Abstract
Objective
To investigate the effect of a levonorgestrel-releasing intrauterine system (Mirena®) on aromatase and cyclooxygenase-2 (Cox-2) expression in the endometrium of patients with adenomyosis who were submitted to endometrial resection at the time of insertion, compared to a group not submitted to endometrial resection and a group of controls with adenomyosis not submitted to any previous hormonal treatment.
Patients and methods
Patients with adenomyosis (n = 89) were included in this study. Twenty- two patients had been using Mirena® for 5 years but had not been submitted to endometrial resection prior to insertion of the device. Twenty-four patients were submitted to endometrial resection at the time of Mirena® insertion. The remaining 43 patients with adenomyosis had undergone no previous hormonal treatment and served as a control group. Cox-2 and aromatase expression were determined in the endometrium by immunohistochemistry.
Results
Use of Mirena® for 5 years reduced aromatase expression in the endometrium; however, this reduction was significantly greater in the uteri previously submitted to endometrial resection. The reduction in Cox-2 expression was significant only in the uteri submitted to endometrial resection followed by the insertion of Mirena®.
Conclusion
Endometrial resection followed by the insertion of Mirena® was associated with greater rates of amenorrhea in patients with adenomyosis, which in turn were associated with a more effective inhibition of aromatase and Cox-2 expression in the endometrium.
Introduction
Adenomyosis is a frequent cause of abnormal uterine bleeding and pain in women of reproductive age. Although hysterectomy has always been advocated as an effective treatment, less invasive procedures such as endometrial resection have been proposed as an alternative; however, these procedures may be associated with lower success rates.
Nevertheless, the poor rates of amenorrhea may be due more to the intrinsic capacity of the endometrium to regenerate rather than to any fault in the actual technique.Citation1 Several recent studies have shown the presence of aromatase expression in the endometrium of patients with adenomyosis, myomas, and endometrial polyps.Citation2,Citation3 Locally produced estrogens upregulate several angiogenic and inflammatory factors in the endometrium, thus enabling this tissue to regenerate more effectively following hysteroscopic surgery. This would explain the resumption of bleeding and the low rates of amenorrhea achieved with endometrial ablation. It is therefore important that aromatase expression be suppressed in order to achieve better rates of amenorrhea following endometrial resection. Several different progestins have been shown to inhibit aromatase, vascular endothelial growth factor, and cyclooxygenase-2 (Cox-2) expression in the endometrium.Citation2,Citation4–Citation6 This effect may help improve the surgical efficacy of endometrial resection for the treatment of menorrhagia by creating a long-lasting suppressive effect on the activity of these enzymes and growth factors in the endometrium. This can be achieved by inserting a levonorgestrel-releasing intrauterine system (Mirena®) immediately following endometrial resection. The insertion of Mirena® would also provide effective contraception for patients undergoing endometrial resection.
In the present study, the effect of Mirena® on aromatase and Cox-2 expression in the endometrium was evaluated in the fifth year of use of this device by patients previously submitted to endometrial resection at the time of insertion. These patients were compared with another group of women with adenomyosis in the fifth year of use of Mirena® who had not been submitted to endometrial ablation and to a control group of women who had undergone endometrial resection for adenomyosis but who had not been submitted to any hormonal treatment in the 3 months preceding the study.
Patients and methods
Patients (n = 89) aged 30–48 years with a history of menorrhagia and adenomyosis were included in this observational, case-control study. Enrolled patients had already completed their families and were seeking an alternative to hysterectomy. For the purpose of analysis, the patients included in the study were separated into three groups.
