Abstract
Objective
To compare fetal and neonatal cardiac functions in terms of global, systolic, and diastolic function between the preeclampsia and normotensive blood pressure of pregnancies.
Methods
A prospective cohort study was conducted at a university hospital in Northeast Thailand. Twenty-nine pregnancies diagnosed as preeclampsia with or without severe features were compared with 29 normotensive pregnancies. Global cardiac, systolic, and diastolic function were assessed at prenatal and postnatal periods, by a professionally trained obstetrician and pediatric cardiologist, respectively.
Results
The fetal left modified myocardium performance index (Mod-MPI) in preeclampsia and normotensive blood pressure were 0.60±0.08 and 0.59±0.08 (p-value=0.341), respectively, while fetal right Mod-MPI were 0.57±0.16 and 0.54±0.21 (p-value=0.861), respectively. There were no statistically significant differences in terms of fetal isovolumic contraction time (ICT), isovolumic relaxation time (IRT), ejection time (ET), aortic peak systolic velocity (Ao PSV), pulmonary artery peak systolic velocity (PA PSV), mitral valve (MV) E:A ratio, or tricuspid valve (TV) E:A ratios between the two groups. Neonatal mitral valve E peak systolic velocity (MV-E PV) in preeclamptic and normotensive blood pressure groups were significantly different at 51.1±8.02 cm/s and 43.56±5.21cm/s (p-value=0.036), respectively, whereas neonatal left Mod-MPI, mitral valve A peak systolic velocity (MV-A PV), MV E:A ratio, and Ao PSV were not significantly different (p-value=0.436, 0.119, 0.379, and 0.709), respectively.
Conclusion
Neonatal MV-E PV of the preeclampsia group was significantly higher than the normotensive blood pressure group, while there were no statistically significant differences in terms of global cardiac and diastolic functions during the fetal period between two groups.
Introduction
Recently, incidences of preeclampsia have been reported in 2–5% of all pregnancies.Citation1 Neonates born from women suffering with preeclampsia were found to have more complications, such as growth restrictions, preterm birth, birth asphyxia, neonatal seizure, and increased rate of neonatal intensive care unit (NICU) admission than births from normotensive women.Citation2 Impaired trophoblastic invasion of maternal spiral arteries increases vascular resistance in uteroplacental circulation and may affect fetal cardiac function by causing increased cardiac afterload.Citation3 Acute hypertensive events in the women may cause fetal myocardial injury, resulting in heart failure, pulmonary and systemic congestion, and cardiorespiratory insufficiency.Citation4 Consequently, long-term complications in this group might increase the risk of hypertension in the young, ischemic stroke, coronary heart disease, and thickening of the myocardial wall.Citation1
Fetal and neonatal cardiac function assessment are composed of systolic, diastolic, and global function. Global cardiac function can be evaluated by the modified myocardium performance index (Mod-MPI), whereas diastolic function can be assessed by flow patterns through the mitral and tricuspid valves (mitral valve (MV) E:A ratio, tricuspid valve (TV) E:A ratios and isovolumic relaxation time (IRT)).Citation5 The Mod-MPI described by Hernandez-Andrade et alCitation6 as a Doppler index of combined systolic and diastolic function assessment can identify volume fluctuations of preload and afterload.Citation5 Previous studies differed as to whether preeclampsia affected fetal or neonatal cardiac functions.Citation3,Citation7,Citation8 Therefore, the primary objective was to compare fetal cardiac function in terms of Mod-MPI, systolic, and diastolic functions between the preeclampsia and normotensive blood pressure groups, while the secondary objective was to compare neonatal left side cardiac functions between the two groups.
