Abstract
Background
Therapies for invasive breast cancer may be associated with an incremental survival advantage that should be weighed against the risk of toxicities when making treatment decisions. The objective of this study was to elicit patient preferences for a comprehensive profile of attributes associated with chemotherapies for breast cancer.
Methods
This was a cross-sectional study of 121 patients with stage I-IV breast cancer who completed an internet-based conjoint survey that assessed the following attributes: ten grade III/IV toxicities, survival advantage, and administration regimen. Literature and expert input were used to identify descriptions for each attribute and respective levels (eg, different risks of toxicities). Subjects rated the attribute levels on a series of scales and indicated preferences in pair-wise comparisons of two hypothetical treatments differing in attribute levels. Ordinary least-squares regression was used to calculate utilities (preference weights) for each attribute level.
Results
Of the twelve attributes, survival was the most important; specifically, a survival advantage of 3 months versus no survival advantage was most influential in the perceived value of chemotherapy. Among toxicities, the differences in the risks of neutropenia with hospitalization, diarrhea, nausea, and fatigue had the most impact on preferences; the risk differences of myalgia, stomatitis, and hand-foot syndrome had the least. In general, a more convenient administration regimen was less important than a 13% chance or more of severe toxicities, but more important than a 10%–12% chance of severe toxicities.
Conclusion
Breast cancer patients place high value on small incremental survival advantages associated with treatment despite the risk of serious toxicities.
Introduction
Breast cancer is the second most common cancer among women in the United States, with approximately 54,010 new cases of carcinoma in situ (noninvasive cancer) and 207,090 cases of invasive cancer diagnosed per year. In addition, breast cancer is the second leading cause of cancer death in women, with about 39,840 deaths occurring per year.Citation1 Five-year survival rates vary by stage: the 5-year survival rate for patients with stage 0 through stage IIA breast cancer ranges from 92 to 100%, while the 5-year survival rate for patients with stage IV metastatic breast cancer is approximately 20%.Citation1 Thus, the goal of therapy for invasive breast cancer is primarily palliative – that is, aimed at alleviating and controlling symptoms as well as improving quality of life.Citation2 Treatments in common use for invasive breast cancer include cytotoxic chemotherapy, hormonal treatments, and targeted therapies/biologics, which can be administered as single agents or in combination with each other.Citation3
Given that current chemotherapies have advantages or disadvantages relative to each other, it would be useful to understand how these influence patient preferences for treatment. Some studies have examined patient preferences with respect to chemotherapy toxicities. For example, one recent study evaluated preferences for health states associated with chronic lymphocytic leukemia treatments using the standard-gamble method.Citation4 Other studies have also examined patient preferences for select groups of chemotherapy toxicities.Citation5,Citation6 However, to date, no study has evaluated the importance of toxicities relative to other characteristics such as efficacy and administration. Conjoint analysis, which is increasingly being used in evaluating medical interventions, involves respondents making trade-offs among product features (attributes) such as mode of administration and risk of adverse events.Citation7,Citation8 The resulting data, or utilities, enable the assessment of the relative importance of each treatment attribute; specifically, they show the influence that each attribute has on overall treatment preferences.
The goal of the present study was to use conjoint analysis to capture patient preferences for attributes associated with chemotherapies for invasive breast cancer. Specifically, the survey aimed to assess the trade-offs that breast cancer patients are willing to make among chemotherapies according to different toxicity, regimen, and efficacy profiles.
Methods
This was a cross-sectional, internet-based survey assessing the chemotherapy treatment preferences of women with breast cancer. Study participants were recruited through newspaper advertisements and online breast cancer support forums. All participants were women with United States residency; at least 18 years of age; had a diagnosis of stage I through IV breast cancer; had received chemotherapy treatment within the past 5 years; and provided informed consent and Health Insurance Portability and Accountability Act- compliant Authorization.Citation9 Participants completed the survey at home and worked at their own pace. However, research team members were available via telephone or email to answer questions or provide technical help if necessary. Participants were compensated US$30.00 for their time. The study followed the tenets of the Declaration of Helsinki, and the protocol was approved by a commercial institutional review board (Independent IRB; Plantation, FL). This research was implemented following published methodological guidelines for conjoint studies.Citation10 The survey design and analysis are detailed below.