Group A consisted of 22 patients who had completed 5 years of Mirena® use. The device was originally inserted in the women in this group as an outpatient procedure to treat excessive menstrual bleeding due to adenomyosis. In this group, hysteroscopy including an endomyometrial biopsy was carried out to establish the diagnosis of adenomyosis at the time of Mirena® insertion. The myometrial biopsy was carried out using a 5 mm forceps used in laparoscopy for ovarian biopsy, which was inserted through the cervix immediately prior to Mirena® insertion. The use of this forceps permits a sufficiently large fragment of the myometrium to be obtained to enable a histological diagnosis of adenomyosis to be made. Eighteen of the patients in this group were submitted to an endometrial resection at the time of insertion of the replacement device. The procedure was carried out using the bipolar resectoscope (Versapoint™ Gynecare, Ethicon, Inc, Somerville, NJ) under paracervical block, associated with intravenous propofol as previously described.Citation6 In brief, the surgical technique consisted of dilating the cervix to 9 mm to allow the introduction of a bipolar resectoscope. A 0.9% solution of isotonic saline was used as the distention medium for the uterine cavity. The endometrium was removed during surgery together with approximately 5 mm of the underlying myometrium. This procedure was carried out in a private hospital setting and the patients were discharged 6 hours after its completion. In the remaining four patients in this group, a replacement Mirena® was inserted but endometrial ablation was not performed; however, an endomyometrial biopsy was taken at the time of the procedure to establish whether or not adenomyosis was present.
Group B consisted of 24 patients with histologically proven adenomyosis in whom Mirena® was inserted at the time of endometrial resection for the treatment of menorrhagia. All the women in this group had been using the device for at least 5 years when they returned to this center to have it replaced. Outpatient hysteroscopy was performed using a Bettocchi hysteroscope (Karl Storz, Tuttlingen, Germany) under paracervical block established with 10 mL of lidocaine 1% prior to reinsertion of a replacement Mirena®. The previous device was easily removed and an endomyometrial biopsy was performed by inserting a 5-mm forceps into the uterus as described previously. A replacement Mirena® was then inserted without any difficulty.
Group C consisted of 43 patients submitted to endometrial resection between March 2009 and September 2011 in whom pathology revealed adenomyosis. Since they had not been submitted to any hormonal treatment for menorrhagia in the 3 months preceding hysteroscopic surgery, these patients were included as untreated controls to determine the prevalence of aromatase and positive Cox-2 expression in the endometrium. This group was necessary because when Mirena® was inserted in groups A and B the pathology laboratory was not equipped to perform immunohistochemistry.
All surgical procedures were carried out by the same group of surgeons. Pathology and immunohistochemical analysis of the endometrium and myometrium were performed by the same pathologist.
Data on the menstrual-related symptoms such as menorrhagia, dysmenorrhea, and the premenstrual syndrome reported by these patients prior to treatment were obtained from their medical records.
During hysteroscopy performed using the Versapoint™ resectoscope, the endometrium and approximately 0.5 cm of the myometrium were removed as described on previous page, fixed in 4% formalin, and sent to pathology for histological and immunohistochemical evaluation. Immunohistochemistry was performed following antigen retrieval to detect the presence of aromatase p450 and Cox-2 expression. Aromatase expression was investigated using a commercially available monoclonal antibody, MCA2077, clone H4 (Serotech, Raleigh, NC), while Cox-2 expression was assessed using CX-294. Antigen retrieval was performed using the Tris- EDTA buffer at pH 8.0. The reaction was revealed using the streptavidin-biotin method. The presence of aromatase expression was rated either as positive if there was any detectable staining reaction in the glandular epithelium or negative when no reaction was observed. Cox-2 expression was graded as 0 when no expression was detected, +1 when there was a light staining in more than 10% of the glands, +2 when staining was moderate, and +3 when there was strong expression in the endometrium. In all cases, slides were evaluated by a pathologist. Placental tissue and a sample of the atrophic endometrium were used as positive and negative controls, respectively, in all immunostaining reactions for aromatase p450. Statistical analysis was performed using the chi-square test to detect differences in percentages and Student’s t-test for comparison of means. Significance was established at P < 0.05. All patients gave their written informed consent authorizing the immunohistochemical studies to be performed on the endometrial tissue, as determined by the hospital’s internal review board. The insertion of Mirena® following endometrial resection has been the standard treatment for menorrhagia in this day hospital for a decade and all patients were adequately counseled prior to the procedure. A previous study conducted by our group had shown that endometrial resection without the concomitant use of Mirena® was associated with much lower amenorrhea rates than when this device was inserted at the time of surgery.Citation6 For this reason, patients submitted to an endometrial resection for the treatment of adenomyosis-related menorrhagia were always adequately counseled to have a Mirena® device inserted right after completion of the procedure.