Method
This prospective cohort study was conducted at Srinagarind Hospital, Khon Kaen University with approval by the KKU Ethics Committee in Human research (HE621565). Inclusion criteria were women who were healthy, 18 years old or older, singleton pregnancy with gestational age 24–40 weeks, and had preeclampsia based on ACOG 2019Citation9 as increased blood pressure ≥140/90 mmHg measured on two or more occasions at least 4 hours apart or blood pressure ≥160/110 mmHg confirmed within a few minutes, and the presence of significant proteinuria (total protein 300 mg or more per 24 hours urine collection or urine protein dipstick reading 2+ or urine protein: creatinine of 0.3 or more) or new onset of thrombocytopenia, impaired liver function, renal insufficiency, pulmonary edema, and unexplained new onset headache unresponsive to medication or visual symptoms. Exclusion criteria included abnormal fetal karyotypes, abnormal fetal structures, abnormal amniotic fluid index (AFI), fetal congenital heart defects, fetal growth restriction (FGR), death of the fetus in utero (DFIU), and receiving MgSO4 or antihypertensive drugs before enrollment. Women with medical conditions such as anemia of any causes, diabetes mellitus, chronic hypertension, gestational hypertension, infection, heart disease, thyroid disease, central nervous system (CNS) disorders, lung disease, autoimmune disease, renal disease, and unstable hemodynamics were also excluded.
Sample size was calculated based on the primary objective to detect the 10% minimally clinical difference of mean fetal left Mod-MPI between preeclampsia and normotensive blood pressure groups with 90% power and α=0.05. The required number of women in the study group was 25. With an expected dropout rate of 5%, 28 women were required per group.
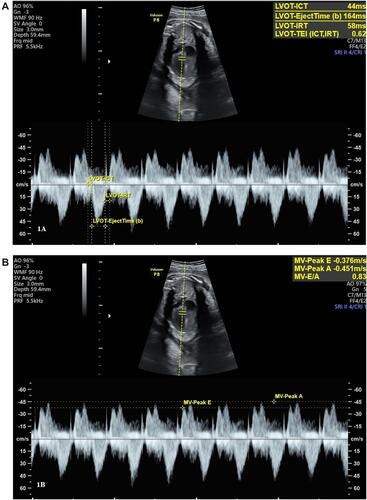
The preeclamptic women were admitted to the labor room, and all eligible subjects provided informed consent before participating in the study. The control group were selected from normotensive blood pressure women, whose gestational age (GA) was compatible with cases. Since the preeclampsia with severe features was diagnosed after 34 weeks of gestation, all pregnancies were terminated. If the diagnosis was made before 34 weeks of gestation with reassured both mother and fetus, expectant management was offered. Before giving any medication in the preeclampsia group, all fetal biometric parameters, fetal heart rate (FHR), were evaluated and fetal echocardiography was performed by using a GE Voluson E8 (GE Medical Systems, Zipf, Austria), equipped with a transabdominal curvilinear transducer of frequency 2–5 MHz. The Mod-MPI was performed on a cross-sectional plane of fetal thorax at the level of four chambers view with an apical projection. The angle of insonation was adjusted to less than 30°. The Doppler velocity was set at 15 cm/s, with WMF of 90 Hz and lowest gain color and mechanical and thermal indices less than 1. A Doppler sample volume size of 3 mm was placed at the internal leaflet of the mitral valve or tricuspid valve, with the pulsed Doppler trace including the positive waveform (E and A waveform) and the negative waveform (aorta or pulmonary artery waveform). The isovolumic contraction time (ICT) was determined from the beginning of the mitral valve closure to the aortic valve opening, ejection time (ET) was measured at the period from the aortic valve opening to closure, and IRT was measured from the aortic valve closure to the mitral valve opening (). The Mod-MPI was estimated from (ICT+IRT)/ET.Citation5 For diastolic function assessment, the peak velocity of E and A waveforms, both left side heart (MV-E PV, MV-A PV) () and right-side heart (TV-E PV, TV-A PV), were measured. The sample gate was placed distal to the aortic or pulmonic valve and then the color Doppler was applied. Peak systolic velocity of the aorta (Ao PSV) and pulmonary artery (PA PSV) were measured. All parameters were measured in triplicate by a single sonographer and the mean of each value was determined.
