Abstract
Background
The purpose of this study was to quantify the impact of estradiol-valerate/dienogest (E2V/DNG; Qlaira®/Natazia®) on work productivity and activities of daily living in European and Australian women with heavy menstrual bleeding.
Methods
Women aged 18–54 years with a confirmed diagnosis of heavy menstrual bleeding and no recognizable pathology were recruited across nine European countries (the Czech Republic, Finland, Germany, Hungary, The Netherlands, Poland, Sweden, UK, and Ukraine) and Australia. The women were randomized to receive either E2V/DNG (n = 149) or placebo (n = 82) for seven treatment cycles (196 days). The outcomes assessed included work productivity (ie, productivity while at work) and activities of daily living, measured on a Likert scale from 0 to 10 (with higher values denoting higher impairment levels) at baseline and at the end of the third and seventh cycles (days 84 and 196). The equivalent monetary value associated with the changes in work productivity and activities of daily living was also calculated.
Results
Across all the countries, greater improvements from baseline to the end of treatment were observed with E2V/DNG treatment than placebo in work productivity (46.0% versus 15.1%) and activities of daily living (55.6% versus 30.8%). In 2008, savings associated with improvements in work productivity and activities of daily living due to E2V/DNG treatment (net of placebo improvement) were estimated to be between US$22–62 and US$18–56 per month (in purchasing power parity of US$), respectively.
Conclusion
E2V/DNG has a consistent positive impact on work productivity and activities of daily living in European and Australian women with heavy menstrual bleeding. These improvements were associated with a reduction in monetary burden of heavy menstrual bleeding compared with the placebo group, consistent with the response to treatment observed.
Introduction
Heavy menstrual bleeding (HMB) is a common complaint among women of reproductive age.Citation1 Although usually defined objectively as blood loss of 80 mL or more per menstruation for the purpose of clinical studies, in routine practice, it may be defined as experience of excessive menstrual blood loss that interferes with the woman’s physical, emotional, and social quality of life.Citation2 Prevalence rates for HMB range between 4% and 52%, with rates reported in studies based on subjective perception of the heaviness of menstruation tending to be higher than those where blood loss was objectively assessed.Citation2 About one in 20 women aged 30–49 years in England and Wales consult their general practitioner for HMB in any given year, corresponding to approximately 1.5 million women.Citation3
HMB imposes a significant humanistic burden in the form of impaired health-related quality of life that arises from greater physical, emotional, and social impairment in women with the condition compared with the general female population.Citation4,Citation5 Impaired work productivity due to HMB can have significant economic implications, both for individual women and for society, beyond the direct medical costs of primary care.Citation5 The indirect cost of abnormal uterine bleeding, which includes HMB, was estimated to be US$12 billion per year in the US alone, with directs cost of US$1 billion.Citation5 However, these estimated costs do not account for intangible costs and productivity loss due to presenteeism (ie, reduced on-the-job productivity), and as such, the overall economic impact of HMB may be largely underestimated.
There is a wide variety of therapeutic interventions, both medical and surgical, used for the treatment of HMB.Citation6–Citation8 Because a significant number of women with HMB have no underlying anatomical pathology, medical therapy should usually be considered as first-line therapy,Citation9 and in particular, for women who desire to maintain their child-bearing potential. Estradiol-valerate/dienogest (E2V/DNG; Qlaira®/Natazia®) is the first combined oral contraceptive approved to treat HMB in women without organic pathology who desire oral contraception. The approval of E2V/DNG in the treatment of HMB was achieved based on two randomized, placebo-controlled, double-blind studies, one conducted in the USA and Canada, and the other in Europe and Australia. Citation10,Citation11 As part of these studies, data on work productivity and activities of daily living were collected in addition to efficacy and safety outcomes.
The objective of this paper was to quantify the impact of HMB in women without organic pathology on work productivity and activities of daily living in a European and Australian setting, and to demonstrate the improvements in these outcomes as a result of E2V/DNG treatment. Respective data from the US/Canadian study are presented elsewhere.
