Abstract
Premenstrual dysphoric disorder (PMDD) and premenstrual syndrome (PMS) refer to physical, cognitive, or affective symptoms that arise in the late luteal phase and remit with menses. The present work is a clinically focused scoping review of the last twenty years of research on treatment for these disorders. A search of key terms using the PubMed/Medline, the Cochrane Library, Embase, and Web of Science databases was performed, and 194 studies of adult women met initial inclusion criteria for review. Research studies concerning medications, pharmacological and non-pharmacological complementary and alternative medicine treatments, and surgical interventions with the most available evidence were appraised and summarized. The most high-quality evidence can be found for the use of selective serotonin reuptake inhibitors (SSRIs) and combined oral contraceptives (COCs), with gonadotropin releasing hormone (GnRH) agonists and surgical interventions showing efficacy for refractory cases. While there is some evidence of the efficacy of alternative and complementary medicine treatments such as nutraceuticals, acupuncture, and yoga, variability in quality and methods of studies must be taken into account.
Introduction
A subset of women experience physical, cognitive, or affective symptoms in the luteal phase of the menstrual cycle that range from mild to severe and disabling.Citation1 Initially described as “premenstrual tension” in the 1930s,Citation2 further observation of common symptoms led to changes in the accepted clinical definition and the use of the new term “premenstrual syndrome” in the 1950s.Citation3 Premenstrual syndrome (PMS) was initially argued to be culturally bound,Citation4 however, epidemiological data have demonstrated that the experience of clinically significant premenstrual symptoms is a phenomenon affecting girls and women globally and in various cultural settings.Citation5 In the past several decades, efforts to establish more rigorous and clearly defined standards of severe premenstrual symptoms have given rise to the additional term premenstrual dysphoric disorder (PMDD), first established by the Diagnostic and Statistical Manual of Mental Disorders 4th edition.Citation6
Diagnosis
There has been considerable debate as well as heterogeneity in guidelines across disciplines regarding the appropriate diagnostic criteria for syndromes of clinically significant premenstrual symptoms. The International Society for the Study of Premenstrual Disorders (ISPMD) is an international consensus group that undertook the task of consolidating expert opinion into operational diagnostic, research, and management guidelines.Citation7,Citation8 The ISPMD describes a spectrum of premenstrual disorders (PMDs) including core and variant PMDs.Citation7 Variant PMDs include premenstrual exacerbations of underlying psychiatric disorders, symptoms occurring in response to exogenous progesterone administration, symptoms arising from ovarian activity other than ovulation, and symptoms arising from ovarian activity although menstruation has been suppressed.Citation7 Core PMDs, the topic of the present paper, are defined as the psychological or somatic symptoms that: 1) arise in the 14 day luteal phase; 2) affect normal daily functioning, affect school, work, or interpersonal relationships, or cause significant distress; and 3) and resolve with the onset of menstruation ().Citation7 Both PMS and PMDD are considered core PMDs.
Table 1 Diagnosis of Premenstrual Dysphoric Disorder
In contrast to the common clinical definition of PMS, which focuses on impairment and does not specify number or type of required symptoms,Citation7 PMDD is strictly defined by the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM5) as requiring a minimum of 5 symptoms that must be present across affective and physical-behavioral symptom categories ().Citation1 The affective symptom category includes affective lability, irritability, depressed mood, and anxiety, while the physical-behavioral symptom category includes anhedonia, difficulty concentrating, anergia, change in appetite or cravings, hypersomnia or insomnia, sense of being overwhelmed or out of control, and physical symptoms such as breast tenderness or swelling, weight gain, bloating, and joint and muscle pain.Citation1 In order to meet criteria for a diagnosis of PMDD, these symptoms must be present in the luteal phase of most menstrual cycles in the prior year and must result in clinically significant distress or interference in daily life.
Per the ISPMD as well as the DSM5, the diagnosis of PMDD is properly established by two months of prospective daily symptom monitoring prior to the initiation of any treatment using a validated tool such as the Daily Record of Severity of Problems (DRSP).Citation9 While screening tools, structured clinical interviews, and self-report scales are available and in use clinically, such retrospective assessments are determined to have limited value due to their subjectivity as well as concerns for recall bias.Citation7,Citation10 Biochemical testing, including hormonal testing, and tracking of physical parameters, such as breast size and total body water, has not been shown to be diagnostically useful.Citation7 As such, prospective symptom monitoring remains the gold standard of diagnosis.
Epidemiology and Morbidity
The DSM5 reports a 1.8% to 5.8% 12-month prevalence of PMDD among menstruating women in the United States.Citation1 PMS with clinically significant distress has been shown to have significantly higher prevalence rates, estimated at 13% to 18%.Citation11 Globally, prevalence rates of PMDD demonstrate some regional differences. Estimates of PMDD across Europe vary, with a 1.1% prevalence reported among a cohort of Spanish women,Citation12 a 2.1% prevalence reported among a cohort of Polish women,Citation13 and a 3.1% prevalence reported among a cohort of Swiss women.Citation14 In India, prevalence has been reported as 8% for PMDD and 43% for PMS per a recent meta-analysis.Citation15 The rate of PMDD in East Asia including Japan, Korea, China, Taiwan, Hong Kong, and Macau per a recent literature review was 1.3% to 2.8%.Citation16 Rates are estimated to be somewhat higher in Latin America,Citation17 with one cohort of Brazilian women demonstrating a prevalence of 17.6% for PMDD.Citation18
Morbidity of severe PMS is significant, with functional impairment similar to other depressive disorders.Citation11 An international study of the impact of severe PMS found an increase in reported work absenteeism and work impairment compared to those reporting only mild symptoms.Citation19 Recent meta-analyses have also shown that PMDD is associated with suicidal ideation, plans to suicide, and suicide attempts globally.Citation20,Citation21
Pathophysiology
The menstrual cycle consists of a series of reoccurring biological events that produces a uterine environment in which conception may occur. The cycle is an average of 28 days and begins with the first day of menstruation, or the shedding of the endometrial lining in the absence of fertilization. The follicular phase, which is generally the first 14 days of the menstrual cycle, is dominated by rising 17-β estradiol, an ovarian steroid hormone and an estrogen, produced by the action of follicle stimulating hormone (FSH) and luteinizing hormone (LH) on the hormone-producing cells of ovarian follicles.Citation22 FSH and LH are released by the anterior pituitary in response to pulses of gonadotropin-releasing hormone (GnRH) from the hypothalamus. 17-β estradiol produces changes in the endometrial and cervical environment in preparation for potential fertilization and acts on the anterior pituitary to decrease the secretion of FSH in a negative feedback cycle.Citation22 The decrease in FSH halts the development of ovarian follicles except for the dominant, FSH-receptor rich follicle, which will continue to produce 17-β estradiol.Citation22 This production from the now mature ovarian follicle results in positive feedback at the anterior pituitary that ultimately leads to the surge in FSH and LH responsible for ovulation, or the release of the oocyte from the mature follicle.Citation22
Following ovulation, the cycle enters the luteal phase, the remaining 14 days of the menstrual cycle. The remaining ovarian follicle becomes the corpus luteum, which continues to secrete diminishing levels of 17-β estradiol but also responds to the low levels of LH by facilitating production of progesterone, the ovarian steroid hormone that dominates the luteal phase.Citation22 Progesterone produces further changes in the uterine environment to facilitate implantation of a fertilized ovum. If no implantation occurs in the optimal window, the corpus luteum undergoes degeneration to become the corpus albicans, incapable of producing further ovarian hormones, leading to the fall of both progesterone and estrogen.Citation22 Without hormonal support, the uterine environment degenerates and is shed in a menstrual period, beginning the cycle again.
The pathophysiology of PMS and PMDD is the subject of active research. Neuroactive metabolites of progesterone, which rise sharply in the late luteal phase, have been heavily implicated. Most evidence implicates the specific metabolite allopregnanolone: its interaction with GABA-A receptors in the brain has been hypothesized to provoke the affective symptoms of PMS and PMDD in a subset of women.Citation23,Citation24 In studies of women with PMDD, altered sensitivity to allopregnanolone has been found compared to controls.Citation25 Despite these findings, the specific mechanism by which allopregnanolone contributes to negative mood symptoms in susceptible women remains elusive, partly due to the variability of responses observed.Citation26
Rationale and Objectives
While distressing and even disabling premenstrual symptoms have been described across time and cultures, clinical consensus on diagnostic guidelines is just beginning to coalesce, allowing for increased rigor in studies of potential treatments. The aims of the present scoping review are to explore the recent literature assessing treatment of adult women with PMS or PMDD in order to provide a clinically relevant synthesis of the most studied interventions to assist healthcare providers who are caring for such patients.
Methods
A scoping review was performed using PubMed/Medline, the Cochrane Library, Embase, and Web of Science databases on 3/16/2022. The search terms included “Premenstrual Dysphoric Disorder” OR premenstrual syndrome OR premenstrual dysphoric disorder OR premenstrual syndrome AND “drug therapy” or medication* or drug* or pharmacol* or “non-pharmacological”. Results were limited to papers published between 2000 and 2022, published in peer-reviewed journals, written in the English language, assessing adult women (no younger than age 18), and assessing a treatment modality of premenstrual symptoms. Research studies, meta-analyses, and review articles were included. Case reports and conference proceedings were excluded. Grey literature was excluded as it is not peer-reviewed.
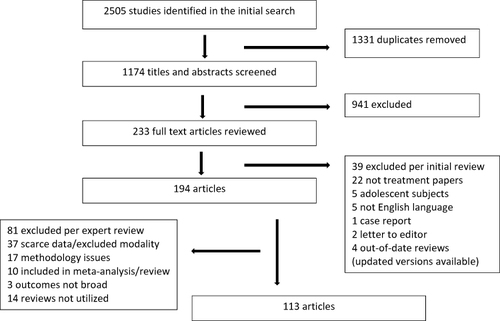
The search was performed by a research librarian, and review of the resulting data was conducted by the lead author. Of 2505 articles identified per the search, 1331 were immediately removed as duplicates, 941 were excluded upon screen of the title and abstract due to failure to meet inclusion criteria listed above, and an additional 39 were excluded upon full text review for failure to meet inclusion criteria (). A total of 194 articles were thus selected by the lead author for further review by individual authors. Individual authors reviewed the articles relevant to their area of expertise. Articles were additionally excluded by authors due to: 1) low quality of evidence compared to other available studies (for example, open-label design, lack of placebo control, etc.) for a given treatment modality; 2) outcome measures that did not address premenstrual symptom reduction; or 3) very scarce evidence available for the treatment modality. Two articles were identified for device-based treatment modalities (bright light therapyCitation27 and vestibular stimulationCitation28); these were excluded from review given a lack of available studies for comparison. Twenty-nine articles identified were systematic or narrative reviews; such articles are included only as per their relevance. Some articles otherwise included in meta-analyses or systematic reviews were not individually reviewed for the purposes of the present work. An additional 81 articles were thus excluded from the pool, leaving 113 articles selected for this scoping review (Appendix 1). Seminal articles published before 2000 were identified by reviewing references from selected studies and included where appropriate.