At 2 days after delivery, all stable neonates were evaluated for vital signs, weight, and measured Mod-MPI using a GE Vivid E9, 12 MHz linear probe. After obtaining written informed consent from their parents, the neonates were laid on the bed with no movement. A linear probe was placed at the neonatal anterior chest wall for assessment. The Doppler sample volume was 2–4 mm, 200–400 Hz of wall filter, and Doppler beam at ≤20°. The mitral inflow velocity pattern was performed at the apical four chamber view, with the Doppler sample volume positioned at the tips of the mitral leaflets.Citation10 The Ao PSV was recorded from the Doppler sample volume placed just below the aortic valve. The left neonatal Mod-MPI was calculated using the same formula as the fetus. All parameters were measured in triplicate and mean values were recorded from both groups. All examinations were performed by one pediatric cardiologist to negate inter-observer variation.
Statistical Analysis
The characteristics of subjects were reported as frequency and percentage for categorical data and mean with standard deviation (SD) for continuous data. Independent t-test and z-test were used for testing the difference between normal blood pressure and preeclampsia groups. Comparing the cardiac functions was conducted by multiple linear regression, fetal Mod-MPI, confounding factors were adjusted by using GA, FHR, and estimated fetal weight, while neonatal Mod-MPI was adjusted by using the neonatal heart rate. All statistical analyses were performed by using STATA software version 15 (StataCorp, College Station, TX).
Results
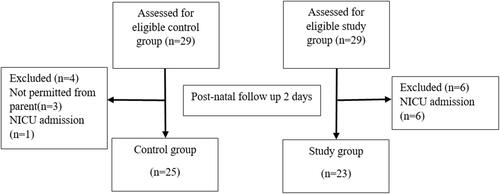
This study was conducted at Srinagarind Hospital, Khon Kaen University, between April 2020 and January 2021. Twenty-nine pregnant women diagnosed with preeclampsia who met the inclusion criteria were enrolled into the study group and 29 pregnant women with normotensive blood pressure who met the inclusion criteria were enrolled as the control group. A flow chart showing enrollment of study participants is in . Characteristics data are shown in . There were no differences in terms of maternal age, primigravida, GA, FHR, neonatal birth weight, neonatal heart rate, or oxygen saturation between the two groups, but mean arterial pressure (MAP) and pre-pregnancy BMI in the preeclampsia group were higher than the control group.
Table 1 Data Characteristics
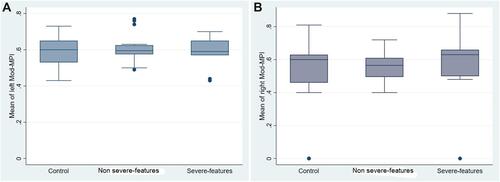
Fetal cardiac functions of both groups are shown in . The fetal left and right Mod-MPI were slightly higher in the preeclampsia group; however, this was not statistically significant. Thirteen fetuses (22%) from both groups had left Mod-MPI exceeding the 90th percentile of normal range. There were no statistically significant differences in fetal ICT, IRT, ET, MV E:A ratio, TV E:A ratio, Ao PSV, or PA PSV between the two groups. For subgroup analysis, 14 pregnancies (48.27%) were preeclampsia with severe features and 15 pregnancies (51.72%) were preeclampsia without severe features. The mean GA at diagnosis of preeclampsia with or without severe features were 35.64±3.11 and 37.07±2.25 weeks (P-value=0.095), respectively, while the MAP were 122.85±7.96 and 107.43±5.90 mmHg (P-value<0.001), respectively. The mean fetal left Mod-MPI of preeclampsia with or without severe features groups were 0.58±0.09 and 0.60±0.07, respectively, with a mean difference of 0.02 (95% CI=−0.04–0.09) (). The mean fetal right Mod-MPI were 0.57±0.25 and 0.56±0.08, respectively, with a mean difference of 0.01 (95% CI=−0.18–0.21) ().
