters
Changes in coagulation factors have been shown to occur during the menopausal transition, such as increases in levels of the coagulation proteins factor VII and fibrinogen.Citation36 The effects of BZA/CE on coagulation parameters were evaluated in the SMART-1 trial. At Month 24, there were significant decreases from baseline in levels of fibrinogen (P < 0.001), protein S activity (P < 0.01), and antithrombin III activity (P < 0.05 and P < 0.01) for BZA 20 mg/CE 0.45 and 0.625 mg compared with placebo.Citation24 Neither dose of BZA/CE affected partial thromboplastin time, prothrombin time, or D-dimer levels. There were small decreases from baseline with BZA 20 mg/CE 0.45 and 0.625 mg in plasminogen activator inhibitor-1 activity (P < 0.05 for both doses) and plasminogen activator inhibitor-1 antigen levels (P < 0.05 for BZA 20 mg/CE 0.625 mg). Plasminogen activity was significantly increased from baseline with BZA 20 mg/CE 0.45 and 0.625 mg (P < 0.001).Citation24
Sleep, QoL, and satisfaction
During menopause, many women experience bothersome symptoms such as VMS, mood disturbances, or sexual dysfunction, which may negatively impact QoL.Citation7 Sleep disruptions, including increased time to fall asleep, night-time awakenings, and daytime tiredness, are also common among postmenopausal women.Citation37 The effects of BZA/CE on sleep parameters were evaluated in the SMART-1 trial using daily diaries and in the SMART-2 trial using the Medical Outcomes Study sleep scale. Impact on QoL measures was assessed in each SMART trial using the Menopause-specific Quality Of Life (MENQOL) questionnaire. In the SMART-3 trial, effects on sexual function were assessed using the Arizona Sexual Experiences scale. Satisfaction with treatment was evaluated in the SMART-2 and SMART-3 trials as measured by the Menopause Symptoms Treatment Satisfaction Questionnaire.
Sleep
In the SMART-1 trial, women treated with BZA 20 mg/ CE 0.45 and 0.625 mg had significant reductions in mean minutes to fall asleep (−11.6 and −10.9 minutes, respectively), increases in mean minutes slept (22.6 and 19.7 minutes, respectively), and increases in quality of sleep score (0.29 and 0.27, respectively) compared with placebo (−5.6 minutes, 10.1 minutes, and 0.13, respectively; P < 0.05) at 13 weeks.Citation38 In a subset of women who had at least seven hot flushes per day at baseline (n = 81), BZA 20 mg/CE 0.45 and 0.625 mg improved mean minutes slept and quality of sleep score, but did not affect time to fall asleep (this analysis was not powered to show statistical significance).Citation38 In the SMART-2 trial, BZA 20 mg/CE 0.45 and 0.625 mg significantly improved time to fall asleep, sleep disturbance, sleep adequacy, and overall sleep problems indexes I and II compared with placebo (P < 0.001 for all) at 12 weeks in the SMART-2 trial as assessed on the Medical Outcomes Study sleep scale.Citation39
QoL
At Week 12 in the SMART-1 trial, BZA 20 mg/CE 0.45 and 0.625 mg significantly improved total (−0.7 and −0.8, respectively) and vasomotor function (−1.2 and −1.5, respectively) MENQOL scores compared with placebo (−0.5 for both domains; P < 0.001).Citation40 Similar results were observed in the SMART-2 trial in which BZA 20 mg/CE 0.45 and 0.625 mg significantly improved total (−1.6 and −1.9, respectively) and vasomotor function (−3.3 and −3.8, respectively) MENQOL scores compared with placebo (−1.0 and −1.6, respectively; P < 0.001).Citation39,Citation40 In the SMART-3 trial, women treated with BZA 20 mg/CE 0.45 and 0.625 mg reported significant improvements in ease of lubrication on the Arizona Sexual Experiences scale (−0.82 and −0.85, respectively) compared with placebo (−0.50; P < 0.05). BZA 20 mg/CE 0.45 and 0.625 mg were also associated with significant improvements from baseline in the total (−1.09 and −1.18, respectively), vasomotor (−1.33 and −1.67, respectively), and sexual (−1.95 and −1.91, respectively) function compared with placebo (−0.67, −0.51, and −1.24, respectively; P < 0.001 for all).Citation41
Treatment satisfaction