Treatment Review
Medication
SSRIs
Evidence from multiple randomized controlled trials (RCTs) has established that selective serotonin reuptake inhibitors (SSRIs), dosed continuously or only in the luteal phase of the menstrual cycle, are the gold standard of treatment for PMDD as per expert guidelines.Citation29 The mechanism by which SSRIs treat PMDD is hypothesized to be distinct from the mechanism by which they are thought to treat other depressive and anxiety disorders, as the effect on symptoms is rapid and achieved at relatively low doses.Citation30 One hypothesis is that SSRIs may modulate the synthesis of allopregnanolone,Citation31 and one open-label trial of sertraline for PMDD demonstrated changes in total peripheral allopregnanolone levels, although these changes varied based on the subjects’ baseline levels.Citation32
Sertraline,Citation33,Citation34 escitalopram,Citation35–37 paroxetine,Citation38–43 and fluoxetineCitation44–46 dosed continuously or only in the luteal phase of the menstrual cycle have all been shown to be efficacious in the treatment of PMDD (). Luteal phase dosing of fluoxetine has also been shown to be efficacious as a dose of 90 mg given 14 days prior to menses and then again 7 days prior to menses.Citation45 SertralineCitation47 dosed continuously, in the luteal phase, or at symptom-onset and fluoxetineCitation48 dosed continuously have additionally been shown to be efficacious in the treatment of severe PMS.
Table 2 SSRI/SNRI Luteal Phase or Symptom-Onset Dosing Showing Efficacy for Treatment of PMDD
Luteal phase dosing of SSRIs has been shown to improve symptoms that persist into the follicular phase even when medication is discontinued with the onset of menses.Citation49 Studies comparing both continuous and luteal phase dosing strategies have shown mixed results, with most studies concluding that continuous and luteal phase dosing are comparableCitation40,Citation41,Citation50 but with one meta-analysis demonstrating increased efficacy of the continuous dosing strategy compared to luteal phase dosing only.Citation51 Symptom-onset dosing, in which medication is started only with the onset of premenstrual symptoms, has shown promise with this strategy demonstrated to be more effective than placebo in trials of sertraline for severe PMSCitation47 and escitalopram for PMDD ().Citation36 One large recent trial of symptom-onset dosing of sertraline for PMDD demonstrated a lack of a significant difference for the primary study outcome of Premenstrual Tension Scale total score compared to placebo, however the symptom-onset group did show significant improvements in secondary outcome measures including total DRSP score.Citation52 Notably, the same study demonstrated no significant discontinuation symptoms in intermittent dosing strategies for sertraline.Citation52
Table 3 Oral Complementary and Alternative Medicine Dosing for Treatment of PMS or PMDD
Serotonin–Norepinephrine Reuptake Inhibitors (SNRIs)
The evidence base for the use of serotonin–norepinephrine reuptake inhibitors (SNRIs) for PMDD is small but promising. Venlafaxine has shown efficacy for the treatment of PMDD in one open-label trial using a continuous dosing strategyCitation53 and one trial of placebo non-responders using a luteal phase dosing strategy ().Citation54 Duloxetine has shown efficacy for the treatment of PMDD in one small single-blind trialCitation55 and one open-label study,Citation56 both using a continuous dosing strategy. Placebo-controlled trials are warranted for further investigation of these agents for the treatment of PMDD, but it is reasonable to use them as a second-line agent if tolerability limits use of SSRIs.
Combined Oral Contraceptives (COCs)
There are ample data that combined oral contraceptives (COCs) are effective treatment for somatic symptoms of the menstrual cycle, including but not limited to dysmenorrhea, gastrointestinal changes, and menorrhagia. However, data concerning the effect of COCs on affective premenstrual symptoms have been inconsistent.Citation57 The data are further complicated by the availability of different combinations of hormones as well as different doses, usage, and timing.
Drospirenone, a progesterone derivative, combined with ethinyl estradiol (EE) is the only current United States Food and Drug Administration (FDA) approved COC for the treatment of PMDD. An early RCT of EE 30 µg and drospirenone 3 mg in a 21 active/7 placebo day pattern versus 28 days of placebo for women with PMDD found significant improvement in the active treatment arm only for acne, appetite, and food craving; although results trended toward improvement in affective symptoms, they did not reach significance.Citation58 A Phase III multicenter study assessing an altered regimen of EE 20 µg and drospirenone 3 mg in a 24 active/4 day placebo pattern versus placebo did find significant improvement in PMDD symptoms as measures by total DRSP score, with maximum reduction in symptoms occurring after 3 months of use.Citation59 Post-hoc analyses of the same trial found significant improvement in functional impairmentCitation60 and symptom clusters of negative emotions, food cravings, and water retention over placebo.Citation61 A smaller study of the same EE/drospirenone 24/4 regimen similarly demonstrated significant improvement in DRSP score for active treatment compared to placebo.Citation62 A more recent randomized open-label trial performed for women with PMS comparing EE 20 µg and drospirenone 3 mg to EE 20 µg and desogestrel 150 µg in a 24 active/4 placebo day pattern given over six cycles showed significant reduction in PMS symptoms in both treatment groups, although the drospirenone arm demonstrated superior efficacy and faster improvement in symptoms.Citation63 A study that set out to assess continuously dosed EE/drospirenone against a 21 active/7 placebo day pattern found no difference in efficacy between these arms, however a strong placebo response was also observed in this study, and neither arm ultimately separated from placebo.Citation64
Levonorgestrel-containing COCs have also been studied for treatment of PMS and PMDD. Studies assessing continuous dosing without placebo days of levonorgestrel 90 µg with EE 20 µg on PMDD and PMS showed mixed results, with two studies, one placebo controlled and one open-label, demonstrating significant efficacy of the active treatment for premenstrual symptoms and two studies demonstrating a failure of active treatment to separate from placebo.Citation65 A recent meta-analysis of COCs for PMDD or PMS concluded that while COCs were shown to be effective for symptom reduction overall, they were not effective for depressive symptoms specifically, and no specific formulation has shown superiority over the others to date.Citation66
Progesterone
To date, progesterone-only interventions have not consistently demonstrated reduction of PMS and PMDD symptoms.Citation67 One open-label study of 37 women given 100 mg of sublingual micronized progesterone on day 11 to day 25 of their cycles across three cycles reported significant efficacy of treatment over placebo, with authors hypothesizing that increased bioavailability of sublingual dosing may be responsible for the observed results.Citation68 One RCT assessing progesterone receptor modulator ulipristal dosed as 5 mg daily over 28 days showed significant increase in remission of PMDD in the treatment arm, a promising result warranting further study.Citation69
Estrogen
There is weak evidence concerning the efficacy of unopposed estrogen to suppress ovulation as a treatment PMS, and it may actually worsen symptoms in some women.Citation70 Estrogen given as either a patch or implant to suppress ovulation with progestogen for endometrial protection shows some efficacy for premenstrual symptoms, although the evidence was judged to be low quality per a recent systematic review.Citation70 Patch dosing tested has ranged from 100 to 200 µg twice weekly in the luteal phase.Citation70 Lower doses appear to be better tolerated than higher doses, but long-term adverse effects have not been adequately assessed.Citation70
Androgens
Dutasteride is a synthetic 4-azasteroid that selectively inhibits 5α-reductase, thereby preventing metabolism of progesterone to allopregnanolone. A small double-blind placebo-controlled study evaluating daily doses of 0.5 mg and 2.5 mg throughout the menstrual cycle for symptoms of PMDD demonstrated significant efficacy for the 2.5 mg dose in ameliorating anxiety, sadness, bloating, irritability, and food cravings.Citation71 Although promising, as dutasteride can negatively impact the development of male fetuses due to its inhibition of testosterone metabolism, caution would need to be exercised in its use for women who may become pregnant.Citation71
Gonadotropin Releasing Hormone (GnRH) Receptor Agonists
Ovulation triggers the hormonal cascade linked to PMDD, and continuous exposure to GnRH receptor agonists acts to suppress ovulation through downregulation of GnRH receptors, ultimately leading to falling levels of gonadal steroids. Suppression of ovulation has been shown to treat PMS, presumably by eliminating the hormonal flux that provokes its symptoms.Citation72 Leuprolide (Lupron) is a GnRH agonist dosed as a monthly intramuscular 3.75 mg injection that treats PMS via ovulatory suppression.Citation72,Citation73 Danazol, an anti-gonadotropic derivative of ethisterone capable of suppressing ovulatory cycles, has also shown some efficacy in treatment of PMS,Citation74,Citation75 but significant negative somatic and mood side effects secondary to its anti-androgenic properties preclude long-term use.Citation29
Overall, prolonged use of GnRH agonists has significant negative side effects related to the induction of a menopausal state, including vasomotor symptoms and bone demineralization.Citation29 The re-introduction of estrogen and progesterone decreases this side effect burden. Such add-back therapy has been shown to not diminish the overall efficacy of GnRH agonists for treatment of PMS,Citation73 despite the potential for induction of PMS symptoms with re-introduction of progesterone in some women.Citation76 Add-back regimens must be personalized for each woman given these individual variations in response; intermittent progesterone add-back may be preferable for progesterone-sensitive women.Citation77,Citation78
Anxiolytics
Although benzodiazepines have a history of clinical use in the treatment of PMDD, the literature assessing their efficacy is sparse and clinically out-of-date. The two major studies demonstrating efficacy of alprazolam over placeboCitation79,Citation80 for premenstrual symptoms were conducted on women diagnosed with late luteal phase disorder (LLPD), the precursor of PMDD defined in the Diagnostic and Statistical Manual of Mental Disorders 3rd edition revised.Citation81 Both studies demonstrated efficacy over placebo in cohorts of women without other psychiatric diagnoses or follicular phase symptoms; one study specifically compared women with follicular phase symptoms to those without and concluded that alprazolam only demonstrated efficacy in the group without follicular phase anxiety or depression.Citation80 Two studies of women with PMS failed to demonstrate any advantage of alprazolam over placebo.Citation82,Citation83 Overall, while benzodiazepines may be useful during the symptomatic phase of PMDD in women without comorbid diagnoses or follicular phase symptoms, the evidence to recommend their use is weak, and they should be considered for adjunctive treatment only in refractory cases.