Materials and methods
Study population
Full detailed methodology including the criteria for patient selection and treatment allocation in this study have been described in detail elsewhere.Citation10 Briefly, this was a multicenter, randomized, double-blind, placebo-controlled study conducted at 34 centers in Europe (Czech Republic, Finland, Germany, Hungary, The Netherlands, Poland, Sweden, UK, and Ukraine) and Australia between February 2006 and May 2008. The study was approved by local ethics committees or institutional review boards of all participating centers, and conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and the International Conference on Harmonization and Good Clinical Practice guidelines. Written informed consent was obtained from participants prior to study entry. All participants underwent a 28-day screening phase and a 90-day run in phase to confirm HMB objectively, as previously described.Citation10 Women meeting the inclusion criteria received either E2V/DNG or placebo for seven 28-day cycles of treatment. The women completed electronic diaries documenting the presence and intensity of menstrual blood loss on a daily basis, and were also required to collect all used sanitary protection (pads and tampons) so that menstrual blood loss could be objectively measured using a modified version of the alkaline hematin method. The primary efficacy variable was the proportion of women with a complete response (ie, restoration of completely normal menstruation, defined using a composite of eight stringent criteria)Citation10 during a 90-day on-treatment phase compared with a 90-day run in phase.
Work productivity and activities of daily living outcomes were measured using the modified Work Productivity and Activity Impairment Questionnaire (mWPAI)Citation12 at baseline, day 84 during treatment, and day 196 at the end of treatment. The mWPAI, with a modified recall period, asked patients to assess subjectively the effect of HMB on their ability to work and perform regular daily activities during the preceding 12 weeks (as opposed to 7 days with the unmodified WPAI). Participants subjectively rated their level of impairment in work productivity and activities of daily living on a 10-point Likert scale, with higher values indicating higher impairment. Because the effects of HMB vary across menstrual cycles (depending on variations in bleeding intensity and the duration of each cycle), data from several cycles were required to account for this potential variability. Although the prolonged 12-week recall window with the mWPAI has not been validated, it has the advantage of increasing the likelihood of capturing the variable effects of HMB on work productivity (both absenteeism and presenteeism) and activities of daily living across the cycles.
Statistical analyses
Work productivity and activities of daily living outcomes were summarized using descriptive statistics. In our study, work productivity refers to presenteeism, ie, impairment while at work or reduced on-the-job productivity, rather than overall work impairment associated with both absenteeism (work time missed) and presenteeism. Due to the small number of observations in some countries, bootstrapping was used to provide estimates that are appropriate for accurate statistical inference. Bootstrapping relies on repeated samples that are randomly created from original data (excluding patients with missing baseline data) to increase the stability of the computed statistic. In this study, 1000 samples were created and used to calculate baseline values, including standard errors and confidence intervals for work productivity and activities of daily living.
Multivariate analyses were undertaken with Bayesian Markov Chain Monte Carlo methods in WinBUGsCitation13 to obtain estimates of the impact of treatment on work productivity and activities of daily living impairment at visit 7 (day 84) and visit 11 (end of treatment) using pooled data from the two Phase III HMB studies evaluating the safety and efficacy of E2V/DNG in the US/Canadian and European/Australian setting.Citation10,Citation11 Impairment of both work productivity and activities of daily living at day 84 and at end of treatment served as dependent variables within the model. Relevant covariates were chosen in a stepwise approach to improve model convergence and provide the best model fit for the observed data, measured using the deviance information criterion.Citation14 Relevant variables included in the model were race, education level, standardized blood loss at baseline, baseline global Psychological General Well-Being Index score, treatment arm, and country-specific random effects. The robustness of the findings was tested using different distributional assumptions; both normal and gamma distribution was assumed for the work productivity and activities of daily living impairment variables.Citation15 Country-specific work productivity and impairment in activities of daily living were then estimated at day 84 and 196 (end of treatment) using the distributional assumption that resulted in a minimum deviance information criterion. The actual baseline work productivity and activities of daily living impairment values were used because the variables available in the clinical trial lacked predictive power.