Table 2 Comparison of Fetal Cardiac Function between Preeclampsia and Normotensive Blood Pressure Groups
Figure 3 Box-plot of (A) fetal left Mod-MPI and (B) fetal right Mod-MPI in preeclampsia with or without severe features and the control groups.
Forty-eight neonates underwent echocardiography while 10 did not. Among these, seven were admitted early in the NICU and three were not permitted by their parents. Mean neonatal heart rates in the preeclampsia and normotensive blood pressure groups were 142.11±10.23 and 138.93±10.24 beats per minute (P-value=0.25), respectively. shows the comparison of neonatal left side cardiac function between the two groups. The mean values of neonatal left Mod-MPI were 0.44±0.11 and 0.50±0.25 in the preeclamptic and normotensive blood pressure groups, (P-value=0.436), respectively, with a mean difference of 0.05 (95% CI=−0.09–0.21). Neonatal MV-E PV of preeclampsia and normotensive blood pressure groups were 51.10±8.02 cm/s and 43.56±5.21 cm/s, respectively, with a mean difference of 7.46 (95% CI=0.54–14.37; P-value=0.036). There were no statistically significant differences in terms of neonatal MV-A PV, MV E:A ratio, and Ao PSV between the two groups.
Table 3 Comparison of Neonatal Left Side Cardiac Functions between Preeclampsia and Normotensive Blood Pressure Groups
Discussion
This prospective cohort study demonstrated no statistically significant differences in fetal and neonatal Mod-MPI between the preeclampsia and normotensive blood pressure groups. Furthermore, subgroup analysis did not show statistically significant differences of left and right Mod-MPI between the preeclampsia with severe features or without severe features and control groups. The neonatal MV-E PV showed significantly higher in neonates of preeclamptic women than normotensive women. However, the neonatal MV E:A ratio was similar between the two groups. Our study is compatible with the studies of Api et alCitation3 and Lee et al.Citation11 In 2009, Api et alCitation3 reported that fetal Mod-MPI values were not different among preeclampsia with or without severe features and normal blood pressure groups. Lee et alCitation11 evaluated fetal Mod-MPI and cardiac output between pregnancies with complications including preeclampsia, FGR, or preterm and normal blood pressure groups. They found no statistically significant differences in fetal Mod-MPI. In contrast to our study, Bhorat et alCitation8 determined fetal Mod-MPI in early onset preeclampsia without FGR and normal blood pressure groups. Their results showed a significant elevation of fetal Mod-MPI in the early onset preeclampsia without FGR group. For subgroup analysis, we demonstrated that type of preeclampsia may not affect fetal Mod-MPI. In our study, most preeclampsia cases were terminated immediately after diagnosis, therefore we did not demonstrate whether time of exposure acute hypertension affected fetal Mod-MPI.
In a healthy fetus, the E:A ratio is normally less than 1, but in the case of cardiac volume overload can invert the E:A ratio.Citation5 Our results showed no significant differences in fetal MV E:A ratio and TV E:A ratio. This finding was also supported by Api et al,Citation3 Balli et al,Citation7 and Lee et al.Citation11 Fetal cardiac systolic function can be evaluated by many parameters such as cardiac output, annular displacement, annular peak velocity, and strain rateCitation5 but the technique for assessment may be difficult to perform. We used the reproducible evaluation technique for both Ao PSV and PA PSV to assess resistance in the upper and lower parts of the fetal body. Doppler measurement of flow through the outflow tracts reflects systolic function.Citation5 We found no significant differences in means of fetal Ao PSV and PA PSV between the two groups. All values in both groups were within the normal reference ranges.Citation12