One single-blind study comparing buspirone 10 mg daily to fluoxetine 20 mg daily for PMS found significant reductions in both groups for self-rated premenstrual symptom scores after one and two months of treatment without significant between-group differences, but the lack of a placebo group limits interpretation of the data.Citation48
Other Medications
A small (n = 20) study investigated adjunctive quetiapine SR, a second generation antipsychotic, starting at 25 mg for inadequate therapeutic response for PMDD in patients treated initially with SSRI or SNRI monotherapy and found greater improvements in mood ratings for the treatment group compared to placebo.Citation84
A small open-label case series of 8 women with treatment-resistant PMDD, 6 of whom had additional comorbid psychiatric diagnoses, treated with the carbonic anhydrase inhibitor acetazolamide 125 mg daily in the 7 to 10 days prior to menses reported remission of symptoms in all studied cases.Citation85
One randomized placebo-controlled study of clonidine, an α-2 adrenergic agonist, for PMDD failed to show efficacy over placebo on any collected mood or premenstrual symptom scale.Citation86
Novel/Investigational Therapeutics
As the underlying pathophysiology of PMDD is increasingly illuminated, the opportunity for rational drug design grows. As women with PMDD demonstrate paradoxical negative mood symptoms in response to rising allopregnanolone in the luteal phase,Citation87 its antagonism was hypothesized to be a potential treatment for PMDD. Sepranolone is isoallopregnanolone, an isomer and antagonist of allopregnanolone that does not itself interact with GABA-A receptors.Citation88 The compound is delivered as a series of subcutaneous injections of either 10 mg or 16 mg in 0.4 mL given every other day through the luteal phase and beginning at ovulation.Citation88
A Phase 2 study of sepranolone for PMDD, in which participants received 5 injections of either placebo or study drug dosed at 10 mg or 16 mg over the course of one menstrual cycle, demonstrated promising results with significant improvement in total DRSP score for the drug group compared to placebo and no significant between-group differences in frequency of adverse effects.Citation88 Unfortunately, an extension of this study using 5 to 7 injections of placebo or study drug over the course of 3 menstrual cycles failed to demonstrate superiority of drug over placebo at the primary endpoint of change in total DRSP score.Citation89 There was a significant effect of drug on improvement in reported distress, and a post-hoc analysis looking at total DRSP score over 9 premenstrual days in the last treatment cycle only showed significant effect of sepranolone 10 mg over placebo.Citation89 Notably, the placebo response in the study was significant, calculated as 30% greater than the placebo response of the original study, which likely contributed to the failure of study drug to separate from placebo.Citation89 Again, sepranolone was demonstrated to be well-tolerated with only injection-site reactions occurring more frequently in the drug versus the placebo group.Citation89
Complementary and Alternative Medicine
The US National Center for Complementary and Alternative Medicine (NCCAM) (currently named the National Center for Complementary and Integrated Health) defines complementary and alternative medicine (CAM) as “approaches that are not typically part of conventional medical care or that may have origins outside of usual Western practice”.Citation90 The WHO utilizes a similar broad definition.Citation91 According to the 2007 National Health Interview Survey, 38.3% of adults in the USA used CAM in the previous year, 33.5% men and 42.8% women.Citation92 CAM use is often in conjunction with conventional medicine and infrequently under the care of a licensed or certified CAM practitioner. In the women’s health and premenstrual disorders’ literature in particular, common use of CAM is reported.Citation93,Citation94
Noted limitations of the CAM literature include studies’ heterogeneity regarding diagnosis, inclusion criteria, dose and duration of treatment, outcome measures, and, importantly, posology, or dose selection, which lead to comparing studies using non-chemically identical products.Citation95 Also, no comprehensive understanding of the mechanism of action of each CAM approach is to date available. Moreover, in the case of non-pharmacological approaches such as exercise or acupuncture, adequate controls are challenging to create.Citation96 Alongside the large placebo response in the PMS literature in general (in some studies as high as 50%), these limitations make interpretation challenging. Although many potential therapeutic CAM candidates have been studied in some form, including anise,Citation97 Echium amoenum,Citation98 ginger,Citation99 Melissa officinalis,Citation100,Citation101 royal jelly,Citation102 curcumin,Citation103 Ginkgo biloba,Citation104 Zataria multiflora,Citation100,Citation105 Nardostachys jatamansi,Citation106 Phaleria macrocarpa,Citation107 oxaloacetate,Citation108 Neptune Krill Oil,Citation109 fennel,Citation110 soy,Citation111 wheat germ,Citation100–112 and lecithin,Citation113 for the purpose of this review we will cover interventions with the most data available for adult women (). Similarly, CAM approaches with minimal data available and for which dosing cannot be quantified, specifically aromatherapy,Citation114 kinesio taping,Citation115,Citation116 external Qi therapy,Citation117,Citation118 and Chinese herbal medicine practiceCitation119 are considered outside the scope of this review.
Herbal Preparations
Chasteberry’s extract (Vitex Agnus-Castus, VAC), obtained from the dried ripe fruit of a plant native to the Mediterranean region and Asia, is the most investigated remedy among pharmacological CAM. Although the exact mechanism of action is not fully known, pathways implicated in the relief of PMS include inhibition of prolactin secretion through dopamine D2 receptor agonismCitation120 and binding of opioidCitation121 and estrogen receptors.Citation122 In addition to positive open-label studies,Citation123,Citation124 numerous RCTsCitation125,Citation126 and subsequent meta-analyses have been conducted. Following a 2017 meta-analysis of 17 RCTs limited by significant heterogeneity (diagnostic criteria, posology, dosage 8–41 mg/day, luteal phase versus continuous use, outcome measures) showing superiority to placebo, equal efficacy to oral contraceptive or fluoxetine, and superiority to other selected CAM intervention,Citation127 a 2019 meta-analysis selecting only 3 RCTs with a sufficiently characterized preparation of VAC also demonstrated efficacy, finding that VAC users were 2.57 times more likely to achieve remission than placebo users.Citation128 In terms of dosage, comparison of fixed doses of the VAC Ze 440 extract demonstrated that 8 mg daily was not significantly different than placebo while 20 mg and 30 mg were similarly efficacious.Citation129
Other herbal preparations thought to have anxiolytic, anti-inflammatory, or anti-spasmodic effect, thus theoretically well positioned as a treatment option based on the array of symptoms of PMS, have received interest. Of the eight clinical trials reported on chamomile, only one had placebo as comparison and conclusive results cannot be drawn given the significant studies’ heterogeneity regarding preparations, doses, outcome measures, and follow ups.Citation130 Conflicting results derive from two small RCTs on curcumin versus placebo.Citation103,Citation131 A case seriesCitation132 and a small open-label studyCitation133 with positive findings are available for Kami-shoyo-san (TJ-24), a Japanese herbal medicine preparation approved in Japan. Saffron relieved PMS symptoms more than placebo in two RCTs,Citation134,Citation135 with one trial additionally demonstrating no significant difference in outcome compared to fluoxetine.Citation134 One RCT of saffron versus placebo resulted in significant improvement in both groups, with saffron failing to separate from placebo.Citation136 Hypericum perforatum (St John’s wort), a herbal supplement influencing the monoamine system and found helpful in some forms of depression, did not differentiate from placebo in a small RCTCitation137 but did in another small RCT only for selected symptoms.Citation138
Vitamins
Vitamin B6 (pyridoxine), a cofactor in the synthesis of monoamines and γ-aminobutyric acid (GABA), is included among the first-line interventions for PMDD in the Royal College of Obstetrician and Gynaecologists (RCOG) guidelines, along with SSRIs, CBT, exercise, and COCs, despite the low level of evidence for its efficacy.Citation139 A 1999 systematic review including 9 low-quality RCTs (lacking details on randomization and mostly underpowered) concluded that pyridoxine was more than twice as effective as placebo for PMS.Citation140 Tested doses ranged from 50 mg/day to 150 mg/day in preparation of pyridoxine alone to 600 mg/day in multivitamin preparations; as no change in efficacy with higher doses was observed,Citation140 lower doses should be prescribed given evidence of adverse effects, such as peripheral neuropathy, of long-term use of high-dose pyridoxine.Citation139 An upper-limit of 100 mg/day has been suggested for adults using pyridoxine.Citation141 A 2016 meta-analysis of 12 heterogeneous case–control studies also demonstrates efficacy of pyridoxine over placebo for treatment of PMS.Citation142
The proposed mechanism of action of vitamin B1 (thiamine) as a treatment for PMS remains unclear, although its function as a cofactor in the metabolism of carbohydrates and amino acids has been suggested to be of interest.Citation143 Thiamine was shown to decrease PMS symptom severity more than placebo in one small RCT of university students,Citation143 and its efficacy is increased when used concomitantly with calcium at doses of 100 mg/day with 500 mg/day of calcium.Citation144 A prospective cohort study of nurses found a lower rate of PMS in participants with high dietary intake of thiamine and riboflavin.Citation145
Inhibition of cyclooxygenase and nitric oxide synthase, effect on calcium metabolism, and prevention of monoamine depletion are included in the potential rationale for the use of vitamin D in PMS. Vitamin D supplementation has been shown to be effective in improving PMS symptoms in interventional studies per a recent meta-analysis, but no significant association between serum 25-hydroxyvitamin D3 and PMS symptoms was observed, perhaps due to prevalent vitamin D deficiency in the population studied.Citation146 A subsequent RCT in a similarly vitamin D deficient sample found no effect of vitamin D 2000 IU dosed every other day over placebo.Citation147 Overall, since available studies are highly heterogeneous in term of dosages, often focused on vitamin D-deficient populations, and also inconsistent regarding the relation between vitamin D serum levels and symptom severity, available evidence is not sufficient to inform recommendations regarding specific dosage range, especially for those without vitamin D deficiency.
Minerals
Variations in calcium homeostasis have been demonstrated to occur over the menstrual cycle in response to hormone fluctuations, with greater variations observed in women with PMS and PMDD compared to controls.Citation148 Such dysregulation of calcium homeostasis has been implicated in affective disorders.Citation148 Oral calcium at doses of 500 mg dailyCitation149 and twice per dayCitation150 has been shown to improve symptoms of PMS in RCTs. Calcium in conjunction with pyridoxine and with thiamineCitation144 has been demonstrated to be more efficacious than either of those vitamins alone. A pilot study comparing calcium 600 mg twice daily compared to fluoxetine found a small effect of calcium and a larger effect of fluoxetine,Citation151 in line with the overall small effect for calcium on total PMS symptoms compared to placebo (48% vs 30% reduction) observed in the largest RCT of calcium for PMS to date.Citation152
Lower serum levels of zinc have been observed in women with PMS compared to controls in some studies, and it has been hypothesized that zinc supplementation may therefore treatment symptoms.Citation153 Limited evidence supports the use of zinc, dosed as 50 mg of elemental zinc during the luteal phaseCitation154 or up to 30 mg of elemental zinc daily,Citation155,Citation156 for PMS.
Some observation of low magnesium levels in women with PMS compared to controls has led to the theory that magnesium supplementation may alleviate symptoms.Citation157 Limited evidence additionally supports use of magnesium supplementation at 250 mg daily,Citation158,Citation159 particularly in combination with pyridoxine,Citation160 for PMS, although to date superiority over placebo has not been demonstrated; overall, improved quality of data is necessary to guide recommendations.Citation161
Other Dietary Supplements
Omega-3 polyunsaturated fatty acids (PUFAs), believed to exert various biological functions including monoaminergic pathway enhancement, and control of pain pathways and inflammation, have been the focus of study for use in PMS. In a recent meta-analysis of 8 RCTs, omega-3 fatty acids were shown to be effective for reducing the severity of symptoms of PMS, particularly in older women and with increased length of treatment, but the authors urge caution in interpretation of these results due to significant heterogeneity of studies.Citation162 A meta-analysis of studies on myo-inositol, a sugar-like molecule involved in stress response and the serotonin-signaling system, for depression and anxiety disorders that included two RCTs of PMDD, reported a trend toward efficacy on depressive symptoms over placebo.Citation163
Proprietary Blends
Femal, a pollen-based herbal medicinal product,Citation164,Citation165 and Femi comfort, a blend of pyridoxine, vitamin E, and evening primrose oil,Citation166 have shown efficacy over placebo for PMS symptoms in RCTs. PMS50, a proprietary blend of primarily B vitamins, magnesium, and chasteberry, improved mood symptoms compared to placebo in an RCT of women with moderate to severe PMS.Citation167
Non-Pharmacological CAM
A 2019 systematic review of RCTs assessing safety and efficacy of acupuncture or acupressure for PMS or PMDD found greater reductions in physical and psychological symptoms compared to sham treatment without sufficient data to comment on adverse effects of treatment; overall, the evidence was noted to be low quality.Citation168 Two meta-analyses conducted similarly noted greater efficacy of acupuncture over placebo in pooled data.Citation169,Citation170 One recent RCT of auriculotherapy failed to separate from placebo.Citation171 A meta-analysis and systematic review of reflexology for PMS found an overall effect for the intervention in reduction of severity of symptoms, with increased effect shown with longer duration of reflexology sessions.Citation172 Small RCTs of yoga for PMS shown positive findings on symptoms as well as dimensions such as sleep quality, but as yoga is a broad intervention compromising multiple techniques, further studies better delineating the specifics of the intervention tested are necessary.Citation173–178
A 2020 meta-analysis of RCTs evaluating the effect of exercise on PMS selected 15 studies of at least eight weeks duration; significant improvement in PMS symptoms was demonstrated compared to controls, but evidence quality was limited, with high heterogeneity of studies.Citation179 Other studies of swimming,Citation180 pilates,Citation181 traditional Chinese exercise Baduanjin,Citation182 and simple aerobic exerciseCitation183–185 have had similar positive results, although quality of data is limited. Given the benefit of regular exercise for overall health, complementary use in the treatment of PMS as part of an integrated, holistic approach is likely to be useful.Citation139
Psychotherapies
Several psychotherapeutic modalities have been shown to be efficacious in the treatment of PMS and PMDD,Citation186–189 although the preponderance of evidence favors cognitive behavioral therapy (CBT).Citation190 One meta-analysis of randomized controlled trials including 5 trials utilizing CBT in women with PMS found significant reductions in symptoms of anxiety and depression, although the quality of the available studies was concluded to be low.Citation191 Mindfulness-based CBT (MCBT) has additionally been shown to be efficacious for PMS in two small randomized controlled studies.Citation192,Citation193 Several controlled studies assessing CBT delivered to a group of subjects have concluded that the intervention remains effective in this format.Citation194,Citation195 Two randomized controlled trials of internet-based CBT have demonstrated superiority of this care delivery model over control interventions,Citation196,Citation197 a finding that has implications for broadening access to therapy. In terms of comparative efficacy, one meta-analysis concluded that CBT showed the same small to moderate effect size as antidepressants in the treatment of PMS and PMDD and hypothesized that improved outcomes could be achieved with combination treatment.Citation198 An additional study assessing CBT versus fluoxetine versus combined treatment for women with PMDD also demonstrated similar efficacy between treatment groups, however no additional effect was observed for combination therapy.Citation199 Psychoeducation alone has been shown to be effective in reducing symptoms of PMS in two randomized control trials.Citation194,Citation200
Surgery
Hysterectomy with Bilateral Salpingectomy/Oophorectomy (BSO)
Hysterectomy with BSO has been successfully utilized as a definitive treatment option for women with PMDD refractory to other interventions.Citation201,Citation202 A retrospective study of 47 women who underwent hysterectomy with BSO reported a 96% satisfaction rate and a 93.6% remission rate, with women reporting an average of nearly 10 years of distressing symptoms prior to the procedure.Citation201 Seventeen women in the cohort additionally suffered from symptoms such as menorrhagia, uterine fibroids, and ovarian cysts, providing additional indications for surgery.Citation201 As with all therapies resulting in ovulatory suppression, hormonal add-back is required following surgery to prevent the complications of medical menopause.Citation201
Endometrial Ablation
Novasure is a procedure to thermally ablate the endometrial lining, usually performed for treatment of menorrhagia. A prospective cohort study of 36 women undergoing the procedure found significant improvement in symptoms of PMS.Citation203 The mechanism of the effect on PMS is not clear, and further research is needed to validate these preliminary findings.
