Abstract
Following the successful development of long-acting steroid-releasing vaginal ring devices for the treatment of menopausal symptoms and contraception, there is now considerable interest in applying similar devices to the controlled release of microbicides against HIV. In this review article, the vaginal ring concept is first considered within the wider context of the early advances in controlled-release technology, before describing the various types of ring device available today. The remainder of the article highlights the key developments in HIV microbicide-releasing vaginal rings, with a particular focus on the dapivirine ring that is presently in late-stage clinical testing.
Introduction to controlled drug release and vaginal rings
The concept of controlled drug release from polymeric materials was first established in the 1960s by Judah Folkman when he discovered that exposure of anesthetic gases to the external surface of a silicone rubber arteriovenous shunt containing circulating rabbit blood caused the rabbits to fall asleep.Citation1 Based on this ability of gaseous molecules to permeate silicone rubber, it was postulated that solid drugs incorporated into silicone rubber (or other elastomeric polymers) could be implanted in the body to provide rate-controlled drug-delivery systems.Citation2,Citation3 The first controlled-release drug-delivery systems to be commercialized were two devices: Alza’s Ocusert® (an ophthalmic insert releasing pilocarpine at a constant rate for the treatment of glaucoma) and Progestasert® (an intrauterine implant providing a constant rate of progesterone delivery). Both were reservoir-type devices fabricated from the thermoplastic polymer poly(ethylene-co-vinyl acetate) (PEVA). The first commercial silicone elastomer device for controlled release was the Population Council’s subdermal contraceptive implant Norplant®, first approved for use in Finland in 1983 and marketed in the USA in 1991. Consisting of six small (2.4 mm × 34 mm) silicone elastomer cylinders, each filled internally with 36 mg of levonorgestrel (a progestin used in many birth-control pills), Norplant® was a direct extension of Folkman’s original concept and provided effective fertility control for five years. A second generation of Norplant®-type devices is currently available, containing either one or two drug-loaded rods and fabricated from either silicone elastomer or PEVA.
In 1970, a US patent assigned to the UpJohn Company was published describing “an improved resilient annular device for intravaginal placement and retention . . . containing an effective amount of a medicament which is capable of passage through the drug-permeable polymeric material” ().Citation4 This first description of the concept of a drug-releasing vaginal ring heralded an intense period of activity in ring development through the 1970s and 1980s, mainly focused around contraception and hormone replacement therapy and involving various ring designs. The World Health Organization (WHO) was at the forefront of this research with their program for the development of a silicone elastomer steroidal contraceptive ring to help contain the burgeoning population growth. The interested reader is directed to Hoffman’s excellent overview on the origins and evolution of controlled-release drug-delivery systems.Citation5
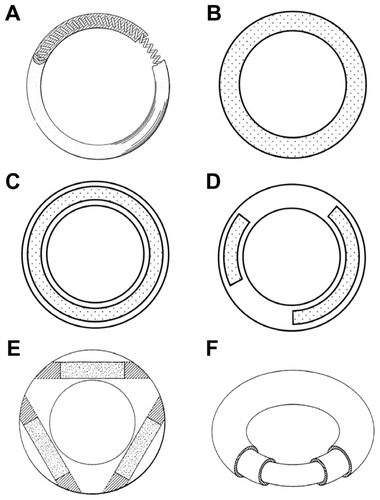
Figure 1 Representative vaginal ring designs. (A) Over-molded metal spring design first described in a 1970 patent.Citation4 (B) Matrix-type ring with solid micronized drug dispersed throughout the entire polymer. (C) Full length reservoir/core ring design, where the drug-loaded core is encapsulated by a nonmedicated, rate-controlling polymer membrane. (D) Multiple partial core ring design, where each core contains a different drug substance. (E) Insertable core ring design, where drug-loaded cores are inserted into a prefabricated ring body before sealing the ends. (F) Sandwich or shell ring design, where a drug-loaded layer is sandwiched between a nonmedicated polymeric central core and a nonmedicated outer rate-controlling polymer membrane.
Vaginal rings
Vaginal rings are flexible, torus-shaped, silicone elastomer or thermoplastic devices that provide long-term, sustained or controlled delivery of pharmaceutical substances to the vagina for either local or systemic effect. Designed to be readily inserted and removed by the woman herself, they are generally positioned in the upper third of the vagina adjacent to the cervix. Although the exact location of ring placement is generally not critical for clinical efficacy, it may have implications for comfort in some women.