Consistent with the WPAI scoring manual,Citation12 a direct relationship was assumed between work productivity or activities of daily living and the Likert Scale response from 0 to 10 in the calculation of the impairment value. For example, a response of 1 on the Likert Scale was assumed to be equivalent to a 10% productivity reduction or impairment in activities of daily living, and a response of 9 was assumed to be equivalent to a 90% productivity reduction or impairment in the activities of daily living. An individual reporting work productivity or activities of daily living of 3 at baseline and 2 at the end of treatment thus had a 10% point improvement in work productivity or impairment in activities of daily living.
Average weekly salary data in a given country were used to provide a common reference point for all countries. However, it was understood that due to the nature of HMB, the weekly estimate would have a monetary impact equivalent to a month’s impact. Where only hourly average salary data were available, an estimate was obtained by assuming a 40-hour work week.
Patients were also asked to record the duration of their bleeding during each cycle. The average number of days with bleeding per month pooled across the two Phase III studies evaluating the safety and efficacy of E2V/DNG in the US/Canadian and European/Australian settings were calculated (over the 3-month efficacy period) as 6.9 at baseline and 5.5 at the end of treatment.
The monetary value associated with work productivity and activities of daily living at baseline was calculated by multiplying the mean salary (obtained from the International Labor Organization [http://www.ilo.org] statistics) by the mean impairment based on the mWPAI response and mean duration of bleeding to obtain the monthly estimate, separately for the E2V/DNG and placebo groups. The monthly monetary estimate of work productivity and activities of daily living was again calculated at the end of treatment using the same method. The gain in work productivity and activities of daily living, in monetary terms, was thus the difference between baseline and end of treatment in each treatment group.
Results
Clinical outcomes
The clinical outcomes of this study have been published in full elsewhere.Citation10 In brief, 108 and 62 women in the E2V/DNG treatment and placebo groups, respectively, were included in the analysis (excluding those subjects with missing data). Of these, a significantly higher proportion of women in the E2V/DNG group (44; 40.7%) had a complete response to treatment (ie, a return to “menstrual normality”) compared with women in the placebo group (1; 1.6%; P < 0.0001).Citation10 During the 90-day efficacy period, mean menstrual blood loss in the E2V/DNG group was reduced by 458.4 ± 409.6 mL compared with baseline, whereas the reduction in the placebo group was 93.2 ± 268.0 mL; adjusted between-treatment difference in volume of menstrual blood loss was 373 mL lower in the E2V/DNG group (95% confidence interval 490–255 mL; P < 0.0001). The marked decline in menstrual blood loss with E2V/DNG, already apparent after cycle 1, was accompanied by significant improvements in hemoglobin, hematocrit, and serum ferritin concentrations.
Work productivity and activities of daily living
Baseline work productivity and impairment in activities of daily living varied considerably across countries; work productivity impairment ranged from 18% in Sweden to 73% in Poland (, where 1 on the Likert Scale was assumed to be equivalent to a 10% impairment value), whereas impairment in activities of daily living ranged from 30% in the UK to 65% in Poland (). Consistent with greater improvement in underlying symptoms with E2V/DNG treatment, improvements from baseline to the end of treatment were observed in all countries in work productivity () and activities of daily living () to a greater extent in the E2V/DNG group. End of treatment reduction in impairment of work productivity ranged from 14% in Sweden to 32% in Poland, and activities of daily living levels ranged from 14% in Sweden to 28% in Poland. Variations across the countries in terms of percentage improvement in work productivity and activities of daily living in both treatment groups are summarized in . Across all the countries, E2V/DNG treatment improved work productivity and activities of daily living from baseline to the end of treatment to a greater extent than placebo ().
Figure 1 Work productivity impairment values at baseline and EOT as measured using the modified Work Productivity and Activity Impairment (mWPAI) questionnaire.
Abbreviation: EOT, end of treatment; AUS, Australia; CZR, Czech Republic; FIN, Finland; GER, Germany; HUN, Hungary; NL, The Netherlands; PL, Poland; SWE, Sweden; UK, United Kingdom; UKR, Ukraine.