In the SMART-2 trial, Menopause Symptoms Treatment Satisfaction Questionnaire results showed that 73.5% of women treated with BZA 20 mg/CE 0.45 mg and 78.2% of women treated with BZA 20 mg/CE 0.625 mg were satisfied with treatment compared with 44.4% of women given placebo (P < 0.001). In particular, women treated with BZA 20 mg/CE 0.45 and 0.625 mg showed significantly higher satisfaction than those given placebo in the categories of ability to control hot flushes during the day (P < 0.001) and during the night (P < 0.001), effect on quality of sleep (P < 0.001), and effect on mood and emotions (P < 0.05).Citation39 Similarly, in the SMART-3 trial, 62.6% of women treated with BZA 20 mg/CE 0.45 mg and 69.4% of women treated with BZA 20 mg/CE 0.625 mg were satisfied with treatment compared with 47.5% of women given placebo (P < 0.05 and P < 0.001, respectively). Significantly greater proportions of women treated with BZA 20 mg/CE 0.45 and 0.625 mg were satisfied with their ability to control hot flushes during the day (P < 0.001) and during the night (P < 0.001), effect on mood or emotions (P < 0.05), and effect on quality of sleep (P < 0.001) compared with placebo at 12 weeks.Citation41
Conclusion
The TSEC is a novel menopausal therapy that has been developed to blend the uterine- and breast-protective effects of a SERM in women with a uterus with the established benefits of estrogens on VMS, VVA, and bone. TSECs, such as BZA/ CE, may be an appropriate alternative to HT for women with concerns about the potential safety risks of HT (combined estrogen/progestin or estradiol/progestin therapy) such as cardiovascular risk and breast stimulation.Citation11,Citation14,Citation15 Tolerability concerns include breast pain and irregular vaginal bleeding.Citation11
Preclinical studies showed that BZA/CE has favorable vasomotor effects and prevents bone loss in OVX rats while minimizing stimulation of uterine and breast tissue in a variety of in vitro and in vivo models. Furthermore, the tissue-selective effects of BZA/CE were distinct from those of RLX/CE or LAS/CE. BZA was shown to be a stronger antagonist of CE-induced breast cancer cell proliferation and mammary gland end bud formation than RLX or LAS. BZA and RLX were more effective in inhibiting CE-induced increases in uterine wet weight than was LAS.
In Phase III clinical studies, BZA 20 mg/CE 0.45 and 0.625 mg were shown to significantly reduce the frequency/ severity of hot flushes and improve VVA measures in symptomatic postmenopausal women. These BZA/CE doses also significantly increased BMD at the lumbar spine and total hip while preventing endometrial stimulation, as shown by low rates of endometrial hyperplasia similar to those with placebo.
Results of the SMART trials showed that treatment with BZA 20 mg/CE 0.45 and 0.625 mg maintained breast safety, with low rates of breast-related AEs and no changes in breast density. In contrast, HT use has been reported to increase mammographic breast density,Citation42–Citation44 and long-term estrogen plus progestin therapy has been associated with an increased risk of breast cancer.Citation11 Study findings also showed that BZA/ CE has a favorable tolerability profile, with incidences of bleeding/spotting and breast tenderness that were low and similar compared with placebo. These results contrast those reported for HT, which has been associated with increased rates of bleeding/spotting as well as breast pain/tenderness compared with placebo.Citation45 BZA/CE also showed positive effects on coagulation factors and improvements in lipid parameters in the SMART trials, including decreases in total and LDL cholesterol and increases in HDL cholesterol. These effects on blood lipids with BZA/CE have been shown to be similar to those observed with CE alone and with CE/MPA.Citation46
BZA/CE has demonstrated significant improvements in sleep parameters, including time to fall asleep and overall sleep scores. Studies of HT have also shown improvements in sleep, although the effects were relatively small.Citation47–Citation50 BZA/CE also showed significant improvements in total and vasomotor function in all three SMART trials; in comparison, the effects of HT on QoL parameters have been mixed.Citation47,Citation48,Citation51–Citation53
Overall, BZA/CE has been shown to be generally safe and well tolerated over 2 years of treatment in postmenopausal women and is associated with high rates of treatment satisfaction. Collectively, these data suggest that BZA/CE is a promising option for the comprehensive treatment of menopausal symptoms and prevention of postmenopausal osteoporosis. Further studies are needed to determine the long-term efficacy and safety of BZA/CE in postmenopausal women.
Acknowledgments
Medical writing support for this manuscript was provided by Katie Gersh, PhD, of MedErgy, and was funded by Pfizer Inc. The authors retained full editorial control over the content of the article.
Disclosure
BSK and SM are employees of Pfizer Inc.