Discussion
The PMS/PMDD treatment literature overall suffers from a paucity of high quality data. Consensus on diagnostic criteria has evolved in the last several decades, and rigorous studies assessing prospectively confirmed PMDD are necessary to continue to build the evidence base for treatment. The robust placebo response seen across studies warrant their own study and inquiry. Combinations of treatments may be more effective than a single approach, but studies assessing the superiority of combined approaches are lacking. Finally, the study of CAM approaches to treatment has been classically limited by posology as well as factors contributing to significant heterogeneity. However, clinicians can use existing data to guide decision making according to the unique needs and preferences of their patients.
For mild symptoms or patients who decline pharmacotherapy, evidence supports psychotherapeutic interventions and non-pharmacological complementary and alternative practices such as exercise, yoga, and acupuncture. For more severe symptoms and if patients are agreeable, treatments with the most robust evidence, namely SSRIs and COCs, should be prioritized. Low-risk pharmacological CAM interventions with promising evidence, such as calcium and pyridoxine supplementation, can be used as adjuncts to bolster treatment or as first approaches for patients who prefer nutraceutical treatment with caution and diligent assessment of potential medical contraindications (eg for proposed calcium supplementation, a history of nephrolithiasis or hypercalcemia). Liaison with primary care is recommended to support safe and informed prescribing of complementary agents, and more research is needed overall to clarify the potential benefit of such multimodal approaches to treatment. Finally, GnRH agonists or even surgical measures may be warranted for severe refractory symptoms, although such interventions require ongoing monitoring and hormonal add-back.
Limitations
The present study is limited in some ways relating to adjustments made for feasibility and clinical utility. Firstly, as the initial search included only studies published from 2000 onwards, relevant data published prior to this date may be excluded. Secondly, excluding treatment modalities with a scarcity of data narrows the scope of the review.
Conclusion
Overall, despite the limitations of the reviewed literature noted above, effective treatments for PMDD and PMS, important and under-recognized disorders, are available and should be utilized. Growing evidence concerning the pathophysiology of PMDD has led to the development of novel investigative compounds, however it remains to be seen as to when and whether such therapies might become available. By assessing the needs of each unique patient and appropriately utilizing an inter-disciplinary approach involving psychiatric and obstetrics–gynecological practitioners as appropriate, individualized treatments can be assembled through careful review of the available evidence.
Disclosure
Dr. Deligiannidis receives research funding from the National Institutes of Health (R01MH118269, R01MH120313) which supported her time for this research and manuscript. Dr. Deligiannidis has also received research funding from Sage Therapeutics, Inc. and Vorso Corporation (contracted research) and serves as a consultant to Sage Therapeutics, Inc., Brii Biosciences, GH Research, Ireland LLC and Brainify.AI. Dr. Carlini, Dr. Lanza di Scalea, Dr. McNally, and Ms. Lester have no disclosures to report for this work.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
- Frank RT. The hormonal causes of premenstrual tension. Arch Neurol Psychiatry. 1931;26(5):1053–1057. doi:10.1001/archneurpsyc.1931.02230110151009
- Greene R, Dalton K. The premenstrual syndrome. Br Med J. 1953;1(4818):1007–1013. doi:10.1136/bmj.1.4818.1007
- Johnson TM. Premenstrual syndrome as a western culture-specific disorder. Cult Med Psychiatry. 1987;11(3):337–356. doi:10.1007/bf00048518
- Dennerstein L, Lehert P, Heinemann K. Global epidemiological study of variation of premenstrual symptoms with age and sociodemographic factors. Menopause Int. 2011;17(3):96–101. doi:10.1258/mi.2011.011028
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994.
- O’Brien PMS, Bäckström T, Brown C, et al. Towards a consensus on diagnostic criteria, measurement and trial design of the premenstrual disorders: the ISPMD Montreal consensus. Arch Womens Ment Health. 2011;14(1):13–21. doi:10.1007/s00737-010-0201-3
- Ismaili E, Walsh S, O’Brien PMS, et al. Fourth consensus of the International Society for Premenstrual Disorders (ISPMD): auditable standards for diagnosis and management of premenstrual disorder. Arch Womens Ment Health. 2016;19:953–958. doi:10.1007/s00737-016-0631-7
- Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health. 2006;9(1):41–49. doi:10.1007/s00737-005-0103-y
- Yonkers KA, Simoni MK. Premenstrual disorders. Am J Obstet Gynecol. 2018;218(1):68–74. doi:10.1016/j.ajog.2017.05.045
- Halbreich U, Borenstein J, Pearlstein T, Kahn LS. The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD). Psychoneuroendocrinology. 2003;28(Suppl 3):1–23.
- Dueñas JL, Lete I, Bermejo R, et al. Prevalence of premenstrual syndrome and premenstrual dysphoric disorder in a representative cohort of Spanish women of fertile age. Eur J Obstet Gynecol Reprod Biol. 2011;156(1):72–77. doi:10.1016/j.ejogrb.2010.12.013
- Skrzypulec-Plinta V, Drosdzol A, Nowosielski K, Plinta R. The complexity of premenstrual dysphoric disorder - risk factors in the population of Polish women. Reprod Biol Endocrinol. 2010;8. doi:10.1186/1477-7827-8-141
- Tschudin S, Bertea PC, Zemp E. Prevalence and predictors of premenstrual syndrome and premenstrual dysphoric disorder in a population-based sample. Arch Womens Ment Health. 2010;13(6):485–494. doi:10.1007/s00737-010-0165-3
- Dutta A, Sharma A. Prevalence of premenstrual syndrome and premenstrual dysphoric disorder in India: a systematic review and meta-analysis. Heal Promot Perspect. 2021;11(2):161–170. doi:10.34172/hpp.2021.20
- Schatz D, Hsiao MC, Liu CY. Premenstrual dysphoric disorder in east Asia: a review of the literature. Int J Psychiatry Med. 2012;43(4):365–380. doi:10.2190/PM.43.4.f
- Bahamondes L, Córdova-Egüez S, Pons JE, Shulman L. Perspectives on premenstrual syndrome/premenstrual dysphoric disorder: outcomes from a meeting of the Latin America experts group. Dis Manag Heal Outcomes. 2007;15(5):263–277. doi:10.2165/00115677-200715050-00001
- de Carvalho AB, de Cardoso TA, Mondin TC, et al. Prevalence and factors associated with Premenstrual Dysphoric Disorder: a community sample of young adult women. Psychiatry Res. 2018;268:42–45. doi:10.1016/j.psychres.2018.06.005
- Heinemann LAJ, Do Minh T, Heinemann K, Lindemann M, Filonenko A. Intercountry assessment of the impact of severe premenstrual disorders on work and daily activities. Health Care Women Int. 2012;33(2):109–124. doi:10.1080/07399332.2011.610530
- Prasad D, Wollenhaupt-Aguiar B, Kidd KN, De Azevedo Cardoso T, Frey BN. Suicidal risk in women with premenstrual syndrome and premenstrual dysphoric disorder: a systematic review and meta-analysis. J Womens Health. 2021;30(12):1693–1707. doi:10.1089/jwh.2021.0185
- Yan H, Ding Y, Guo W. Suicidality in patients with premenstrual dysphoric disorder–a systematic review and meta-analysis. J Affect Disord. 2021;295:339–346. doi:10.1016/j.jad.2021.08.082
- Reed BG, Carr BR. The Normal Menstrual Cycle and the Control of Ovulation. MDText.com, Inc.; 2000.