Survey design
Twelve attributes of eight invasive breast cancer chemotherapy treatments were identified from a comprehensive literature review, the Common Toxicity Criteria grading system, a detailed assessment of breast cancer forum discussions, and consultation with clinical experts. These attributes included selected grade III/IV toxicities, efficacy (survival advantage), and regimen. Attributes were described in lay terminology so that they could be easily understood by patients. Each attribute was presented in different levels that represented the full range of possibilities across available breast cancer treatments. Each toxicity attribute had three levels. Based on the literature and clinician input, potential ranges for the incidence of each of the toxicities were identified, and these were used as the most favorable and least favorable levels, respectively. The regimen attribute had six levels, each describing a different chemotherapy regimen. presents the attributes and the respective levels used in the survey.
Table 1 Attributes and respective levels presented in conjoint survey
An adaptive conjoint analysis (ACA) approach was used to design the survey. This involves a hybrid approach, in which the data are collected in phases.Citation11,Citation12 The respondent completes the ACA computer-assisted questionnaire, and the questionnaire is modified during the responding process on the basis of the respondent’s previous choices. Thus the interview can focus on just those attributes that the respondent considers most important and those attribute levels regarded as most relevant. In the first phase, respondents were asked to rate the levels of each attribute in terms of acceptability on a 7-point scale, from 1, “not at all acceptable”, to 7, “acceptable”. The second phase (paired comparison questions) elicited treatment preferences by asking respondents to make trade-offs among attributes and choose from a pair of hypothetical treatments. In each of these questions, the profiles of two hypothetical treatments – labeled simply chemotherapy A and chemotherapy B – were presented with different levels of the same three attributes (no chemotherapies were named in the survey). Respondents used a 7-point scale to indicate not only their preference, but the strength of their preference; response options ranged from “strongly prefer A” to “strongly prefer B”. The profiles presented to respondents in this second phase were customized based on responses to previous questions. The final section of the survey contained demographic and clinical questions. The survey was pilot-tested with respect to wording and comprehension in a sample of four breast cancer patients; no major changes were necessary.
Analysis
The conjoint data were analyzed using Sawtooth Software SSI Web (v 6.4; Sawtooth Software, Sequim, WA). Analysis of ACA data involved the combination of the initial rating (the acceptability questions in the first phase) and the paired comparison questions. Specifically, using the information from the initial rating questions, prior utilities were calculated. The average for each attribute was subtracted to center its values at zero. For example, desirability values 3, 2, and 1 would be converted to 1, 0, and −1, respectively. These initial estimates are part-worths, where within each attribute the values have a mean of zero, and differences between values are proportional to differences in desirability ratings or rank orders of preference. With respect to the paired comparison questions, a column vector was created for the dependent variable as follows: the respondents’ answers were zero-centered, where the most extreme lowest value was given −4, and the most extreme highest value was +4. Interior ratings were fitted proportionally within that range. Each pair’s question contributed a row to both the independent variable matrix and dependent variable column vector. Ordinary least-square estimates of the n attribute levels were computed by regression of the dependent variable column vector on the matrix of independent variables. The part-worth estimates based on the prior and paired comparison phases were normalized to have equal sums of differences between the best and worst levels of each attribute across all attributes. The two vectors of part-worths were added together, yielding final utilities for each attribute level for each respondent. The absolute values of the final utilities were arbitrary; what was important was the magnitude of difference between them. These weights were “zero-centered,” whereby the utility weight of the attribute level falling in the middle had a value that approximated zero and the more favorable level was higher, or positive, and the less favorable level was lower, or negative. Finally, the Bayes approach was applied to the data to further refine the precision of the utility estimates.
The utilities enable the calculation of the relative importance of each attribute for each respondent in influencing treatment decisions. Specifically, the relative importance was calculated for each respondent by dividing the range for each attribute (utility of highest level–utility of lowest level) by the sum of ranges of all attributes for the respective individual and multiplying it by 100. These estimates indicate how much the difference in importance between the best and worst levels of each attribute affects the decision to choose a treatment. These are ratio data, meaning for example that an attribute with an importance of 10% is twice as important as an attribute with an importance of 5%.
Using the mean utility weights for the attribute levels, we compared the percentages of patients who would prefer the following scenarios differing with respect to selected attributes, while holding all other attributes constant: (1) three levels of efficacy (no additional survival, 1 month additional survival, and 3 months additional survival); (2) three regimens (most convenient [oral], least convenient [21-day cycle; 3-hour infusion on days 1, 8, and 15], and one in between [28-day cycle; 6–10 minute infusion on days 1, 8, and 15]); and (3) a 0% versus a 10% chance of each toxicity.