After delivery, cardiovascular system adaptation from the fetus to neonate occurs during the first week of life.Citation13 After umbilical cord clamping, all fetal shunts are closed, and neonatal systemic circulation starts. Neonatal cardiac compliance increases and can tolerate a marked increase of afterload volume.Citation14 In healthy neonates, Mod-MPI are influenced by preload and afterload but in cases of unstable hemodynamic or tachycardia, data were limited available.Citation14 Normal range neonatal Mod-MPI varies between 0.25 and 0.38.Citation14–Citation16 Our study demonstrated no statistically significant differences in neonatal left Mod-MPI between the two groups. In contrast, KardirCitation15 reported that neonatal left Mod-MPI values of mildly preeclamptic pregnancies were significantly higher than newborns of normotensive pregnancies at 24–48 hours post-delivery. For diastolic adaptation, both E wave and E:A ratio progressively rose during the first week of life and MV E:A ratio was usually more than 1. The E waveform is more sensitive to neonatal preload change.Citation14 Our study showed that preeclampsia might affect neonatal preload because we found significant higher E waves in neonates of maternal preeclampsia than normotensive blood pressure groups (P-value=0.036). Although our neonatal E wave was high in the maternal preeclampsia group, there were no statistically significant differences in MV E:A ratio between the two groups. This could be explained because all cases of preeclampsia could be compensated by the changeable neonatal preload and early terminated of pregnancy after diagnosis so the MV E:A ratio was still unchangeable. We suggested early termination of preeclampsia might prevent neonatal Mod-MPI and MV E:A ratio deterioration. All neonatal Ao PSV values of both groups were within the normal reference range.Citation17
An additional finding, we found 22% of fetuses from both groups had left mod-MPI, ICT, and IRT of exceedingly more than the 90th percentile, while ET, E, and A wave were within the normal reference range. All fetuses were delivered by emergency cesarean section due to fetal non-reassuring status. Moreover, we also observed a slightly higher neonatal left Mod-MPI in these groups compared to the reference range. Although neonatal left Mod-MPI was higher, no additional support was required for them. This could be explained that prolong of left Mod-MPI, IRT and ICT became the early signs cardiac dysfunction reflecting an increase in the time required to relax the myocardiumCitation5 so fetal non-reassuring from hypoxemia state might affect of these values. If the higher Mod-MPI was diagnosed in utero, the obstetrician should consult a pediatric cardiologist for further neonatal evaluation and follow-up.
To the best of our knowledge, this is the first prospective study to evaluate fetal and neonatal Mod-MPI from the fetus in utero through the newborn period. However, this study did not investigate the duration of exposure hypertension that may affect fetal cardiac remodeling. Neonatal right Mod-MPI was not provided because of the difficulty in placing a Doppler sample gate between the long-distance tricuspid and pulmonary valves. A follow-up longitudinal echocardiography study is recommended to investigate the significantly high MV-E PV in neonates born from preeclamptic mothers.
Conclusion
No statistically significant differences were recorded for fetal global cardiac function, systolic, and diastolic function between the preeclampsia and normal blood pressure groups. Subgroup analyses of fetal left Mod-MPI between the preeclampsia with or without severe features were also not significantly different. Neonatal MV-E PV of the preeclampsia group was significantly higher than the normotensive group, whereas the Mod-MPI, MV E:A ratio were not different.
Details of Ethics Approval
Ethical approvals were obtained from Khon Kaen University Ethics Committee for Human Research based on the Declaration of Helsinki and the ICH Good clinical Practice Guidelines (April 7, 2020, Reference No. HE621565).
Acknowledgments
We would like to thank the research team, MFM unit, Khon Kaen University without whom this study would not have been possible.
Disclosure
The authors have no conflicts of interest for this work to report.