Results
Histology and hysteroscopy findings
In the group of untreated patients with a histological diagnosis of adenomyosis, the endometrium was in the proliferative phase in 28 cases and in the secretory phase in the remaining 15. On the other hand, in patients using Mirena®, the most common histological pattern reported consisted of an eutopic endometrium with atrophic glands in the presence of stroma with a decidual reaction or atrophic changes ( and ). These results are summarized in .
Figure 2 Secretory endometrium in a patient with adenomyosis not previously treated with hormones. (A) Hysteroscopy; (B) Adenomyosis; (C) Secretory eutopic endometrium.
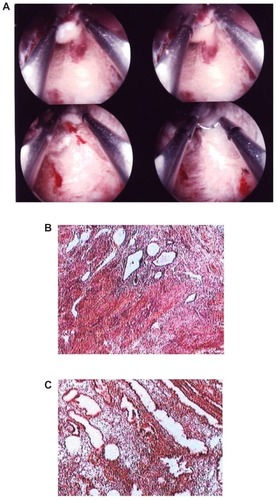
Table 1 Endometrial histology in patients with adenomyosis according to group
In eight patients of group B who had adenomyosis at the time of endometrial resection and Mirena® insertion, examination of the myometrium failed to detect the presence of ectopic endometrial tissue when the device was replaced 5 years later.
The uterine cavity appeared normal at hysteroscopy in 80% of the patients in the untreated control group; however, in 20% of cases an endometrial polyp was also detected.
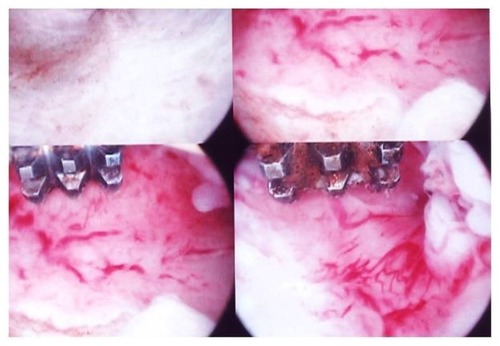
In patients using Mirena® who had not previously been submitted to endometrial resection, the endometrium was thin; however, the presence of dilated blood vessels was detected, a finding that was not present in the group of women who had an endometrial resection at the time of insertion ().
Menstrual symptoms in Mirena® users
The incidence of amenorrhea at the fifth year of use was 84% in the group of patients who had an endometrial resection at the time of Mirena® insertion and only 19% in the group not submitted to this procedure (P < 0.0001). Complete resolution of dysmenorrhea was reported by 91% of the patients who had undergone endometrial resection followed by Mirena® insertion, while in the group not submitted to resection, only 20% of patients reported complete disappearance of this symptom at the fifth year of use (P = 0.0005). The occurrence of dysmenorrhea was consistently associated with the presence of uterine bleeding.