Results
Of 121 participants who completed the conjoint survey, 108 (89%) were included in the final data analysis. Thirteen participants were excluded because their responses in the first section of the survey were illogical (eg, a participant rating a 24% chance of fatigue as more acceptable than a 0% chance) in at least two instances. reports the demographic and clinical characteristics of the study population who were included in the analysis. The mean age was 50 years, with a mean time of 80 months since cancer diagnosis; 60% had attained at least a college-level education. With respect to treatment experience, about 35% of patients were on chemotherapy at the time of their participation in the study, and the mean time since the sample’s last chemotherapy administration was approximately 19 months.
Table 2 Demographic and clinical characteristics
The mean utilities for each attribute level are presented in (the actual values are arbitrary; the magnitude of the differences among them are what should be evaluated). In general, the utilities for each attribute level were ordered in the direction that was expected, where the most favorable attribute level had the highest utility and the least favorable level had the lowest. The efficacy attribute had the highest utility value for its most favorable level (“additional survival benefit of 3 months”) and lowest utility value for its least favorable level (“no additional survival benefit”), with means of 97.05 and −83.77, respectively. This finding indicates that patients view a change in survival advantage from 0% to 3 months as more important than a change from the least favorable to the most favorable level for all other attributes.
Table 3 Mean utilities of attribute levelsTable Footnotea
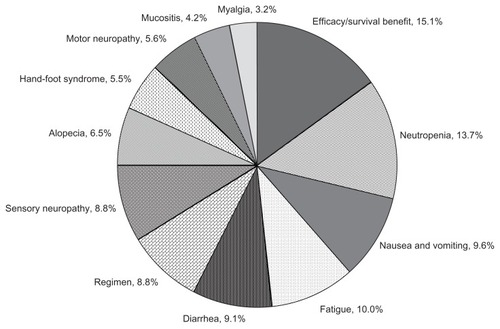
presents the “percentage importance” estimates for each attribute. Overall, the most important attributes in driving treatment preferences were survival advantage, neutropenia/hospitalization, and toxicities such as nausea/vomiting, fatigue, and diarrhea. Least important attributes were the toxicities of mucositis/stomatitis, myalgia, and hand-foot syndrome. In general, the difference between the most inconvenient to the most convenient administration regimen was less important than a 13% chance or more of severe toxicities, but more important than a 10%–12% chance of severe toxicities. The exception to this finding was myalgia, which had a maximum level of 15% chance of occurring; the difference between 0% versus 15% chance of myalgia was still less important than the difference between the most inconvenient and most convenient regimen (percentage importances = 8.8% versus 3.2%).
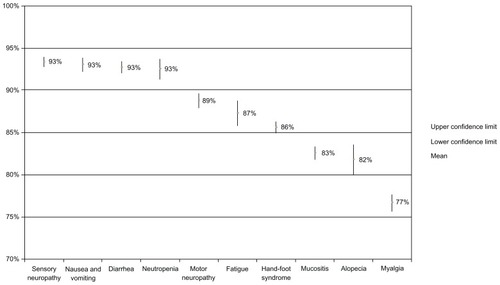
In sensitivity analyses, we created scenarios for each toxicity (ie, each attribute that was a side effect) in which we compared preferences for a 0% risk of that toxicity versus a 10% risk of that toxicity, holding the risk of all other toxicity attributes constant. Specifically, we obtained preference estimates for two product profiles in which one toxicity differed by 10% and the remaining attributes were held constant; we repeated this for each side effect. shows the percentages of patients who would prefer a treatment given a 0% chance versus a 10% chance of each toxicity occurring. The findings show that patients most preferred to avoid neutropenia/hospitalization, diarrhea, nausea/vomiting, and sensory neuropathy (ie, they most preferred no risk of these toxicities relative to no risk of the other toxicities). An additional analysis found no substantial differences in relative importance estimates of toxicities between those who had experienced the side effect versus those who had not (data not shown).
Figure 2 Percentages of patients preferring treatment given a 0% chance of the selected toxicity versus a 10% chance, holding all other attributes constant.