The simplest vaginal ring design contains solid drug particles (usually in a micronized form) dispersed throughout the entire polymeric matrix. The drug-release yield from these so-called “homogeneous” or “matrix” rings () is governed by a permeation mechanism for which the release rate is dependent upon (1) the drug’s solubility in the polymer, (2) the ability of the solvated drug to diffuse through the polymer, (3) the drug-loading within the device, and (4) the ring surface area. The drug-permeation process involves dissolution of the solid drug in the polymer, followed by diffusion of the solubilized molecules through the elastomeric polymer network. Drug near the surface of a matrix ring is released first, creating a drug-depleted layer through which solubilized molecules within the ring body must first diffuse in order to be effectively released. As time progresses the surface area of this inward-moving depletion boundary decreases, causing an increase in the thickness of the depletion layer, resulting in decreased drug-release rates over time.
The “sandwich” and “core” vaginal ring designs (also known as “shell” and “reservoir,” respectively) were developed to provide constant daily release rates throughout the period of use, conforming to “zero order” release kinetics. The sandwich design () consists of a narrow drug-loaded polymer layer positioned between a nonmedicated inner central core and a nonmedicated outer membrane. The position of the drug core close to the surface ensures efficient delivery of drugs having poor polymer diffusion characteristics. Core-type rings contain the drug(s) within one or more central cores that are encapsulated by a drug-free outer polymer membrane (). Several individual drug-loaded cores of various lengths may be incorporated into the same core ring, thereby allowing delivery of multiple actives at predetermined and independent release rates. The release rates of both the core and sandwich-ring designs may be further modified by changing the thickness of the rate-controlling outer membrane.
The range of materials used in the manufacture of vaginal rings is limited by the requirements for biocompatibility, flexibility, and high drug permeability. Commercial ring products are made from either silicone (polydimethylsiloxane) elastomer or PEVA. More recently, thermoplastic polyurethane materials have also being investigated for ring fabrication.Citation6–Citation9
Silicone elastomer vaginal rings
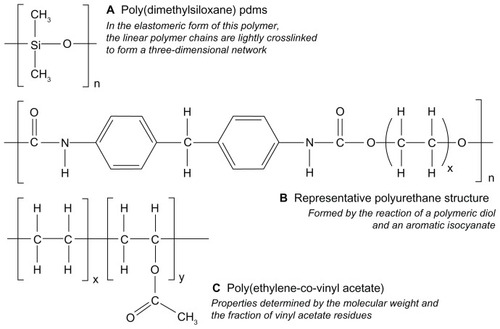
Silicones represent a category of synthetic polymers comprising a main chain of alternating silicon and oxygen atoms, and having two organic groups covalently bonded to the silicon atom ().Citation10 The most common silicone derivative is that having two methyl groups, called polydimethylsiloxane. The linear polymeric forms of silicones are liquids at room temperature, even for large molecular weights. Elastomeric silicone materials are produced by incorporating reactive functional groups into the linear polymers that may be subsequently “cross-linked” using suitable cross-linking molecules. Silicone polymers, and particularly the elastomeric forms, have a long history of use in both medical devices and drug-delivery devices, stemming from their excellent biocompatibility and biodurability.Citation11 Silicone elastomer rings are generally made by elevated-temperature reaction injection molding, using either condensation or addition-cure chemistries.Citation10
Figure 2 Chemical structures representing (A) silicone, (B) polyurethane and (C) poly(ethylene-co-vinyl acetate) materials used in the fabrication of drug-releasing vaginal rings.