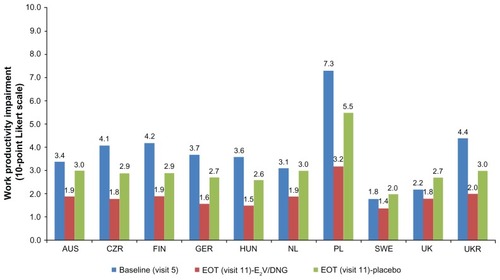
Figure 2 Activities of daily living impairment values at baseline and EOT as measured using the modified Work Productivity and Activity Impairment (mWPAI) questionnaire.
Abbreviations: EOT, end of treatment; E2V/DNG, estradiol-valerate/dienogest; AUS, Australia; CZR, Czech Republic; FIN, Finland; GER, Germany; HUN, Hungary; NL, The Netherlands; PL, Poland; SWE, Sweden; UK, United Kingdom; UKR, Ukraine.
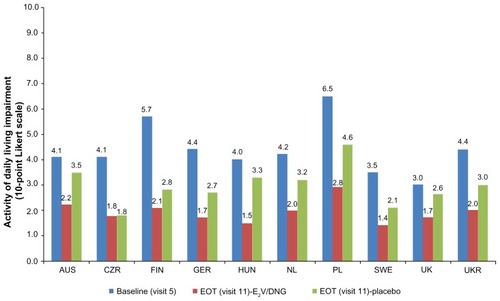
Figure 3 Reduction in work productivity and activities of daily living impairment across all countries (baseline to end of treatment) by treatment group (negative values indicate improvement).
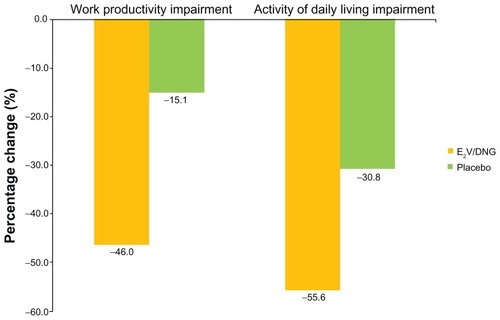
Table 1 Change in work productivity and activities in daily living from baseline to end of treatment (baseline results from bootstrapping; end of treatment results from Bayesian analysis)
Monetary outcomes
Monthly monetary gains (or savings) in work productivity and activities of daily living (in purchasing power parity US$, 2008)Citation16 resulting from treatment of HMB with E2V/DNG are presented (). Savings associated with improvements in work productivity and activities of daily living due to E2V/DNG treatment (net of placebo improvement) were estimated to be between US$22–62 and US$18–56 per month, respectively. Monetary gains (or savings) resulting from treatment of HMB with E2V/DNG in 2008 local currency are also presented in . Gains stemming from E2V/DNG treatment (net of change observed in the placebo group) were estimated to range from 20% (Hungary) to 32% (UK) of the work productivity monetary loss at baseline. E2V/DNG treatment (net of placebo improvement) was associated with an improvement in activities of daily living and associated monetary gains of between 14% (Hungary) and 22% (Poland) compared with baseline.
Table 2 Savings due to E2V/DNG treatment related to improvements in work productivity and activities of daily living, in 2008 local currency and US dollars
Discussion
This paper evaluated the impact of E2V/DNG on work productivity and activities of daily living in European and Australian women suffering from HMB without underlying organic pathology. Based on data from a double-blind, randomized, placebo-controlled Phase III trial, the results demonstrate that E2V/DNG has a consistent, significant, and positive impact on work productivity and activities of daily living across the countries assessed. In many of the countries, the improvement in work productivity and activities of daily living from baseline to end of treatment was found to exceed 50% for both outcomes.
Furthermore, this study shows that there is a substantial burden associated with HMB, in that productivity loss and impairment in activities of daily living at baseline (ie, pretreatment) consistently reached the 35%–45% range (100% denoting complete productivity loss or activities of daily living impairment), with the highest country-specific average at almost 75%. This finding is consistent with previous research indicating high burden levels associated with HMB, based primarily on US women. On a per patient basis, annual lost productivity due to HMB and/or “dysfunctional uterine bleeding”, encompassing the value of lost income and lost household activities, has been estimated to be $2291 per affected woman in the USA.Citation17 In addition, there is also productivity loss associated with missed school or curtailed educational pursuits. Nonetheless, indirect cost data associated with absenteeism or presenteeism specific to education (as opposed to employment) are lacking and constitute a gap in current HMB research.