- Schmidt PJ, Martinez PE, Nieman LK, et al. Premenstrual dysphoric disorder symptoms following ovarian suppression: triggered by change in ovarian steroid levels but not continuous stable levels. Am J Psychiatry. 2017;174(10):980–989. doi:10.1176/appi.ajp.2017.16101113
- Bäckström T, Sanders D, Leask R, Davidson D, Warner P, Bancroft J. Mood, sexuality, hormones, and the menstrual cycle. II. Hormone levels and their relationship to the premenstrual syndrome. Psychosom Med. 1983;45(6):503–507. doi:10.1097/00006842-198312000-00004
- Timby E, Bäckström T, Nyberg S, Stenlund H, Wihlbäck ACN, Bixo M. Women with premenstrual dysphoric disorder have altered sensitivity to allopregnanolone over the menstrual cycle compared to controls—a pilot study. Psychopharmacology. 2016;233(11):2109–2117. doi:10.1007/s00213-016-4258-1
- McEvoy K, Osborne LM. Allopregnanolone and reproductive psychiatry: an overview. Int Rev Psychiatry. 2019;31:1–8. doi:10.1080/09540261.2018.1553775
- Krasnik C, Montori VM, Guyatt GH, Heels-Ansdell D, Busse JW. The effect of bright light therapy on depression associated with premenstrual dysphoric disorder. Am J Obstet Gynecol. 2005;193(3):658–661. doi:10.1016/j.ajog.2005.01.055
- Johny M, Kumar SS, Rajagopalan A, Mukkadan JK. Vestibular stimulation for management of premenstrual syndrome. J Nat Sci Biol Med. 2017;8(1):82–86. doi:10.4103/0976-9668.198365
- Nevatte T, O’Brien PMS, Bäckström T, et al. ISPMD consensus on the management of premenstrual disorders. Arch Womens Ment Health. 2013;16(4):279–291. doi:10.1007/s00737-013-0346-y
- Steinberg EM, Cardoso GMP, Martinez PE, Rubinow DR, Schmidt PJ. Rapid response to fluoxetine in women with premenstrual dysphoric disorder. Depress Anxiety. 2012;29(6):531–540. doi:10.1002/da.21959
- Pinna G, Costa E, Guidotti A. SSRIs act as selective brain steroidogenic stimulants (SBSSs) at low doses that are inactive on 5-HT reuptake. Curr Opin Pharmacol. 2009;9(1):24–30. doi:10.1016/j.coph.2008.12.006
- Gracia CR, Freeman EW, Sammel MD, Lin H, Sheng L, Frye C. Allopregnanolone levels before and after selective serotonin reuptake inhibitor treatment of premenstrual symptoms. J Clin Psychopharmacol. 2009;29(4):403–405. doi:10.1097/JCP.0b013e3181ad8825
- Halbreich U, Bergeron R, Yonkers KA, Freeman E, Stout AL, Cohen L. Efficacy of intermittent, luteal phase sertraline treatment of premenstrual dysphoric disorder. Obstet Gynecol. 2002;100(6):1219–1229. doi:10.1016/s0029-7844(02)02326-8
- Freeman EW, Rickels K, Sondheimer SJ, Polansky M, Xiao S. Continuous or intermittent dosing with sertraline for patients with severe premenstrual syndrome or premenstrual dysphoric disorder. Am J Psychiatry. 2004;161(2):343–351. doi:10.1176/appi.ajp.161.2.343
- Eriksson E, Ekman A, Sinclair S, et al. Escitalopram administered in the luteal phase exerts a marked and dose-dependent effect in premenstrual dysphoric disorder. J Clin Psychopharmacol. 2008;28(2):195–202. doi:10.1097/JCP.0b013e3181678a28
- Freeman EW, Sondheimer SJ, Sammel MD, Ferdousi T, Lin H. A preliminary study of luteal phase versus symptom-onset dosing with escitalopram for premenstrual dysphoric disorder. J Clin Psychiatry. 2005;66(6):769–773. doi:10.4088/JCP.v66n0616
- Ladea M, Sarpe MC, Dumitrescu MR. The efficacy and the tolerability of escitalopram in the treatment of premenstrual dysphoric disorder. Gineco.ro. 2008;4(4):253–258.
- Cohen LS, Soares CN, Yonkers KA, Bellew KM, Bridges IM, Steiner M. Paroxetine controlled release for premenstrual dysphoric disorder: a double-blind, placebo-controlled trial. Psychosom Med. 2004;66(5):707–713. doi:10.1097/01.psy.0000140005.94790.9c
- Steiner M, Ravindran AV, LeMelledo J-M, et al. Luteal phase administration of paroxetine for the treatment of premenstrual dysphoric disorder: a randomized, double-blind, placebo-controlled trial in Canadian women. J Clin Psychiatry. 2008;69(6):991–998. doi:10.4088/JCP.v69n0616
- Landén M, Nissbrandt H, Allgulander C, Sörvik K, Ysander C, Eriksson E. Placebo-controlled trial comparing intermittent and continuous paroxetine in premenstrual dysphoric disorder. Neuropsychopharmacology. 2007;32(1):153–161. doi:10.1038/sj.npp.1301216
- Wu KY, Liu CY, Hsiao MC. Six-month paroxetine treatment of premenstrual dysphoric disorder: continuous versus intermittent treatment protocols. Psychiatry Clin Neurosci. 2008;62(1):109–114. doi:10.1111/j.1440-1819.2007.01785.x
- Pearlstein TB, Bellew KM, Endicott J, Steiner M. Paroxetine controlled release for premenstrual dysphoric disorder: remission analysis following a randomized, double-blind, placebo-controlled trial. Prim Care Companion J Clin Psychiatry. 2005;7(2):53–60. doi:10.4088/pcc.v07n0203
- Steiner M, Hirschberg AL, Bergeron R, Holland F, Gee MD, Van Erp E. Luteal phase dosing with paroxetine controlled release (CR) in the treatment of premenstrual dysphoric disorder. Am J Obstet Gynecol. 2005;193(2):352–360. doi:10.1016/j.ajog.2005.01.021
- Cohen LS, Miner C, Brown EW, et al. Premenstrual daily fluoxetine for premenstrual dysphoric disorder: a placebo-controlled, clinical trial using computerized diaries. Obstet Gynecol. 2002;100(3):435–444. doi:10.1016/s0029-7844(02)02166-x
- Miner C, Brown E, McCray S, Gonzales J, Wohlreich M. Weekly luteal-phase dosing with enteric-coated fluoxetine 90 mg in premenstrual dysphoric disorder: a randomized, double-blind, placebo-controlled clinical trial. Clin Ther. 2002;24(3):417–433. doi:10.1016/S0149-2918(02)85043-3
- Steiner M, Steinberg S, Stewart D, et al. Fluoxetine in the treatment of premenstrual dysphoria. N Engl J Med. 1995;332(23):1529–1534. doi:10.1056/nejm199506083322301
- Kornstein SG, Pearlstein TB, Fayyad R, Farfel GM, Gillespie JA. Low-dose sertraline in the treatment of moderate-to-severe premenstrual syndrome: efficacy of 3 dosing strategies. J Clin Psychiatry. 2006;67(10):1624–1632. doi:10.4088/JCP.v67n1020
- Nazari H, Yari F, Jariani M, Marzban A, Birgandy M. Premenstrual syndrome: a single-blind study of treatment with buspirone versus fluoxetine. Arch Gynecol Obstet. 2013;287(3):469–472. doi:10.1007/s00404-012-2594-x
- Yonkers KA, Pearlstein T, Fayyad R, Gillespie JA. Luteal phase treatment of premenstrual dysphoric disorder improves symptoms that continue into the postmenstrual phase. J Affect Disord. 2005;85(3):317–321. doi:10.1016/j.jad.2004.10.006
- Marjoribanks J, Brown J, O’Brien PMS, Wyatt K. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6):1–159. doi:10.1002/14651858.CD001396.pub3
- Shah NR, Jones JB, Aperi J, Shemtov R, Karne A, Borenstein J. Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder: a meta-analysis. Obstet Gynecol. 2008;111(5):1175–1182. doi:10.1097/AOG.0b013e31816fd73b
- Yonkers KA, Kornstein SG, Gueorguieva R, Merry B, Van Steenburgh K, Altemus M. Symptom-onset dosing of sertraline for the treatment of premenstrual dysphoric disorder: a randomized clinical trial. JAMA psychiatry. 2015;72(10):1037–1044. doi:10.1001/jamapsychiatry.2015.1472
- Hsiao MC, Liu CY. Effective open-label treatment of premenstrual dysphoric disorder with venlafaxine. Psychiatry Clin Neurosci. 2003;57(3):317–321. doi:10.1046/j.1440-1819.2003.01123.x
- Cohen LS, Soares CN, Lyster A, Cassano P, Brandes M, Leblanc GA. Efficacy and tolerability of premenstrual use of venlafaxine (flexible dose) in the treatment of premenstrual dysphoric disorder. J Clin Psychopharmacol. 2004;24(5):540–543. doi:10.1097/01.jcp.0000138767.53976.10
- Ramos MG, Hara C, Rocha FL. Duloxetine treatment for women with premenstrual dysphoric disorder: a single-blind trial. Int J Neuropsychopharmacol. 2009;12(08):1081. doi:10.1017/S1461145709000066
- Mazza M, Harnic D, Catalano V, Janiri L, Bria P. Duloxetine for premenstrual dysphoric disorder: a pilot study. Expert Opin Pharmacother. 2008;9(4):517–521. doi:10.1517/14656566.9.4.517
- Lete I, Lapuente O. Contraceptive options for women with premenstrual dysphoric disorder: current insights and a narrative review. Open Access J Contracept. 2016;7:117–125. doi:10.2147/oajc.s97013
- Freeman EW, Kroll R, Rapkin A, et al. Evaluation of a unique oral contraceptive in the treatment of premenstrual dysphoric disorder. J Womens Health Gender Based Med. 2001;10(6):561–569. doi:10.1089/15246090152543148
- Yonkers KA, Brown C, Pearlstein TB, Foegh M, Sampson-Landers C, Rapkin A. Efficacy of a new low-dose oral contraceptive with drospirenone in premenstrual dysphoric disorder. Obstet Gynecol. 2005;106(3):492–501. doi:10.1097/01.AOG.0000175834.77215.2e
- Marr J, Heinemann K, Kunz M, Rapkin A. Ethinyl estradiol 20 μg/drospirenone 3 mg 24/4 oral contraceptive for the treatment of functional impairment in women with premenstrual dysphoric disorder. Int J Gynecol Obstet. 2011;113:103–107. doi:10.1016/j.ijgo.2010.10.029
- Marr J, Niknian M, Shulman LP, Lynen R. Premenstrual dysphoric disorder symptom cluster improvement by cycle with the combined oral contraceptive ethinylestradiol 20 mcg plus drospirenone 3 mg administered in a 24/4 regimen. Contraception. 2011;84(1):81–86. doi:10.1016/j.contraception.2010.10.010
- Pearlstein TB, Bachmann GA, Zacur HA, Yonkers KA. Treatment of premenstrual dysphoric disorder with a new drospirenone-containing oral contraceptive formulation. Contraception. 2005;72(6):414–421. doi:10.1016/j.contraception.2005.08.021
- Wichianpitaya J, Taneepanichskul S. Clinical study A comparative efficacy of low-dose combined oral contraceptives containing desogestrel and drospirenone in premenstrual symptoms. Obstet Gynecol Int. 2013;2013:1–9. doi:10.1155/2013/487143
- Eisenlohr-Moul TA, Girdler SS, Johnson JL, Schmidt PJ, Rubinow DR. Treatment of premenstrual dysphoria with continuous versus intermittent dosing of oral contraceptives: results of a three-arm randomized controlled trial. Depress Anxiety. 2017;34(10):908–917. doi:10.1002/da.22673
- Freeman EW, Halbreich U, Grubb GS, et al. An overview of four studies of a continuous oral contraceptive (levonorgestrel 90 mcg/ethinyl estradiol 20 mcg) on premenstrual dysphoric disorder and premenstrual syndrome. Contraception. 2012;85(5):437–445. doi:10.1016/j.contraception.2011.09.010
- de Wit AE, de Vries YA, de Boer MK, et al. Efficacy of combined oral contraceptives for depressive symptoms and overall symptomatology in premenstrual syndrome: pairwise and network meta-analysis of randomized trials. Am J Obstet Gynecol. 2021;225(6):624–633. doi:10.1016/j.ajog.2021.06.090
- Ford O, Lethaby A, Roberts H, Mol BWJ. Progesterone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;2012(3). doi:10.1002/14651858.CD003415.pub4
- Horbatiuk O, Binkovska A, Herych O, et al. Using micronized progesterone for treatment of premenopausal age women suffering from severe premenstrual syndrome. Curr Issues Pharm Med Sci. 2017;30(3):138–141. doi:10.1515/cipms-2017-0025
- Comasco E, Kallner HK, Bixo M, et al. Ulipristal acetate for treatment of premenstrual dysphoric disorder: a proof-of-concept randomized controlled trial. Am J Psychiatry. 2021;178(3):256–265. doi:10.1176/appi.ajp.2020.20030286
- Naheed B, Kuiper JH, Uthman OA, O’Mahony F, O’Brien PMS. Non-contraceptive oestrogen-containing preparations for controlling symptoms of premenstrual syndrome. Cochrane Database Syst Rev. 2017;2017(3). doi:10.1002/14651858.CD010503.pub2
- Martinez PE, Rubinow DR, Nieman LK, et al. 5α-reductase inhibition prevents the luteal phase increase in plasma allopregnanolone levels and mitigates symptoms in women with premenstrual dysphoric disorder. Neuropsychopharmacology. 2016;41(4):1093–1102. doi:10.1038/npp.2015.246
- Brown CS, Ling FW, Andersen RN, Farmer RG, Arheart KL. Efficacy of depot leuprolide in premenstrual syndrome: effect of symptom severity and type in a controlled trial. Obstet Gynecol. 1994;84(5):779–786.