Two estrogen-releasing, reservoir-type silicone elastomer vaginal rings have been commercialized for the treatment of menopausal symptoms related to estrogen deficiency. Estring® (Pfizer, New York, NY) contains a full-length reservoir core () and releases 7.5 μg/day of 17β-estradiol continuously over 3 months for the treatment of local symptoms. Femring® (Warner Chilcott UK Ltd, Larne, Northern Ireland) contains a single partial-length reservoir core (single-core version of ) and releases either 50 μg/day or 100 μg/day (depending on core length) of the estradiol prodrug 17β-estradiol-3-acetate over 3 months for the treatment of both systemic and local symptoms. Both rings show a high degree of user acceptability and are preferred by women over estrogen vaginal gels.Citation12–Citation15 A 1-year combination contraceptive ring device, worn for 3 weeks per cycle and simultaneously releasing 15 mcg/day ethinyl estradiol and 150 mcg/day nestorone, is also in clinical development.Citation16–Citation19
The first report of a microbicide-releasing vaginal ring described the continuous in vitro release over 8 days of the non-ionic surfactant nonoxynol-9 from a matrix-type silicone elastomer device.Citation20 Unlike the solid antiretroviral compounds currently being tested as HIV microbicides, nonoxynol-9 is a liquid at a room temperature, such that its release from the ring followed unconventional release kinetics. Nonoxynol-9 has since been discontinued as a microbicide candidate since studies showed that it increased the risk of HIV transmission following frequent application.Citation21,Citation22 A subsequent study showed that dapivirine (also known as TMC120) could be effectively released in vitro over 71 days from a silicone elastomer reservoir-type ring device.Citation23 Moreover, it was calculated that the constant daily rate observed of 130 μg release could effectively be maintained for at least 1 year, and theoretically for up to 4 years.
Many of the lead-candidate antiretroviral compounds being developed as HIV microbicides (eg, dapivirine, maraviroc, UC781, and MC1220) have physicochemical properties (specifically, high hydropobicity and low molecular weight) very similar to those of steroid molecules, suggesting that silicone elastomer vaginal ring formulations may also be useful for the controlled release of microbicides. Although numerous antiretroviral compounds have been tested in rings in vitro, only the dapivirine-matrix and reservoir-type silicone elastomer rings have reached the clinical stages of development (). Two Phase I safety studies have been completed for a 200 mg dapivirine reservoir ring (study IPM 001) (),Citation24 a 25 mg dapivirine reservoir ring (studies IPM 008 and IPM 01) (),Citation24,Citation25 and a 25 mg dapivirine matrix ring (study IPM 018) ().Citation21 Although these rings had different designs, they were both manufactured using a condensation-cure silicone elastomer system specifically designed for the incorporation and release of drug molecules. The IPM 001 and IPM 008 studies demonstrated good safety and tolerability for both the 25 mg and 200 mg dapivirine reservoir-type rings, compared with a placebo ring, during the 7-day study period. Reported vaginal-fluid concentrations of dapivirine at various locations along the cervicovaginal tract (introitus, cervix, ring position) were in the range 0.7–7.1 μg/mL, with levels highest close to the ring and lowest at the introitus. Vaginal- and cervical-tissue concentrations on day 7 were between 0.3 μg/g and 3.5 μg/g. Plasma concentrations, which are a particularly important consideration from the perspective of the potential emergence of strains resistant to the drug in HIV-positive users, were less than 50 pg/mL. Dapivirine concentrations in vaginal fluid and tissue were more than three orders of magnitude greater than the in vitro EC50 against a wild-type HIV-1 strain.Citation26,Citation27 Dapivirine levels measured with the 25 mg continuous core device () were generally higher (but did not reach statistical significance) than those for the 200 mg two-core ring device (). This is consistent with the nature of membrane-type diffusion-controlled drug-delivery systems, where drug release kinetics are determined by the length of the reservoir and the thickness of the rate-controlling membrane, but not the drug-loading (which only influences the duration of release).Citation28