The episodic nature of HMB and the fact that the disease affects women who are active participants in the labor force requires a broader approach to evaluating the burden of HMB than simply examining its clinical characteristics, treatment, and costs of medical care. Although clinical aspects of the disease will remain important, the high impact of HMB on productivity outcomes documented in our findings underscores the need to incorporate measures of productivity loss in the evaluation of health care interventions for HMB. Clear documentation of improvements in productivity outcomes associated with new therapeutic options should, therefore, be considered important from the public health perspective.
Available data have demonstrated that successful treatment of HMB increases participation in the labor force and decreases work absenteeism.Citation18–Citation23 Although not directly assessed, the available data from our study would be indicative of increased participation in the labor force and decreased work absenteeism with E2V/DNG treatment relative to placebo. Treatment with E2V/DNG was associated with at least a 34% improvement in the HMB-related monetary burden in work productivity and activities of daily living compared with placebo. Although both E2V/DNG and placebo were associated with monthly gains greater than at baseline, the monthly gains associated with the E2V/DNG treatment arm were greater than those in the placebo group. Our data suggest that treating HMB with E2V/DNG may be beneficial to society through enhanced work productivity and reduced economic losses.
A strength of our study was that the analysis drew upon data collected prospectively during a randomized clinical trial. Randomized trials are generally accepted as the gold standard in determining treatment effect because they provide the least biased evidence.Citation24 In addition, although some of the individual countries had small sample sizes which could lead to high uncertainty regarding country-specific estimates of work productivity and activities of daily living, bootstrap and Bayesian methods were used to provide more reliable estimates. However, a limitation of clinical trials is that they usually recruit a narrowly defined patient population, and as such, may not be applicable to the general population of women with HMB in actual clinical practice.Citation25
In summary, our results show that E2V/DNG treatment improves work productivity and activities of daily living in European and Australian women with HMB without underlying organic pathology to a greater extent than placebo. The greater improvements in work productivity and activities of daily living with E2V/DNG treatment are also consistent with the greater response to treatment achieved over placebo. However, observational studies in women with self-reported HMB (as opposed to clinical trials assessing HMB confirmed with objective measures) are needed to confirm our findings in the real-world setting.
Disclosure
This study was funded by Bayer HealthCare Pharmaceuticals, Berlin, Germany, the manufacturer of E2V/DNG. Editorial assistance with the preparation of this manuscript was provided by Richard Glover of inScience Communications, Springer Healthcare. Funding for this editorial assistance was provided by Bayer HealthCare Pharmaceuticals. RW, DS, and SS are employees of United BioSource Corporation which was contracted to perform the analysis. DJV is contracted to United BioSource Corporation. IF is a consultant and speaker for Bayer HealthCare Pharmaceuticals, Schering Plough, and Daiichi Sankyo Pharmaceuticals, and has received research support from the National Institutes of Health, the Australian National Health and Medical Research Council, the Population Council, Bayer HealthCare Pharmaceuticals, and Schering Plough. AF and KW-J are current employees of Bayer HealthCare Pharmaceuticals.