- Wyatt KM, Dimmock PW, Ismail KMK, Jones PW, O’Brien PMS. The effectiveness of GnRHa with and without “add-back” therapy in treating premenstrual syndrome: a meta analysis. BJOG. 2004;111(6):585–593. doi:10.1111/j.1471-0528.2004.00135.x
- Hahn PM, Van Vugt DA, Reid RL. A randomized, placebo-controlled, crossover trial of danazol for the treatment of premenstrual syndrome. Psychoneuroendocrinology. 1995;20(2):193–209. doi:10.1016/0306-4530(94)00053-D
- Anwar S, Shami N, Asif S. Use of low dose danazol in luteal phase only as management of premenstrual syndrome and mastalgia. Pak J Med Heal Sci. 2015;9(3):864–865.
- Henshaw C, Foreman D, Belcher J, Cox J, O’Brien S. Can one induce premenstrual symptomatology in women with prior hysterectomy and bilateral oophorectomy? J Psychosom Obstet Gynaecol. 1996;17(1):21–28. doi:10.3109/01674829609025660
- Mitwally MFM, Gotlieb L, Casper RF. Prevention of bone loss and hypoestrogenic symptoms by estrogen and interrupted progestogen add-back in long-term GnRH-agonist down-regulated patients with endometriosis and premenstrual syndrome. Menopause. 2002;9(4):236–241. doi:10.1097/00042192-200207000-00004
- Segebladh B, Borgström A, Nyberg S, Bixo M, Sundström-Poromaa I. Evaluation of different add-back estradiol and progesterone treatments to gonadotropin-releasing hormone agonist treatment in patients with premenstrual dysphoric disorder. Am J Obstet Gynecol. 2009;201(2):139.e1–139.e8. doi:10.1016/j.ajog.2009.03.016
- Harrison WM, Endicott J, Nee J. Treatment of premenstrual dysphoria with alprazolam: a controlled study. Arch Gen Psychiatry. 1990;47(3):270–275. doi:10.1001/archpsyc.1990.01810150070011
- Berger CP, Presser B. Alprazolam in the treatment of two subsamples of patients with late luteal phase dysphoric disorder: a double-blind, placebo-controlled crossover study. Obstet Gynecol. 1994;84(3):379–385.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Vol. 3rd. Washington, DC: American Psychiatric Association; 1987.
- Schmidt PJ, Grover GN, Rubinow DR. Alprazolam in the treatment of premenstrual syndrome: a double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1993;50(6):467–473. doi:10.1001/archpsyc.1993.01820180069007
- Evans SM, Haney M, Levin FR, Foltin RW, Fischman MW. Mood and performance changes in women with premenstrual dysphoric disorder: acute effects of alprazolam. Neuropsychopharmacology. 1998;19(6):499–516. doi:10.1016/S0893-133X(98)00064-5
- Jackson C, Pearson B, Girdler S, et al. Double-blind, placebo-controlled pilot study of adjunctive quetiapine SR in the treatment of PMS/PMDD. Hum Psychopharmacol. 2015;30(6):425–434. doi:10.1002/hup.2494
- Sani G, Kotzalidis GD, Panaccione I, et al. Low-dose acetazolamide in the treatment of premenstrual dysphoric disorder: a case series. Psychiatry Investig. 2014;11(1):95. doi:10.4306/pi.2014.11.1.95
- Bunevicius R, Hinderliter AL, Light KC, Pedersen CA, Girdler SS. Lack of beneficial effects of clonidine in the treatment of premenstrual dysphoric disorder: results of a double-blind, randomized study. Hum Psychopharmacol. 2005;20(1):33–39. doi:10.1002/hup.652
- Bäckström T, Bixo M, Strömberg J. GABAA receptor-modulating steroids in relation to women’s behavioral health. Curr Psychiatry Rep. 2015;17(11):1–7. doi:10.1007/s11920-015-0627-4
- Bixo M, Ekberg K, Poromaa IS, et al. Treatment of premenstrual dysphoric disorder with the GABAA receptor modulating steroid antagonist Sepranolone (UC1010)—A randomized controlled trial. Psychoneuroendocrinology. 2017;80:46–55. doi:10.1016/J.PSYNEUEN.2017.02.031
- Bäckström T, Ekberg K, Hirschberg AL, et al. A randomized, double-blind study on efficacy and safety of sepranolone in premenstrual dysphoric disorder. Psychoneuroendocrinology. 2021;133:105426. doi:10.1016/j.psyneuen.2021.105426
- Complementary, alternative, or integrative health: what’s in a name? National Center for Complementary and Alternative Medicine. Available from: https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-A-name. Accessed December 7, 2022.
- WHO global report on traditional and complementary medicine 2019. World Health Organization; 2019. Available from: https://apps.who.int/iris/bitstream/handle/10665/312342/9789241515436-eng.pdf?sequence=1&isAllowed=y. Accessed December 7, 2022.
- Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;10(12):1–23.
- Kraemer GR, Kraemer RR. Premenstrual syndrome: diagnosis and treatment experiences. J Womens Health. 1998;7(7):893–907. doi:10.1089/jwh.1998.7.893
- Upchurch DM, Rainisch BW. The importance of wellness among users of complementary and alternative medicine: findings from the 2007 National Health Interview Survey. BMC Complement Altern Med. 2015;15(1). doi:10.1186/s12906-015-0886-y
- Van Breemen RB. Development of safe and effective botanical dietary supplements. J Med Chem. 2015;58(21):8360–8372. doi:10.1021/acs.jmedchem.5b00417
- Deligiannidis KM, Freeman MP. Complementary and alternative medicine for the treatment of depressive disorders in women. Psychiatr Clin North Am. 2010;33(2):441–463. doi:10.1016/j.psc.2010.01.002
- Farahmand M, Khalili D, Ramezani Tehrani F, Amin G, Negarandeh R. Could Anise decrease the intensity of premenstrual syndrome symptoms in comparison to placebo? A double-blind randomized clinical trial. J Complement Integr Med. 2021;17(4). doi:10.1515/jcim-2019-0077
- Farahmand M, Khalili D, Ramezani Tehrani F, Amin G, Negarandeh R. Effectiveness of Echium amoenum on premenstrual syndrome: a randomized, double-blind, controlled trial. BMC Complement Med Ther. 2020;20(1). doi:10.1186/s12906-020-03084-2
- Khayat S, Kheirkhah M, Behboodi Moghadam Z, Fanaei H, Kasaeian A, Javadimehr M. Effect of treatment with ginger on the severity of premenstrual syndrome symptoms. ISRN Obstet Gynecol. 2014;2014:1–5. doi:10.1155/2014/792708
- Maleki-Saghooni N, Karimi FZ, Behboodi Moghadam Z, Mirzaii Najmabadi K. The effectiveness and safety of Iranian herbal medicines for treatment of premenstrual syndrome: a systematic review. Avicenna J Phytomed. 2018;8(2):96–113.
- Mirghafourvand M, Malakouti J, Charandabi SMA, Khalili AF, Homayi SG. The efficacy of lemon balm (Melissa officinalis L.) alone and combined with lemon balm—Nepeta menthoides on premenstrual syndrome and quality of life among students: a randomized controlled trial. J Herb Med. 2016;6(3):142–148. doi:10.1016/j.hermed.2016.07.001
- Taavoni S, Barkhordari F, Goushegir A, Haghani H. Effect of Royal Jelly on premenstrual syndrome among Iranian medical sciences students: a randomized, triple-blind, placebo-controlled study. Complement Ther Med. 2014;22(4):601–606. doi:10.1016/j.ctim.2014.05.004
- Bahrami A, Zarban A, Rezapour H, Agha Amini Fashami A, Ferns GA. Effects of curcumin on menstrual pattern, premenstrual syndrome, and dysmenorrhea: a triple-blind, placebo-controlled clinical trial. Phyther Res. 2021;35(12):6954–6962. doi:10.1002/ptr.7314
- Ozgoli G, Selselei EA, Mojab F, Majd HA. A randomized, placebo-controlled trial of Ginkgo biloba L. in treatment of premenstrual syndrome. J Altern Complement Med. 2009;15(8):845–851. doi:10.1089/acm.2008.0493
- Sodouri M, Alavi N. Effects of Zataria multi-flora, Shirazi thyme, on the severity of premenstrual syndrome; 2013. Available from: ncbi.nlm.nih.gov. Accessed December 7, 2022.
- Malik R, Firdose KF, Bhat MDA. Efficacy of Nardostachys jatamansi DC. in the management of premenstrual syndrome: a randomized controlled study. J Herb Med. 2018;14:17–21. doi:10.1016/j.hermed.2018.09.003
- Tjandrawinata RR, Nofiarny D, Susanto LW, Hendri P, Clarissa A. Symptomatic treatment of premenstrual syndrome and/or primary dysmenorrhea with DLBS1442, a bioactive extract of Phaleria macrocarpa. Int J Gen Med. 2011;4:465. doi:10.2147/ijgm.s21053
- Tully L, Humiston J, Cash A. Oxaloacetate reduces emotional symptoms in premenstrual syndrome (PMS): results of a placebocontrolled, cross-over clinical trial. Obstet Gynecol Sci. 2020;63(2):195–204. doi:10.5468/ogs.2020.63.2.195
- Sampalis F, Bunea R, Pelland MF, Kowalski O, Duguet N, Dupuis S. Evaluation of the effects of Neptune Krill OilTM on the management of premenstrual syndrome and dysmenorrhea. Altern Med Rev. 2003;8(2):171–179.
- Delaram M, Heydarnejad MS. Herbal remedy for premenstrual syndrome with fennel (Foeniculum vulgare) - Randomized, placebo-controlled study. Adv Clin Exp Med. 2011;20(4):509–512.
- Bryant M, Cassidy A, Hill C, Powell J, Talbot D, Dye L. Effect of consumption of soy isoflavones on behavioural, somatic and affective symptoms in women with premenstrual syndrome. Br J Nutr. 2005;93(5):731–739. doi:10.1079/bjn20041396
- Ataollahi M, Ali Akbari SA, Mojab F, Majd HA. The effect of wheat germ extract on premenstrual syndrome symptoms. Iran J Pharm Res. 2015;14(1):159–166.