References
- Timpka S, Macdonald‐Wallis C, Hughes AD, et al. Hypertensive disorders of pregnancy and offspring cardiac structure and function in adolescence. J Am Heart Assoc. 2016;5(11):e003906. doi:10.1161/JAHA.116.003906
- Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25(4):391–403. doi:10.1016/j.bpobgyn.2011.01.006
- Api O, Emeksiz MB, Api M, Ugurel V, Unal O. Modified myocardial performance index for evaluation of fetal cardiac function in pre-eclampsia. Ultrasound Obstet Gynecol. 2009;33(1):51–57. doi:10.1002/uog.6272
- Murase M, Ishida A, Morisawa T. Left and right ventricular myocardial performance index (Tei index) in very-low-birth-weight infants. Pediatr Cardiol. 2009;30(7):928–935. doi:10.1007/s00246-009-9464-8
- Crispi F, Gratacós E. Fetal cardiac function: technical considerations and potential research and clinical applications. Fetal Diagn Ther. 2012;32(1–2):47–64. doi:10.1159/000338003
- Hernandez-Andrade E, Figueroa-Diesel H, Kottman C, et al. Gestational-age-adjusted reference values for the modified myocardial performance index for evaluation of fetal left cardiac function. Ultrasound Obstet Gynecol. 2007;29(3):321–325. doi:10.1002/uog.3947
- Balli S, Kibar AE, Ece I, Oflaz MB, Yilmaz O. Assessment of fetal cardiac function in mild preeclampsia. Pediatr Cardiol. 2013;34(7):1674–1679. doi:10.1007/s00246-013-0702-8
- Bhorat IE, Bagratee JS, Reddy T. Assessment of fetal myocardial performance in severe early onset pre-eclampsia (EO-PET) with and without intrauterine growth restriction across deteriorating stages of placental vascular resistance and links to adverse outcomes. Eur J Obstet Gynecol Reprod Biol. 2017;210:325–333. doi:10.1016/j.ejogrb.2017.01.014
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133(1):1.
- Tsutsumi T, Ishii M, Eto G, Hota M, Kato H. Serial evaluation for myocardial performance in fetuses and neonates using a new Doppler index. Pediatr Int. 1999;41(6):722–727. doi:10.1046/j.1442-200x.1999.01155.x
- Lee JY, Kim YL, Jeong JE, Ahn JW. Prediction of pregnancy complication occurrence using fetal cardiac output assessments made by ultrasonography at 20 to 24 weeks of gestation. Obstet Gynecol Sci. 2017;60(4):336–342. doi:10.5468/ogs.2017.60.4.336
- Parasuraman R, Osmond C, Howe DT. Gestation-specific reference intervals for right and left ventricular ejection force from 12 to 40 weeks of gestation. J Obstet Gynaecol Res. 2012;38(1):160–164. doi:10.1111/j.1447-0756.2011.01660.x
- Bokiniec R, Własienko P, Borszewska-Kornacka MK, Madajczak D, Szymkiewicz-Dangel J. Myocardial performance index (Tei index) in term and preterm neonates during the neonatal period. Kardiol Pol. 2016;74(9):1002–1009. doi:10.5603/KP.a2016.0056
- Mertens L, Seri I, Marek J, et al. Targeted neonatal echocardiography in the neonatal intensive care unit: practice guidelines and recommendations for training: writing group of the American Society of Echocardiography (ASE) in collaboration with the European Association of Echocardiography (EAE) and the Association for European Pediatric Cardiologists (AEPC). J Am Soc Echocardiogr. 2011;24(10):1057–1078. doi:10.1016/j.echo.2011.07.014
- Mutlu K, Karadas U, Yozgat Y, et al. Echocardiographic evaluation of cardiac functions in newborns of mildly preeclamptic pregnant women within postnatal 24–48 hours. J Obstet Gynaecol. 2018;38(1):16–21. doi:10.1080/01443615.2017.1322564
- Bokiniec R, Własienko P, Szymkiewicz-Dangel J, Borszewska-Kornacka MK. Echocardiographic analysis of left ventricular function in term and preterm neonates at week 40 of postconceptional life. Kardiol Pol. 2019;77(4):445–450. doi:10.5603/KP.a2019.0040
- Pees C, Glagau E, Hauser J, Michel-Behnke I. Reference values of aortic flow velocity integral in 1193 healthy infants, children, and adolescents to quickly estimate cardiac stroke volume. Pediatr Cardiol. 2013;34(5):1194–1200. doi:10.1007/s00246-012-0628-6