Aromatase expression
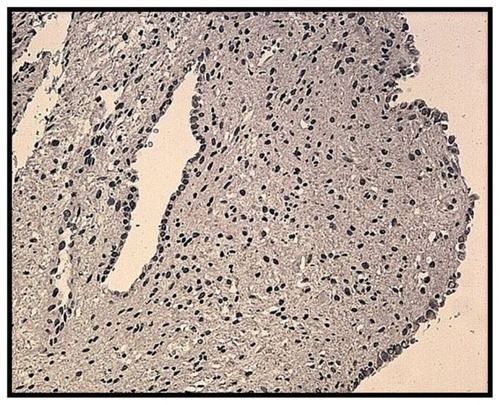
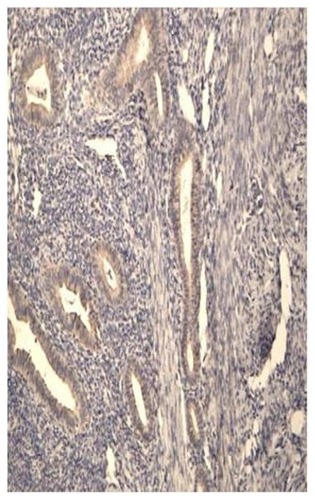
The staining reaction for aromatase was detected using immunohistochemistry not only in the glandular epithelium of the eutopic endometrium but also in the ectopic glands embedded in the myometrium in the untreated controls with adenomyosis (). In all cases there was agreement between the positive staining in both the eutopic and ectopic endometrium. In the untreated group, aromatase expression was present in 23/28 cases during the proliferative phase and in 9/15 cases in the luteal phase of the menstrual cycle, a difference that was not statistically significant (P = 0.2). For this reason, the results were pooled together and analyzed as a single group of untreated control patients (group C).
Figure 4 Aromatase expression in the glandular epithelium of a patient with adenomyosis during the proliferative phase.
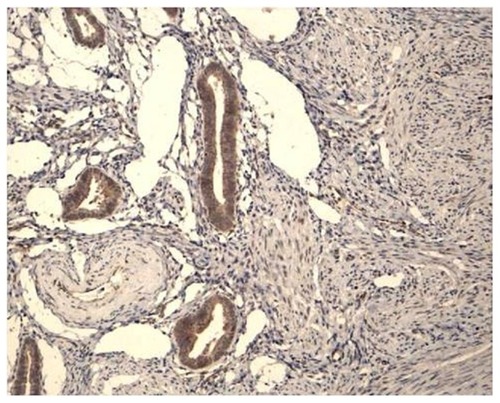
In contrast, in patients with adenomyosis using Mirena®, there were significantly fewer cases in which a positive staining reaction for aromatase could still be detected in both the eutopic endometrium and in the ectopic glands embedded in the myometrium at the end of the fifth year of use when compared to the untreated patients with adenomyosis (group C) (). Although this decrease in the number of cases was statistically significant in both group A and group B, it was significantly greater in the group previously submitted to an endometrial resection when compared to the group of women who had not undergone resection, being detected in only 1/24 of cases in the former. The difference between groups A and B was statistically significant (P < 0.01). These results are summarized in .
Figure 5 Positive aromatase expression in the eutopic endometrium of a patient with adenomyosis (A) and negative expression in a patient after using a Mirena® device for 5 years (B).
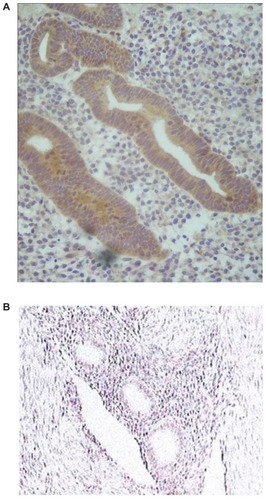
Table 2 The effect of Mirena® and endometrial resection on aromatase expression in the glandular epithelium of adenomyosis lesions
Cox-2 expression
The mean intensity of Cox-2 expression did not vary significantly between the proliferative and luteal phases of the menstrual cycle in the untreated patients with adenomyosis, and for this reason the results were analyzed together (group C) ( and ). However, in the patients who had been using Mirena® for 5 years, a significant reduction occurred in the mean intensity of Cox-2 expression only in the group of patients who had been submitted to endometrial resection at the time of Mirena® insertion (group B) (). Conversely, in patients not submitted to this procedure, the mean intensity scores for Cox-2 in the glandular epithelium were not significantly different from those found in the untreated controls and significantly higher than those found in group B. These results are summarized in .