References
- National Institute for Clinical ExcellenceHeavy Menstrual Bleeding Costing report: Implementing NICE guidance in England2007 Available from: http://www.nice.org.uk/page.aspx?o=CG044CostReportAccessed October 9, 2009
- National Collaborating Centre for Women’s and Children’s HealthHeavy Menstrual Bleeding. Clinical Guideline. National Institute for Health and Clinical ExcellenceLondon, UKRCOG Press2007
- National Institute for Clinical ExcellenceFluid-filled Thermal Balloon and Microwave Endometrial Ablation Techniques for Heavy Menstrual BleedingTechnology Appraisal 78London, UKNational Institute for Clinical Excellence2004
- FraserISLanghamSHealth-related quality of life and economic burden of abnormal uterine bleedingExpert Rev Obstet Gynecol200942179189
- LiuZDoanQVBlumenthalPDuboisRWA systematic review evaluating health-related quality of life, work impairment, and healthcare costs and utilization in abnormal uterine bleedingValue Health200710318319417532811
- ACOG Practice BulletinClinical management guidelines for obstetrician- gynecologists. Number 81, May 2007Obstet Gynecol200710951233124817470612
- AmannMAnguinoHBaumanRAChronic Abnormal Uterine Bleeding in Nongravid WomenPasadena, CAKaiser Permanente Southern California2006
- VilosGALefebvreGGravesGRSOGC Clinical practice guidelines: guidelines for the management of abnormal uterine bleedingJ Obstet Gynaecol Can2001238704709
- ClarkeJTreatment of heavy menstrual bleedingBMJ2010341c377120716600
- FraserISRomerTParkeSEffective treatment of heavy and/or prolonged menstrual bleeding with an oral contraceptive containing estradiol valerate and dienogest: a randomized, double-blind Phase III trialHum Reprod201126102698270821784734
- JensenJTParkeSMellingerUMachlittAFraserISEffective treatment of heavy menstrual bleeding with estradiol valerate and dienogest: a randomized controlled trialObstet Gynecol2011117477778721422847
- Reilly AssociatesWork productivity and activity impairment questionnaire scoring2002 Available from: http://www.reillyassociates.net/WPAI_Scoring.htmlAccessed March 3, 2011
- WinBUGs Available from: http://www.mrc-bsu.cam.ac.uk/bugs/winbugs/contents.htmlAccessed May 3, 2012
- SpiegelhalterDJBestNGCarlinBPVan der LindeABayesian measures of model complexity and fitJ R Stat Soc Ser B Stat Soc2002644583616
- WasiakRFilonenkoAStullDEMethodological considerations in impact assessment of estradiol valerate/dienogest (E2V/DNG, Qlaira®) on work productivity (WP) and activities of daily living (ADL) outcomes in multinational clinical trials in heavy and/or prolonged menstrual bleeding (HPMB)Presented at the 13th Annual European Congress of the International Society for Pharmacoeconomics and Outcomes ResearchPrague, Czech RepublicNovember 6–9, 2010
- United Nations Statistics DivisionPurchasing power parities (PPP) conversion factor, local currency unit to international dollarMillennium development goals indicators2010 Available from: http://unstats.un.org/unsd/mdg/SeriesDetail.aspx?srid=699Accessed March 3, 2011
- FrickKDClarkMASteinwachsDMFinancial and quality-of-life burden of dysfunctional uterine bleeding among women agreeing to obtain surgical treatmentWomens Health Issues2009191707819111789
- el SenounGSMousaHAMahmoodTAMedium-term follow-up of women with menorrhagia treated by rollerball endometrial ablationActa Obstet Gynecol Scand2000791087988311304973
- GoldrathMHEvaluation of hydrothermablator and rollerball endometrial ablation for menorrhagia 3 years after treatmentJ Am Assoc Gynecol Laparosc200310450551114738639
- LofferFDThree-year comparison of thermal balloon and rollerball ablation in treatment of menorrhagiaJ Am Assoc Gynecol Laparosc200181485411172114
- MousaHAAbou El SenounGMMahmoodTAMedium-term clinical outcome of women with menorrhagia treated by rollerball endometrial ablation versus abdominal hysterectomy with conservation of at least one ovaryActa Obstet Gynecol Scand200180544244611328222
- SambrookAMBainCParkinDECooperKGA randomised comparison of microwave endometrial ablation with transcervical resection of the endometrium: follow up at a minimum of 10 yearsBJOG200911681033103719438487
- SculpherMJDwyerNByfordSStirratGMRandomised trial comparing hysterectomy and transcervical endometrial resection: effect on health related quality of life and costs two years after surgeryBr J Obstet Gynaecol199610321421498616131
- AbelUKochAThe role of randomization in clinical studies: myths and beliefsJ Clin Epidemiol199952648749710408986
- FleurenceRLNaciHJansenJPThe critical role of observational evidence in comparative effectiveness researchHealth Aff (Millwood)201029101826183320921482