Figure 3 Schematic diagrams (to scale) describing the design and dimensions of dapivirine-releasing vaginal rings. The design in (A) was evaluated in clinical study IPM 001 () and reported in Romano et al.Citation24 The design in (B) was evaluated in IPM 008 and IPM 018 (), and reported in Romano et alCitation24 and Nel et al.Citation25 The design in (C) is the 25 mg dapivirine matrix ring due to progress to Phase III in 2012 as part of study MTN 020 (). It was previously tested in IPM 013, IPM 018, IPM 024, and IPM 027 (), and has been reported in Nel et al.Citation25 The design in (D) is the reservoir-type dapivirine-releasing ring first reported in the literature by Malcolm et al.Citation23
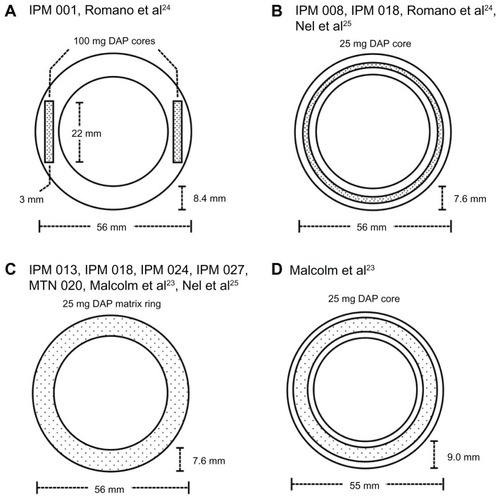
Table 1 Completed, ongoing and planned microbicide ring clinical studies
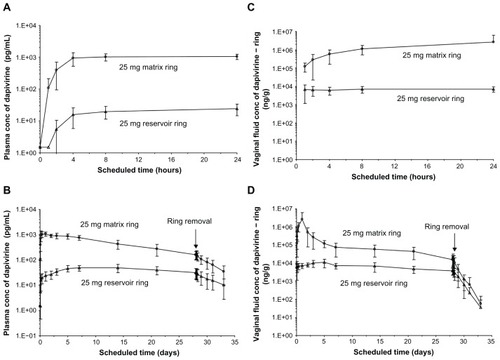
Reservoir rings are associated with several disadvantages compared with matrix-type rings, most notably their complexity of manufacture (a multistep process) and the relatively low (but constant) release rates. In the IPM 018 study (), the pharmacokinetics of matrix and reservoir rings, each containing 25 mg dapivirine (), were compared.Citation25 The results clearly illustrated differences in dapivirine release rates, with significantly higher plasma and vaginal-fluid levels achieved with the matrix format, owing to the presence and rapid release of the drug at the device surface (). Vaginal-fluid levels peaked at ~1000 μg/g 24 hours after placement of the matrix ring, and declined steadily to around ~10 μg/g on day 28, immediately prior to ring removal (). In general, vaginal-fluid levels for the reservoir ring were between one and two orders of magnitude lower than those for the matrix ring. Plasma levels of dapivirine ranged from 10–1000 pg/mL ().
Figure 4 Reported pharmacokinetic profiles for the 25 mg matrix and 25 mg reservoir rings tested in IPM 018. (A and B) show plasma concentrations (conc) over 24 hours and 33 days, respectively. (C and D) show vaginal-fluid concentrations over 24 hours and 33 days, respectively.
© 2009 Lippincott Williams and Wilkins. Reprinted with permission. Nel A, Smythe S, Young K, et al. Safety and pharmacokinetics of dapivirine delivery from matrix and reservoir intravaginal rings to HIV-negative women.Citation25
The propanol by-product associated with condensation-type silicone elastomers can lead to a “drug burst effect,” where the incorporated solid drug is dissolved in the propanol, transported to the device surface, and subsequently deposited following evaporation of the propanol.Citation29–Citation31 This solvent-aided deposition of the drug can artificially increase its release rate during the early period of use, beyond that attributed to the normal polymer diffusion-controlled process. Subsequent studies have confirmed that a substantial drug burst occurred with the early prototype condensation-cure dapivirine-releasing silicone elastomer rings, prompting a move to addition-cure silicone elastomer systems (which produce no solvent by-product during the silicone-curing reaction) for the future clinical development of the dapivirine matrix ring. Preliminary pharmacokinetic data (unpublished) from the IPM013 and IPM024 Phase 1 studies for the addition-cure 25 mg-dapivirine ring showed detection of dapivirine in both vaginal fluid and plasma 1.5 hours after ring insertion; the maintenance of dapivirine concentrations in vaginal fluid was in the 10–50 mcg/g range over the 35-day study period. The ASPIRE Phase III study (MTN 020, ) of the addition-cure silicone elastomer 25 mg dapivirine ring will be launched at several African sites in 2012 and involve almost 3500 women. First results are anticipated in late 2014 or early 2015.