Figure 7 Immunohistochemical grading of Cox-2 expression in the eutopic endometrium of patients with adenomyosis. (A) 1+ expression and (B) 3+ expression.
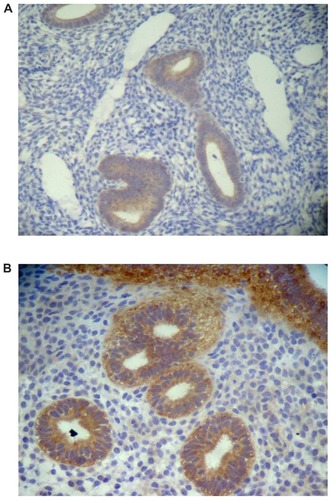
Figure 8 Cox-2 negative expression in a patient using Mirena® who had been previously submitted to an endometrial resection.
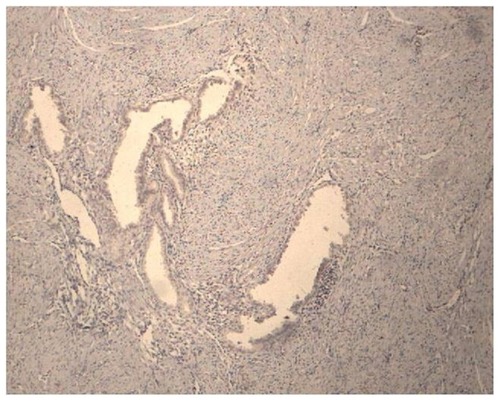
Table 3 Mean Cox-2 expression in the endometrium of patients with adenomyosis in use of Mirena®
Discussion
The findings of the present study show that the use of Mirena® following endometrial resection in uteri with adenomyosis is associated with higher rates of amenorrhea and lower rates of dysmenorrhea compared to a group of women who had Mirena® inserted but who were not submitted to endometrial resection. Although this was not a prospective clinical trial designed specifically to address the question of whether endometrial resection improves rates of amenorrhea in Mirena® users, the amenorrhea rates reported here are similar to those cited in the literature for patients with adenomyosis who had not been submitted to prior endometrial ablation.Citation7 The presence of Mirena® inhibited the expression of aromatase and p450, thus exerting a local anti-estrogenic effect on the endometrium, which is beneficial in the treatment of adenomyosis. The suppression of aromatase in this tissue may explain the efficacy of Mirena® in halting the progression of endometriosis, since the seeding of aromatase-positive cells through retrograde menstruation may play an important role in the development of this disease.Citation8
Aromatase expression in the endometrium is activated by exposure to prostaglandin E2, thus establishing a link between inflammation and the development of this pathology.Citation9 The greater Cox-2 expression in the endometrium of women using Mirena® who had not been submitted previously to ablation correlates positively with the occurrence of uterine bleeding, which is consistent with the role of prostaglandins and the ensuing inflammation in the pathogenesis of both menorrhagia and breakthrough bleeding during the use of oral contraceptives and the progestin-only pill.Citation10–Citation12
The rate of amenorrhea reported here during the fifth year of Mirena® use also compares favorably with rates reported previously by our group 1 year after insertion of this device.Citation6 These rates are also far superior to those achieved with the insertion of Mirena® alone in patients with adenomyosis, myomas, or menorrhagia.Citation13–Citation15
The presence of adenomyosis specimens removed at hysterectomy from patients with persistent bleeding following the insertion of Mirena® or microwave endometrial ablationCitation16–Citation19 is noteworthy and suggests that the presence of adenomyosis increases the risk of failure of both forms of treatment due to the resumption of menorrhagia, probably caused by persistent Cox-2 activation and the less effective inhibition of aromatase in patients not submitted to endometrial resection. Another factor which may contribute to the high incidence of amenorrhea in patients using Mirena® after endometrial resection would be the direct exposure of the ectopic glands in the myometrium to levels of levonorgestrel higher than those achieved when the endometrium is intact. This would lead to a more effective inhibition of both aromatase and Cox-2 in the ectopic glands deeply embedded in the myometrium, resulting in better rates of amenorrhea. This is in agreement with the findings of a previous study showing that the levonorgestrel levels in the endometrium of patients using Mirena® are many times higher than levels in the myometrium, which are similar to those found in extrauterine tissue.Citation20 This suggests that the intact endometrium