- Schmidt K, Weber N, Steiner M, et al. A lecithin phosphatidylserine and phosphatidic acid complex (PAS) reduces symptoms of the premenstrual syndrome (PMS): results of a randomized, placebo-controlled, double-blind clinical trial. Clin Nutr ESPEN. 2018;24:22–30. doi:10.1016/j.clnesp.2018.01.067
- Es-Haghee S, Shabani F, Hawkins J, et al. The effects of aromatherapy on premenstrual syndrome symptoms: a systematic review and meta-analysis of randomized clinical trials. Evid Based Complement Altern Med. 2020;2020:1–13. doi:10.1155/2020/6667078
- Choi JH. Effects of kinesio taping and hot packs on premenstrual syndrome in females. J Phys Ther Sci. 2017;29(9):1514–1517. doi:10.1589/jpts.29.1514
- Lim C, Park Y, Bae Y. The effect of the kinesio taping and spiral taping on menstrual pain and premenstrual syndrome. J Phys Ther Sci. 2013;25(7):761–764. doi:10.1589/jpts.25.761
- Jang HS, Lee MS. Effects of qi therapy (external qigong) on premenstrual syndrome: a randomized placebo-controlled study. J Altern Complement Med. 2004;10(3):456–462. doi:10.1089/1075553041323902
- Jang HS, Lee MS, Kim MJ, Chong ES. Effects of Qi-therapy on premenstrual syndrome. Int J Neurosci. 2004;114(8):909–921. doi:10.1080/00207450490450163
- Jing Z, Yang X, Ismail KMK, Chen X, Wu T. Chinese herbal medicine for premenstrual syndrome. Cochrane Database Syst Rev. 2009;(1). doi:10.1002/14651858.CD006414.pub2
- Merz PG, Gorkow C, Schrödter A, et al. The effects of a special Agnus castus extract (BP1095E1) on prolactin secretion in healthy male subjects. Exp Clin Endocrinol Diabetes. 1996;104(6):447–453. doi:10.1055/s-0029-1211483
- Webster DE, He Y, Chen SN, Pauli GF, Farnsworth NR, Wang ZJ. Opioidergic mechanisms underlying the actions of Vitex agnus-castus L. Biochem Pharmacol. 2011;81(1):170–177. doi:10.1016/j.bcp.2010.09.013
- Ibrahim NA, Shalaby AS, Farag RS, Elbaroty GS, Nofal SM, Hassan EM. Gynecological efficacy and chemical investigation of Vitex agnus-castus L. fruits growing in Egypt. Nat Prod Res. 2008;22(6):537–546. doi:10.1080/14786410701592612
- Momoeda M, Sasaki H, Tagashira E, Ogishima M, Takano Y, Ochiai K. Efficacy and safety of vitex agnus-castus extract for treatment of premenstrual syndrome in Japanese patients: a prospective, open-label study. Adv Ther. 2014;31(3):362–373. doi:10.1007/s12325-014-0106-z
- Ambrosini A, Di Lorenzo C, Coppola G, Pierelli F. Use of Vitex agnus-castus in migrainous women with premenstrual syndrome: an open-label clinical observation. Acta Neurol Belg. 2013;113(1):25–29. doi:10.1007/s13760-012-0111-4
- He Z, Chen R, Zhou Y, et al. Treatment for premenstrual syndrome with Vitex agnus castus: a prospective, randomized, multi-center placebo controlled study in China. Maturitas. 2009;63(1):99–103. doi:10.1016/j.maturitas.2009.01.006
- Ma L, Lin S, Chen R, Wang X. Treatment of moderate to severe premenstrual syndrome with Vitex agnus castus (BNO 1095) in Chinese women. Gynecol Endocrinol. 2010;26(8):612–616. doi:10.3109/09513591003632126
- Verkaik S, Kamperman AM, van Westrhenen R, Schulte PFJ. The treatment of premenstrual syndrome with preparations of Vitex agnus castus: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217(2):150–166. doi:10.1016/j.ajog.2017.02.028
- Csupor D, Lantos T, Hegyi P, et al. Vitex agnus-castus in premenstrual syndrome: a meta-analysis of double-blind randomised controlled trials. Complement Ther Med. 2019;47:102190. doi:10.1016/j.ctim.2019.08.024
- Schellenberg R, Zimmermann C, Drewe J, Hoexter G, Zahner C. Dose-dependent efficacy of the Vitex agnus castus extract Ze 440 in patients suffering from premenstrual syndrome. Phytomedicine. 2012;19(14):1325–1331. doi:10.1016/j.phymed.2012.08.006
- Khalesi ZB, Beiranvand SP, Bokaie M. Efficacy of chamomile in the treatment of premenstrual syndrome: a systematic review. J Pharmacopuncture. 2019;22(4):204–209. doi:10.3831/KPI.2019.22.028
- Khayat S, Fanaei H, Kheirkhah M, Moghadam ZB, Kasaeian A, Javadimehr M. Curcumin attenuates severity of premenstrual syndrome symptoms: a randomized, double-blind, placebo-controlled trial. Complement Ther Med. 2015;23(3):318–324. doi:10.1016/j.ctim.2015.04.001
- Yamada K, Kanba S. Herbal medicine (Kami-shoyo-san) in the treatment of premenstrual dysphoric disorder [6]. J Clin Psychopharmacol. 2002;22(4):442. doi:10.1097/00004714-200208000-00023
- Yamada K, Kanba S. Effectiveness of kamishoyosan for premenstrual dysphoric disorder: open-labeled pilot study. Psychiatry Clin Neurosci. 2007;61(3):323–325. doi:10.1111/j.1440-1819.2007.01649.x
- Rajabi F, Rahimi M, Sharbafchizadeh M, Tarrahi M. Saffron for the management of premenstrual dysphoric disorder: a randomized controlled trial. Adv Biomed Res. 2020;9(1):60. doi:10.4103/abr.abr_49_20
- Agha-Hosseini M, Kashani L, Aleyaseen A, et al. Crocus sativus L. (saffron) in the treatment of premenstrual syndrome: a double-blind, randomised and placebo-controlled trial. BJOG. 2008;115(4):515–519. doi:10.1111/j.1471-0528.2007.01652.x
- Pirdadeh Beiranvand S, Shams Beiranvand N, Behboodi Moghadam Z, et al. The effect of Crocus sativus (saffron) on the severity of premenstrual syndrome. Eur J Integr Med. 2016;8(1):55–61. doi:10.1016/j.eujim.2015.06.003
- Canning S, Waterman M, Orsi N, Ayres J, Simpson N, Dye L. The efficacy of hypericum perforatum (st john’s wort) for the treatment of premenstrual syndrome: a randomized, double-blind, placebo-controlled trial. CNS Drugs. 2010;24(3):207–225. doi:10.2165/11530120-000000000-00000
- Ryoo JG, Chun S, Lee YJ, Suh HS. The effects of St. John’s wort on premenstrual syndrome in single women: a randomized double-blind, placebo-controlled study. Clin Psychopharmacol Neurosci. 2010;8(1):30–37.
- Royal College of Obstetricians and Gynaecologists. Management of premenstrual syndrome: green-top guideline no. 48. BJOG. 2017;124(3):e73–e105. doi:10.1111/1471-0528.14260
- Wyatt KM, Dimmock PW, Jones PW, Shaughn O’Brien PM. Efficacy of vitamin B-6 in the treatment of premenstrual syndrome: systematic review. BMJ. 1999;318(7195):1375–1381. doi:10.1136/bmj.318.7195.1375
- Office of Dietary Supplements, National Institutes of Health. Vitamin B6 - health professional fact sheet; 2022. Available from: https://ods.od.nih.gov/factsheets/VitaminB6-HealthProfessional/. Accessed June 11, 2022.
- Samieipoor S, Kiani F, Sayehmiri K, et al. Effects of vitamin B6 on premenstrual syndrome: a systematic review and meta-analysis. J Chem Pharm Sci. 2016;9(3):1346–1353.
- Abdollahifard S, Rahmanian Koshkaki A, Moazamiyanfar R. The effects of vitamin B1 on ameliorating the premenstrual syndrome symptoms. Glob J Health Sci. 2014;6(6):144–153. doi:10.5539/gjhs.v6n6p144
- Samieipour S, Tavassoli E, Heydarabadi A, et al. Effect of calcium and vitamin B1 on the severity of premenstrual syndrome: a randomized control trial. Int J Pharm Technol. 2016;8(3):18706–18717.
- Chocano-Bedoya PO, Manson JE, Hankinson SE, et al. Dietary B vitamin intake and incident premenstrual syndrome. Am J Clin Nutr. 2011;93(5):1080–1086. doi:10.3945/ajcn.110.009530
- Arab A, Golpour-Hamedani S, Rafie N. The association between vitamin D and premenstrual syndrome: a systematic review and meta-analysis of current literature. J Am Coll Nutr. 2019;38(7):648–656. doi:10.1080/07315724.2019.1566036
- Abdollahi R, Abiri B, Sarbakhsh P, Kashanian M, Vafa M. The effect of vitamin D supplement consumption on premenstrual syndrome in vitamin D-deficient young girls: a randomized, double-blind, placebo-controlled clinical trial. Complement Med Res. 2019;26(5):336–342. doi:10.1159/000500016
- Bocchieri E, Thys-Jacobs S. Role of calcium metabolism in prementrual syndrome. Expert Rev Endocrinol Metab. 2008;3(5):645–655. doi:10.1586/17446651.3.5.645
- Shobeiri F, Araste FE, Ebrahimi R, Jenabi E, Nazari M. Effect of calcium on premenstrual syndrome: a double-blind randomized clinical trial. Obstet Gynecol Sci. 2017;60(1):100. doi:10.5468/ogs.2017.60.1.100
- Ghanbari Z, Haghollahi F, Shariat M, Foroshani AR, Ashrafi M. Effects of calcium supplement therapy in women with premenstrual syndrome. Taiwan J Obstet Gynecol. 2009;48(2):124–129. doi:10.1016/S1028-4559(09)60271-0
- Yonkers KA, Pearlstein TB, Gotman N. A pilot study to compare fluoxetine, calcium, and placebo in the treatment of premenstrual syndrome. J Clin Psychopharmacol. 2013;33(5):614–620. doi:10.1097/JCP.0b013e31829c7697
- Thys-Jacobs S, Starkey P, Bernstein D, Tian J. Calcium carbonate and the premenstrual syndrome: effects on premenstrual and menstrual symptoms. Premenstrual Syndrome Study Group. Am J Obstet Gynecol. 1998;179(2):444–452. doi:10.1016/S0002-9378(98)70377-1
- Fathizadeh S, Amani R, Haghighizadeh MH, Hormozi R. Comparison of serum zinc concentrations and body antioxidant status between young women with premenstrual syndrome and normal controls: a case-control study. Int J Reprod Biomed. 2016;14(11):699–704. doi:10.29252/ijrm.14.11.699
- Siahbazi S, Behboudi-Gandevani S, Moghaddam-Banaem L, Montazeri A. Effect of zinc sulfate supplementation on premenstrual syndrome and health-related quality of life: clinical randomized controlled trial. J Obstet Gynaecol Res. 2017;43(5):887–894. doi:10.1111/jog.13299
- Jafari F, Amani R, Tarrahi MJ. Effect of zinc supplementation on physical and psychological symptoms, biomarkers of inflammation, oxidative stress, and brain-derived neurotrophic factor in young women with premenstrual syndrome: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res. 2020;194(1):89–95. doi:10.1007/s12011-019-01757-9
- Jafari F, Tarrahi MJ, Farhang A, Amani R. Effect of zinc supplementation on quality of life and sleep quality in young women with premenstrual syndrome: a randomized, double-blind, placebo-controlled trial. Arch Gynecol Obstet. 2020;302(3):657–664. doi:10.1007/s00404-020-05628-w
- Rosenstein DL, Elin RJ, Hosseini JM, Grover G, Rubinow DR. Magnesium measures across the menstrual cycle in premenstrual syndrome. Biol Psychiatry. 1994;35(8):557–561. doi:10.1016/0006-3223(94)90103-1
- Ebrahimi E, Khayati Motlagh S, Nemati S, Tavakoli Z. Effects of magnesium and vitamin b6 on the severity of premenstrual syndrome symptoms. J Caring Sci. 2012;1(4):183–189. doi:10.5681/jcs.2012.026
- Quaranta S, Buscaglia MA, Meroni MG, Colombo E, Cella S. Pilot study of the efficacy and safety of a modified-release magnesium 250mg tablet (Sincromag®) for the treatment of premenstrual syndrome. Clin Drug Investig. 2007;27(1):51–58. doi:10.2165/00044011-200727010-00004
- Fathizadeh N, Ebrahimi E, Valiani M, Tavakoli N, Yar MH. Evaluating the effect of magnesium and magnesium plus vitamin B6 supplement on the severity of premenstrual syndrome. Iran J Nurs Midwifery Res. 2010;15(Suppl 1):401–405.