References
- BurgerHGThe endocrinology of the menopauseMaturitas19962321291368735351
- GoldEBColvinAAvisNLongitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women’s health across the nationAm J Public Health20069671226123516735636
- Mac BrideMBRhodesDJShusterLTVulvovaginal atrophyMayo Clin Proc2010851879420042564
- RiggsBLKhoslaSMeltonLJIIIA unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging menJ Bone Miner Res19981357637739610739
- LewisVUndertreatment of menopausal symptoms and novel options for comprehensive managementCurr Med Res Opin200925112689269819775194
- National Osteoporosis FoundationFast Facts on Osteoporosis2010 Available from: http://www.nof.org/node/40Accessed November 17, 2011
- UtianWHPsychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive reviewHealth Qual Life Outcomes200534716083502
- SucklingJLethabyAKennedyRLocal oestrogen for vaginal atrophy in postmenopausal womenCochrane Database Syst Rev20064CD00150017054136
- SwansonSGDrosmanSHelmondFAStathopoulosVMTibolone for the treatment of moderate to severe vasomotor symptoms and genital atrophy in postmenopausal women: a multicenter, randomized, double-blind, placebo-controlled studyMenopause200613691792517006377
- North American Menopause SocietyManagement of osteoporosis in postmenopausal women: 2010 position statement of The North American Menopause SocietyMenopause2010171255420061894
- North American Menopause SocietyEstrogen and progestogen use in postmenopausal women: 2010 position statement of The North American Menopause SocietyMenopause201017224225520154637
- VoigtLFWeissNSChuJDalingJRMcKnightBvan BelleGProgestagen supplementation of exogenous oestrogens and risk of endometrial cancerLancet199133887622742771677110
- WeiderpassEAdamiHOBaronJARisk of endometrial cancer following estrogen replacement with and without progestinsJ Natl Cancer Inst199991131131113710393721
- RossouwJEAndersonGLPrenticeRLRisks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trialJAMA2002288332133312117397
- RolnickSJKopherRADeForTAKelleyMEHormone use and patient concerns after the findings of the Women’s Health InitiativeMenopause200512439940416037754
- KharodeYBodinePVMillerCPLyttleCRKommBSThe pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis preventionEndocrinology2008149126084609118703623
- KommBSLyttleCRDeveloping a SERM: stringent preclinical selection criteria leading to an acceptable candidate (WAY-140424) for clinical evaluationAnn N Y Acad Sci200194931732611795370
- KommBSKharodeYPBodinePVHarrisHAMillerCPLyttleCRBazedoxifene acetate: a selective estrogen receptor modulator with improved selectivityEndocrinology200514693999400815961563
- BerrodinTJChangKCKommBSFreedmanLPNagpalSDifferential biochemical and cellular actions of premarin estrogens: distinct pharmacology of bazedoxifene-conjugated estrogens combinationMol Endocrinol2009237585
- ChangKCNWangYBodinePVNagpalSKommBSGene expression profiling studies of three SERMs and their conjugated estrogen combinations in human breast cancer cells: insights into the unique antagonistic effects of bazedoxifene on conjugated estrogensJ Steroid Biochem Mol Biol20101181–211712419914376
- CrabtreeJSPeanoBJZhangXKommBSWinnekerRCHarrisHAActivity of three selective estrogen receptor modulators on hormonedependent responses in the mouse uterus and mammary glandMol Cell Endocrinol20082871–2404618367319
- PeanoBJCrabtreeJSKommBSWinnekerRCHarrisHAEffects of various selective estrogen receptor modulators with or without conjugated estrogens on mouse mammary glandEndocrinology200915041897190319022889
- KommBSVlasserosFSamadfamRChouinardLSmithSYSkeletal effects of bazedoxifene paired with conjugated estrogens in ovariectomized ratsBone201149337638621658483
- LoboRAPinkertonJVGassMLEvaluation of bazedoxifene/ conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profileFertil Steril20099231025103819635615
- ArcherDFLewisVCarrBROlivierSPickarJHBazedoxifene/ conjugated estrogens (BZA/CE): incidence of uterine bleeding in postmenopausal womenFertil Steril20099231039104419635614
- LindsayRGallagherJCKaganRPickarJHConstantineGEfficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal womenFertil Steril20099231045105219635616
- PickarJHYehI-TBachmannGSperoffLEndometrial effects of a tissue selective estrogen complex containing bazedoxifene/ conjugated estrogens as a menopausal therapyFertil Steril20099231018102419635613
- PinkertonJVUtianWHConstantineGDOlivierSPickarJHRelief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trialMenopause20091661116112419546826