may act as a barrier, preventing the diffusion of levonorgestrel to the underlying myometrium, which receives levonorgestrel only through the systemic circulation. This may explain why the rates of amenorrhea in the present study were far higher in the group of women with adenomyosis submitted to endometrial resection compared to those using Mirena® alone. In the latter, because the endometrium remains intact, the levonorgestrel levels in the myometrium may not be high enough to effectively suppress both aromatase and Cox-2 expression in the deeply embedded ectopic glands. This would explain the increased efficacy of Mirena® in inhibiting these enzymes in uteri with adenomyosis when the endometrium is removed prior to insertion of the device. Although this was not a subject of the present study, it is likely that endometrial resection may improve the diffusion of levonorgestrel into the myometrium; however, for the present moment this hypothesis has to be considered speculative. This may also explain the reduction in breakthrough or irregular bleeding with this combined method compared to rates reported in the literature when Mirena® is used without prior endometrial resection in patients with adenomyosis or myomas.Citation21 The decrease in aromatase expression in adenomyosis uteri bearing a Mirena® device seems to occur even when Cox-2 expression is not suppressed by the treatment. Since prostaglandins are an important inductor for the activation of the aromatase gene in the endometrium, a direct effect of the progestin inhibiting gene transcription would be the likely mechanism.Citation8,Citation9,Citation11
The combination of Mirena® with endometrial resection represents a viable and effective alternative therapy to hysterectomy for the management of menorrhagia and dysmenorrhea in patients with adenomyosis and menorrhagia. In patients undergoing endometrial resection alone or Mirena® insertion alone for the treatment of menorrhagia, it is important to remember that success rates are lower than when both methods are used together. This has been shown in a previous study conducted by our group to compare the amenorrhea and success rates of the combination of Mirena® and endometrial resection versus endometrial resection alone for the treatment of adenomyosis-related menorrhagia.Citation6 Similar results were also reported more recently by another group.Citation22
In conclusion, Mirena® is an effective option for the suppression of aromatase and Cox-2 expression in the endometrium of patients with adenomyosis; however, inhibition appears to be greater when this device is inserted immediately following endometrial resection. Nevertheless, it should be noted that endometrial resection is an alternative to hysterectomy and is not an option for women who still want to preserve their fertility. In this case, particularly when used to treat adenomyosis-related menorrhagia, the insertion of Mirena® alone is a viable option, since fertility returns promptly after removal of the device.Citation23
Because of the negative impact on quality of life caused by the pain and excessive bleeding associated with adenomyosis, hysterectomy remains the standard treatment for this pathology.Citation24 Development of alternative methods of treatment capable of offering effective relief of these symptoms without constituting a major surgical procedure would be desirable. In this aspect, the combination of Mirena® with endometrial resection fulfills these requisites, since it is a less invasive procedure compared to hysterectomy and its success rate is high. However, further prospective studies must be conducted before changes in standard medical practice can be contemplated.
Disclosure
All authors report no conflicts of interest in this work.