- Alshammari E. Magnesium supplementation for premenstrual syndrome and premenstrual dysphoric disorder. Int J Pharm Res. 2020;13(1). doi:10.31838/ijpr/2021.13.01.095
- Mohammadi MM, Dehghan Nayeri N, Mashhadi M, Varaei S. Effect of omega-3 fatty acids on premenstrual syndrome: a systematic review and meta-analysis. J Obstet Gynaecol Res. 2022;48(6):1293–1305. doi:10.1111/jog.15217
- Mukai T, Kishi T, Matsuda Y, Iwata N. A meta-analysis of inositol for depression and anxiety disorders. Hum Psychopharmacol. 2014;29(1):55–63. doi:10.1002/hup.2369
- Gerhardsen G, Hansen AV, Killi M, Fornitz GG, Pedersen F, Roos SB. The efficacy of Femal in women with premenstrual syndrome: a randomised, double-blind, parallel-group, placebo-controlled, multicentre study. Adv Ther. 2008;25(6):595–607. doi:10.1007/s12325-008-0072-4
- Winther K, Hedman C. Assessment of the effects of the herbal remedy femal on the symptoms of Premenstrual Syndrome: a randomized, double-blind, placebo-controlled study. Curr Ther Res. 2002;63(5):344–353. doi:10.1016/S0011-393X(02)80038-8
- Kashani L, Saedi N, Akhondzadeh S. Femicomfort in the treatment of premenstrual syndromes: a double-blind, randomized and placebo controlled trial. Iran J Psychiatry. 2010;5(2):47–50.
- Sadeghi F, Javid AZ, Nazarinasab M, Haghighi‐Zadeh MH. Effects of PMS50 supplementation on psychological symptoms of students with premenstrual syndrome. Int J Gynecol Obstet. 2022;156(2):247–255. doi:10.1002/ijgo.13703
- Armour M, Ee CC, Hao J, Wilson TM, Yao SS, Smith CA. Acupuncture and acupressure for premenstrual syndrome. Cochrane Database Syst Rev. 2018;8:CD005290. doi:10.1002/14651858.CD005290.pub2
- Zhang J, Cao L, Wang Y, Jin Y, Xiao X, Zhang Q. Acupuncture for premenstrual syndrome at different intervention time: a systemic review and meta-analysis. Evid Based Complement Altern Med. 2019;2019. doi:10.1155/2019/6246285
- Kim SY, Park HJ, Lee H, Lee H. Acupuncture for premenstrual syndrome: a systematic review and meta-analysis of randomised controlled trials. BJOG. 2011;118(8):899–915. doi:10.1111/j.1471-0528.2011.02994.x
- Korelo RIG, Moreira NB, de Miguel BA, et al. Effects of Auriculotherapy on treatment of women with premenstrual syndrome symptoms: a randomized, placebo-controlled clinical trial. Complement Ther Med. 2022;66:102816. doi:10.1016/j.ctim.2022.102816
- Hasanpour M, Mohammadi MM, Shareinia H. Effects of reflexology on premenstrual syndrome: a systematic review and meta-analysis. Biopsychosoc Med. 2019;13(1). doi:10.1186/s13030-019-0165-0
- Simsek Kucukkelepce D, Unver H, Nacar G, Tashan ST. The effects of acupressure and yoga for coping with premenstrual syndromes on premenstrual symptoms and quality of life. Complement Ther Clin Pract. 2021;42:101282. doi:10.1016/j.ctcp.2020.101282
- Ghaffarilaleh G, Ghaffarilaleh V, Sanamno Z, Kamalifard M. Yoga positively affected depression and blood pressure in women with premenstrual syndrome in a randomized controlled clinical trial. Complement Ther Clin Pract. 2019;34:87–92. doi:10.1016/j.ctcp.2018.11.007
- Ghaffarilaleh G, Ghaffarilaleh V, Sanamno Z, Kamalifard M, Alibaf L. Effects of yoga on quality of sleep of women with premenstrual syndrome. Altern Ther Health Med. 2019;25(5):40–47.
- Sharma B, Misra R, Singh K, Sharma R. Comparative study of effect of anuloma-viloma (pranayam) and yogic asanas in premenstrual syndrome. Indian J Physiol Pharmacol. 2013;57:384–389.
- Bharati M. Comparing the effects of yoga & oral calcium administration in alleviating symptoms of premenstrual syndrome in medical undergraduates. J Caring Sci. 2016;5(3):179–185. doi:10.15171/jcs.2016.019
- Kamalifard M, Yavari A, Asghari-Jafarabadi M, Ghaffarilaleh G, Kasb-Khah A. The effect of yoga on women’s premenstrual syndrome: a randomized controlled clinical trial. Int J Womens Health Reprod Sci. 2017;5(3):205–211. doi:10.15296/ijwhr.2017.37
- Pearce E, Jolly K, Jones LL, Matthewman G, Zanganeh M, Daley A. Exercise for premenstrual syndrome: a systematic review and meta-analysis of randomised controlled trials. BJGP Open. 2020;4(3):bjgpopen20X101032. doi:10.3399/bjgpopen20X101032
- Maged AM, Abbassy AH, Sakr HRS, et al. Effect of swimming exercise on premenstrual syndrome. Arch Gynecol Obstet. 2018;297(4):951–959. doi:10.1007/s00404-018-4664-1
- Çitil ET, Kaya N. Effect of pilates exercises on premenstrual syndrome symptoms: a quasi-experimental study. Complement Ther Med. 2021;57:102623. doi:10.1016/j.ctim.2020.102623
- Zhang H, Zhu M, Song Y, Kong M. Baduanjin exercise improved premenstrual syndrome symptoms in Macau women. J Tradit Chin Med. 2014;34(4):460–464. doi:10.1016/s0254-6272(15)30047-9
- Vaghela N, Mishra D, Sheth M, Dani VB. To compare the effects of aerobic exercise and yoga on premenstrual syndrome. J Educ Health Promot. 2019;8(1). doi:10.4103/jehp.jehp-50-19
- Samadi Z, Taghian F, Valiani M. Effects of pilates and aerobic exercise on symptoms of premenstrual syndrome in non-athlete girls. J Isfahan Med Sch. 2013;30(213):1880–1891.
- Mohebbi Dehnavi Z, Jafarnejad F, Sadeghi Goghary S. The effect of 8weeks aerobic exercise on severity of physical symptoms of premenstrual syndrome: a clinical trial study. BMC Womens Health. 2018;18(1). doi:10.1186/s12905-018-0565-5
- Han J, Cha Y, Kim S. Effect of psychosocial interventions on the severity of premenstrual syndrome: a meta-analysis. J Psychosom Obstet Gynecol. 2019;40(3):176–184. doi:10.1080/0167482X.2018.1480606
- Shobeiri F, Ebrahimi R, Arasteh FE, Nazari S, Nazari S. Frequency of premenstrual syndrome and effectiveness of group counseling in reducing the severity of symptoms in female students. J Postgrad Med Inst. 2018;32(1):80–86.
- Bakir N, Vural P, Körpe G. The effects of emotional freedom techniques on coping with premenstrual syndrome: a randomized control trial. Wiley Online Libr. 2021. doi:10.1111/ppc.12957
- Shareh H, Ghodsi M, Keramati S. Emotion-focused group therapy among women with premenstrual dysphoric disorder: a randomized clinical trial. Psychother Res. 2022;32(4):440–455. doi:10.1080/10503307.2021.1980239
- Kancheva Landolt N, Ivanov K. Short report: cognitive behavioral therapy - A primary mode for premenstrual syndrome management: systematic literature review. Psychol Heal Med. 2021;26(10):1282–1293. doi:10.1080/13548506.2020.1810718
- Busse JW, Montori VM, Krasnik C, Patelis-Siotis I, Guyatt GH. Psychological intervention for premenstrual syndrome: a meta-analysis of randomized controlled trials. Psychother Psychosom. 2009;78(1):6–15. doi:10.1159/000162296
- Panahi F, Faramarzi M. The effects of mindfulness-based cognitive therapy on depression and anxiety in women with premenstrual syndrome. Depress Res Treat. 2016;2016. doi:10.1155/2016/9816481
- Askari S, Behroozi N, Abbaspoor Z. The effect of mindfulness-based cognitive-behavioral therapy on premenstrual syndrome. Iran Red Crescent Med J. 2018;20(2):57538. doi:10.5812/ircmj.57538
- Başoğul C, Aydın Özkan S, Karaca T. The effects of psychoeducation based on the cognitive-behavioral approach on premenstrual syndrome symptoms: a randomized controlled trial. Perspect Psychiatr Care. 2020;56(3):515–522. doi:10.1111/ppc.12460
- Izadi-Mazidi M, Davoudi I, Mehrabizadeh M. Effect of group cognitive-behavioral therapy on health-related quality of life in females with premenstrual syndrome. Iran J Psychiatry Behav Sci. 2016;10(1):e4961. doi:10.17795/ijpbs-4961
- Weise C, Kaiser G, Janda C, et al. Internet-based cognitive-behavioural intervention for women with premenstrual dysphoric disorder: a randomized controlled trial. Psychother Psychosom. 2019;88(1):16–29. doi:10.1159/000496237
- Borji-Navan S, Mohammad-Alizadeh-Charandabi S, Esmaeilpour K, Mirghafourvand M, Ahmadian-Khooinarood A. Internet-based cognitive-behavioral therapy for premenstrual syndrome: a randomized controlled trial. BMC Womens Health. 2022;22(1):5. doi:10.1186/s12905-021-01589-7
- Kleinstäuber M, Witthöft M, Hiller W. Cognitive-behavioral and pharmacological interventions for premenstrual syndrome or premenstrual dysphoric disorder: a meta-analysis. J Clin Psychol Med Settings. 2012;19(3):308–319. doi:10.1007/s10880-012-9299-y
- Hunter MS, Ussher JM, Browne SJ, Cariss M, Jelley R, Katz M. A randomized comparison of psychological (cognitive behavior therapy), medical (fluoxetine) and combined treatment for women with premenstrual dysphoric disorder. J Psychosom Obstet Gynecol. 2002;23(3):193–199. doi:10.3109/01674820209074672
- Simsek Kücükkelepce D, Timur Tashan S. The effects of health belief model‐based education and acupressure for coping with premenstrual syndrome on premenstrual symptoms and quality of life: a randomized‐controlled trial. Perspect Psychiatr Care. 2021;57(1):189–197. doi:10.1111/ppc.12546
- Cronje WH, Vashisht A, Studd JWW. Hysterectomy and bilateral oophorectomy for severe premenstrual syndrome. Hum Reprod. 2004;19(9):2152–2155. doi:10.1093/humrep/deh354
- Reid RL. When should surgical treatment be considered for premenstrual dysphoric disorder? Menopause Int. 2012;18(2):77–81. doi:10.1258/mi.2012.012009
- Lukes AS, McBride RJ, Herring AH, Fried M, Sherwani A, Dell D. Improved premenstrual syndrome symptoms after novasure endometrial ablation. J Minim Invasive Gynecol. 2011;18(5):607–611. doi:10.1016/j.jmig.2011.06.001