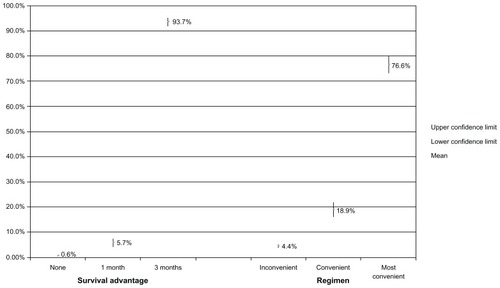
presents the percentages of patients preferring each of three regimens that differ in efficacy (survival advantage) and the percentages of patients preferring three regimens, ie, an oral regimen, a regimen somewhat in “the middle” with respect to convenience (28-day cycle; 6–10 minute infusion on days 1, 8, and 15), and the most inconvenient (21-day cycle; 3-hour infusion on days 1, 8, and 15). The findings show that the difference between a 1-month and 3-month survival advantage substantially influenced patient preferences; the percentages of patients preferring each of these regimens increased from 5.7% to 93.7%, respectively. Also, an oral regimen was highly preferred; 76.6% of patients would choose an oral regimen versus 18.9% who would choose the “middle” regimen.
Discussion
To the best of our knowledge, this study is the first to assess patient preferences for invasive breast cancer treatments considering a variety of treatment features. Specifically, this study assessed the trade-offs that breast cancer patients are willing to make among the risks of severe toxicities, administration regimen, and efficacy when choosing a chemotherapy. Among the chemotherapy attributes evaluated in this study, those that were most influential in driving patient preferences for treatment were improved survival, the risk of neutropenia leading to hospitalization, and the risks of severe fatigue, nausea and vomiting, and diarrhea. Least important were the risks of severe myalgia, mucositis, and hand-foot syndrome. The study findings are useful in better understanding patient preferences in oncology and may enhance the medical decision-making process.
For most patients, the difference between having no additional survival advantage and having a survival advantage of 3 months was about three times more important than an approximate 10% versus 0% risk of experiencing several severe toxicities, including severe myalgia, mucositis, hand-foot syndrome, and motor neuropathy. In addition, the incremental improvement in survival was one-and-a-half times as important as an approximate 15%–20% versus 0% risk of having severe nausea and vomiting, fatigue, diarrhea, or sensory neuropathy. The finding of the high relative importance of improved efficacy is consistent with previous conjoint analyses of medical therapiesCitation13,Citation14 and a review of previous literature,Citation15 which found that patients are willing to accept the risk of serious adverse events in exchange for improved efficacy. For example, Johnson et alCitation12 evaluated the treatment perceptions among patients with multiple sclerosis, and they found that most patients indicated they are willing to accept risks of life-threatening adverse events in exchange for improvements in their health outcomes.
This study builds upon previous work that has examined rankings among chemotherapy side effects.Citation16 Specifically, Sun et alCitation16 evaluated preferences for 27 different side effects among patients with ovarian cancer. They found that severe nausea and vomiting was the least preferred toxicity and that “numbness in hands/feet” was among the most distressful side effects. Similarly, the current study showed that patients would most want to avoid the chance of severe nausea and vomiting and the chance of severe numbness in the arms and legs (sensory neuropathy) among the various potential toxicities evaluated, assuming that they each had an equal chance of occurring.
Our study found no substantial differences in the relative importance of toxicities between those who had experienced the toxicity versus those who had not. This finding differed from a previous study that examined utility (time trade-off) estimates for chemotherapy-related ototoxicity, nephrotoxicity, and neurotoxicity among ovarian cancer patients.Citation17 Specifically, the study found that the most favorable assessment of a particular toxicity was reported from individuals who experienced the selected toxicity. This may be attributable to the fact that patients were asked to assume that the toxicity was permanent; as such, patients with experience may have felt they could better tolerate the effect than naïve patients. In contrast, in the current study, each of the side effects had a specified duration, and thus this may not have been significant enough to translate into differences between those experienced versus not experienced with each toxicity. Moreover, our findings were consistent with the finding of no substantial differences observed in relative importance of eleven of 12 side effects between HIV treatment-experienced, who may have experienced treatment side effects, versus treatment-naïve patients.Citation18
The current study also found that a more convenient administration regimen was more important to patients than severe side effects when the respective risk was less than 13%. Similarly, improved convenience has been found to be influential in treatment choices in other studies.Citation13,Citation14 For example, in a study of patients with idiopathic thrombocytopenic purpura, patients were willing to accept significant treatment-related risks in exchange for improvements in treatment efficacy and convenient administration.Citation13
Our study was not without limitations. First, each of our toxicity attributes was described using durations from a few days to 6 months after treatment end, and these durations represented clinical averages. These average durations may not be reflective of individual experiences, however, as our discussions with clinicians revealed that side effect experience is highly variable between patients. In addition, the participants in our sample did not represent the full range of breast cancer patients; the majority were well-educated, most were Caucasian, and they were all healthy enough to complete a computer-based survey at home. It is unclear whether the preferences of severely ill cancer patients (eg, those who are bedridden) or other ethnic groups would be different from those expressed by patients in our sample. Because of these limitations, while our study has yielded important findings with implications for the medical community, future research is necessary to evaluate the generalizability of our conclusions.