References
- OnogluATaskinOInalMComparison of the long-term histopathologic and morphologic changes after endometrial rollerball ablation and resection: a prospective randomized trialJ Minim Invasive Gynecol2007141394217218227
- MaiaHJrCasoyJCorreiaTEffect of the menstrual cycle and oral contraceptives on aromatase and cyclooxygenase-2 expression in adenomyosisGynecol Endocrinol2006221054755117135033
- MaiaHJrPimentelKCasoyJAromatase expression in the eutopic endometrium of myomatous uteri: the influence of the menstrual cycle and oral contraceptive useGynecol Endocrinol200723632032417616855
- BukulmezOHardyDBCarrBRWordRAMendelsonCRInflammatory status influences aromatase and steroid receptor expression in endometriosisEndocrinology200814931190120418048499
- FechnerSHusenBTholeHExpression and regulation of estrogen-converting enzymes in ectopic human endometrial tissueFertil Steril200788Suppl 41029103817316633
- MaiaHJrMaltezACoelhoGAthaydeCCoutinhoEMInsertion of Mirena® after endometrial resection in patients with adenomyosisAm Assoc Gynecol Laparosc2003104512516
- BednarekPHJensenJTSafety, efficacy and patient acceptability of the contraceptive and non-contraceptive uses of the LNG-IUSInt J Womens Health20101455821072274
- MaiaHJrCasoyJValente FilhoJIs aromatase expression the cause of endometriosis related infertility?Gynecol Endocrinol20092525325719340622
- NobleLSTakayamaKZeitounKMProstaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cellsClin Endocrinol Metab1997822600606
- SmithOPJabourHNCritchleyHPCyclooxygenase enzyme expression and E series prostaglandin receptor signalling are enhanced in heavy menstruationHum Reprod20072251450145617264103
- MaiaHCorreiaTFreitasLAthaydeCCoutinhoEMCyclooxygenase-2 expression in the endometrium and its relationship to bleeding in users of continuous oral contraceptivesGynecol Endocrinol20062229610016603435
- CritchleyHOKellyRWBrennerRMBairdDTAntiprogestins as a model for progesterone withdrawalSteroids20036810–131061106814667999
- GunesMOzdegirmenciOKayikciogluFHaberalAKaplanMThe effect of levonorgestrel intrauterine system on uterine myomas: a 1-year follow-up studyJ Minim Invasive Gynecol200815673573818971138
- KriplaniASinghBMLalSAgarwalNEfficacy, acceptability and side effects of the levonorgestrel intrauterine system for menorrhagiaInt J Gynaecol Obstet200797319019417382331
- KohSCSinghKThe effect of levonorgestrel-releasing intrauterine system use on menstrual blood loss and the hemostatic, fibrinolytic/inhibitor systems in women with menorrhagiaJ Thromb Haemost20075113313817010149
- SharmaBPrestonJRayCMicrowave endometrial ablation for menorrhagia: outcome at 2 years – experience of a district general hospitalJ Obstet Gynaecol200424891691916147652
- StamatellosIKoutsougerasGKaramanidisDStamatopoulosPTimpanidisIBontisJResults after hysteroscopic management of premenopausal patients with dysfunctional uterine bleeding or intrauterine lesionsExp Obstet Gynecol20073413538
- RizkallaHFHigginsMKelehanPO’HerlihyCPathological findings associated with the presence of a Mirena intrauterine system at hysterectomyInt J Gynecol Pathol2008271747818156979
- DengSLangJHLengJHLiuZFSunDWZhuLEffects of levonorgestrel-releasing intrauterine system on pain and recurrence associated with endometriosis and adenomyosisZhonghua Fu Chan Ke Za Zhi2006411066466817199919
- NilssonCGHaukkamaaMVierolaHLuukkainenTTissue concentrations of levonorgestrel in women using a levonorgestrel-releasing IUDClin Endocrinol (Oxf)19821765295366819901
- ShengJZhangWYZhangJPLuDThe LNG-IUS study on adenomyosis: a 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosisContraception200979318919319185671
- LowSSmithKTCRE and Mirena: is the combination better?Gynecol Surg200632146147
- SturridgeFGuillebaudJGynecological aspects of the levonorgestrel-releasing intra uterine deviceBr J Obstet Gynaecol199710432852899091003
- AzzizRAdenomyosis: current perspectivesObstet Gynecol Clin North Am19891612212352664619