- KaganRWilliamsRSPanKMirkinSPickarJHA randomized, placebo- and active-controlled trial of bazedoxifene/conjugated estrogens for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal womenMenopause201017228128919779382
- DreyerPVieiraJGBone turnover assessment: a good surrogate marker?Arq Bras Endocrinol Metabol20105429910520485896
- MandelsonMTOestreicherNPorterPLBreast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancersJ Natl Cancer Inst200092131081108710880551
- McCormackVAdos Santos SilvaIBreast density and parenchymal patterns as markers of breast cancer risk: a meta-analysisCancer Epidemiol Biomarkers Prev20061561159116916775176
- HarveyJAPinkertonJVBaracatECShiHMirkinSChinesAAEvaluation of changes in mammographic breast density associated with bazedoxifene/conjugated estrogens in postmenopausal womenEndocr Rev2011323P1P79
- PinkertonJAConstantineGDKommBSYuHPickarJHBreast effects of bazedoxifene/conjugated estrogens in a randomized, controlled trial of postmenopausal womenPoster presented at: The 19th Annual Meeting of the North American Menopause SocietySeptember 24–27, 2008Lake Buena Vista, FL, USA2008 Abstract P-46
- PinkertonJVTaylorHPanKChinesAMirkinSBreast parameters with bazedoxifene/conjugated estrogens in randomized, controlled trials of postmenopausal womenMenopause201017612211222
- SpencerCPGodslandIFStevensonJCIs there a menopausal metabolic syndrome?Gynecol Endocrinol19971153413559385535
- KronenbergFHot flashes: phenomenology, quality of life, and search for treatment optionsExp Gerontol1994293–43193367925752
- PinkertonJVChinesAARacketaJMirkinSBazedoxifene/conjugated estrogens (BZA/CE): effect on sleep parameters in postmenopausal womenMenopause201017612371238
- UtianWYuHBobulaJMirkinSOlivierSPickarJHBazedoxifene/ conjugated estrogens and quality of life in postmenopausal womenMaturitas200963432933519647382
- PinkertonJVChinesAARacketaJMirkinSMenopause-related quality of life and satisfaction in postmenopausal women treated with bazedoxifene/conjugated estrogens (BZA/CE)Menopause20101761219
- BachmannGBobulaJMirkinSEffects of bazedoxifene/conjugated estrogens on quality of life in postmenopausal women with symptoms of vulvar/vaginal atrophyClimacteric201013213214019863455
- TopalNBAyhanSTopalUBilginTEffects of hormone replacement therapy regimens on mammographic breast density: the role of progestinsJ Obstet Gynaecol Res200632330530816764621
- MartinLJMinkinSBoydNFHormone therapy, mammographic density, and breast cancer riskMaturitas2009641202619709825
- FreedmanMSan MartinJO’GormanJDigitized mammography: a clinical trial of postmenopausal women randomly assigned to receive raloxifene, estrogen, or placeboJ Natl Cancer Inst2001931515611136842
- BarnabeiVMCochraneBBAragakiAKMenopausal symptoms and treatment-related effects of estrogen and progestin in the Women’s Health InitiativeObstet Gynecol20051051063107315863546
- LoboRABushTCarrBRPickarJHEffects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate on plasma lipids and lipoproteins, coagulation factors, and carbohydrate metabolismFertil Steril2001761132411438314
- HaysJOckeneJKBrunnerRLEffects of estrogen plus progestin on health-related quality of lifeN Engl J Med2003348191839185412642637
- BrunnerRLGassMAragakiAEffects of conjugated equine estrogen on health-related quality of life in postmenopausal women with hysterectomy: results from the Women’s Health Initiative Randomized Clinical TrialArch Intern Med2005165171976198616186467
- Polo-KantolaPErkkolaRHeleniusHIrjalaKPoloOWhen does estrogen replacement therapy improve sleep quality?Am J Obstet Gynecol19981785100210099609575
- BarnabeiVMGradyDStovallDWMenopausal symptoms in older women and the effects of treatment with hormone therapyObstet Gynecol200210061209121812468165
- GelfandMMMoreauMAyotteNJHilditchJRWongBALauCYClinical assessment and quality of life of postmenopausal women treated with a new intermittent progestogen combination hormone replacement therapy: a placebo-controlled studyMenopause2003101293612544674
- HlatkyMABoothroydDVittinghoffESharpPWhooleyMAQuality-of-life and depressive symptoms in postmenopausal women after receiving hormone therapy: results from the Heart and Estrogen/ Progestin Replacement Study (HERS) trialJAMA2002287559159711829697
- HainesCJYimSFChungTKA prospective, randomized, placebo-controlled study of the dose effect of oral oestradiol on menopausal symptoms, psychological well being, and quality of life in postmenopausal Chinese womenMaturitas200344320721412648884