Conclusion
In summary, this survey evaluated a comprehensive set of severe toxicities and other features that are observed with chemotherapies for invasive breast cancer. Our study showed that, despite the risk of serious toxicities, a small incremental survival advantage is highly influential in patient preferences for chemotherapy. The findings from this research may be useful in incorporating patients’ views into medical decision making processes, patient education, cost resource allocations, and drug development. A better understanding of patients’ preferences may help to improve patient satisfaction and compliance with treatment regimens.
Disclosure
Kathy Beusterien and Jessica Grinspan are employed by Oxford Outcomes, Inc, an ICON plc company, which consults for Eisai, the study sponsor. Thomas Tencer is a former employee of Eisai, the study sponsor. Adam Brufsky and Constance Visovsky report no conflicts of interest in this work.
References
- American Cancer SocietyBreast cancer overview Available from: http://www.cancer.org/Cancer/BreastCancer/OverviewGuide/Accessed June 1, 2010
- LigibelJABreast cancer metastasisHealthScout Available from: http://www.healthscout.com/ency/68/484/main.htmlAccessed June 1, 2010
- MayerMTreatments in common use for metastatic breast cancer Available from: http://www.advancedbc.org/content/treatmentscommon-use-metastatic-breast-cancer-gAccessed June 1, 2010
- BeusterienKBDaviesJLeachMPopulation preference values for treatment outcomes in chronic lymphocytic leukaemia: a cross-sectional utility studyHealth Qual Life Outcomes201085020482804
- ArmstrongTAlmadronesLGilbertMRChemotherapy-induced peripheral neuropathyOncol Nurs Forum200532230531115759068
- SunCCBodurkaDCDonatoMLPatient preferences regarding side effects of chemotherapy for ovarian cancer: do they change over time?Gynecol Oncol200287111812812468352
- GreenPESrinivasanVConjoint analysis in marketing: new developments with implications for research and practiceJournal of Marketing1990544319
- RyanMFarrarSUsing conjoint analysis to elicit preferences for health careBMJ20003201530153310834905
- US Department of Health & Human ServicesHealth Information Privacy Available from: http://www.hhs.gov/ocr/privacyAccessed June 1, 2010
- BridgesJFHauberABMarshallDConjoint analysis applications in health – a checklist: a report of the ISPOR GoodResearch Practices for Conjoint Analysis Task ForceValue Health201114440341321669364
- GreenPEKriegerAMAgarwalMKAdaptive Conjoint Analysis: some cautions and caveatsJ Market199154319
- Sawtooth SoftwareACA/Web v6.0 Technical PaperSequim, WASawtooth Software, Inc2007 Available from http://sawtoothsoftware.comAccessed August 15, 2009
- JohnsonFRVan HoutvenGOzdemirSMultiple sclerosis patients’ benefit-risk preferences: serious adverse event risks versus treatment efficacyJ Neurol2009256455456219444531
- HauberABJohnsonFRGrotzingerKMOzdemirSPatients‘ benefit-risk preferences for chronic idiopathic thrombocytopenic purpura therapiesAnn Pharmacother201044347948820124463
- SasaneMTencerTFrenchAMaroTBeusterienKMPatient reported outcomes in chemotherapy-induced peripheral neuropathy: A reviewJ Support Oncol201086e15e21
- SunCCBodurkaDCWeaverCBRankings and symptom assessments of side effects from chemotherapy: insights from experienced patients with ovarian cancerSupport Care Cancer20051321922715538640
- CalhounEAFishmanDALurainJRWelshmanEEBennettCLA comparison of ovarian cancer treatments: analysis of utility assessments of ovarian cancer patients, at-risk population, general population, and physiciansGynecol Oncol20049316416915047231
- BeusterienKMDziekanKSchraderSPatient preferences among third agent HIV medications: a US and German perspectiveAIDS Care200719898298817851994