Following the success of antiretroviral combinations for the treatment of HIV infection, there is also considerable interest in combination microbicide products, including vaginal rings, that simultaneously release two or more microbicide compounds having different mechanisms of action. The first clinical trial of such a ring (IPM 026/MTN 013, ), comprising 25 mg dapivirine and 100 g maraviroc (an entry inhibitor) in an addition-cure silicone elastomer matrix format () is planned for 2012 with 48 healthy women using either the combination ring, a 25 mg dapivirine-only ring, a 100 mg maraviroc-only ring, or a placebo ring continuously for 28 days.
Microbicide-releasing silicone elastomer vaginal rings have also been designed for use in macaques, enabling important pharmacokinetic and challenge studies to be performed in a more clinically relevant nonhuman-primate model.Citation32–Citation34
Thermoplastic vaginal rings
Thermoplastics are polymer substances that melt (and flow) upon heating and that harden again upon subsequent cooling. While most thermoplastic materials are not suitable for the fabrication of vaginal rings owing to their poor flexural and mechanical characteristics, a subset known as “thermoplastic elastomers” has been successfully applied to vaginal ring construction. Most notably, ethylene vinyl acetate copolymers (PEVA) are used as both the drug matrix and the rate-limiting sheath in the combination (etonogestrel + ethinyl estradiol) contraceptive vaginal ring device Nuvaring®. A number of other subdermal, implantable drug-delivery devices are also fabricated from PEVA, including Implanon® and Virtasert®. The vinyl acetate content (typically ranging from approximately 10% to 40%) and the molecular weight characteristics of the PEVA material play a major role in determining the mechanical properties, the ease of processing, and the drug release rates of the finished drug-delivery device.
Nuvaring® is a nonbiodegradable, flexible, transparent, colorless, contraceptive vaginal ring (), developed by Organon Inc and first marketed in 2002. It contains two active compounds, progestin etonogestrel (11.7 mg) and estrogen ethinyl estradiol (2.7 mg), both located within a central PEVA (28% w/w vinyl acetate) core as a supersaturated eutectic mixture, and surrounded by a nonmedicated polyethylene-co-vinyl acetate (9% w/w vinyl acetate) sheath layer. The slow permeation of the steroid molecules through the low-vinyl-acetate-content nonmedicated sheath provides for controlled release, resulting in mean daily release rates of 120 μg etonogestrel and 15 μg ethinyl estradiol over the 3-week period of use. The ring is used continuously for 3 weeks, and then removed for 1 week before being replaced with a new ring. Nuvaring® is highly efficacious, with a Pearl index of 1.18 and an efficacy of 99.1%. Off-label 28-day continuous use of Nuvaring® is also being evaluated.Citation35
Despite the commercial success of Nuvaring®, there have been very few reports of the use of PEVA for microbicide-releasing vaginal rings, probably owing to ongoing difficulties in accessing clinical-grade materials for testing in humans. A comparison of the pharmacokinetics of the nonnucleoside reverse-transcriptase inhibitor UC781 in rabbits following vaginal administration of ring segments fabricated from PEVA, polyurethane, and silicone elastomer showed similar in vivo release rates and kinetics.Citation8 Recently, a combination PEVA matrix-type ring providing simultaneous release of the microbicide candidate UC781 and the contraceptive progestin levonorgestrel has been reported.Citation36 If the proof of concept of a microbicide vaginal ring device can be realized with the dapivirine-releasing silicone elastomer ring, then these multipurpose prevention technologies that combine HIV protection with effective contraceptive regimes will be actively pursued.
Table 2 Summary of microbicide-releasing vaginal ring devices either previously evaluated or under ongoing investigation
Thermoplastic polyurethanes are also being evaluated for use in microbicide-releasing vaginal rings. As with PEVA, the properties of polyurethanes can be readily tailored by controlling their chemical structure (), simply by adjusting the components of the reaction mixture (polymeric diol, a chain extender, and isocyanate) used in their synthesis. To date, polyurethane rings releasing either UC781, dapivirine, tenofovir, or a combination have been demonstrated in in vitro studies.Citation6,Citation7,Citation9,Citation37 Constant daily release of dapivirine from matrix-type ring prototypes was sustained over 30 days, with the release rate dependent on the initial drug-loading.Citation6 Novel segmented polyurethane vaginal rings have also been evaluated for simultaneous release of dapivirine and tenofovir. Each drug was incorporated into its own rod segment before joining the rods to prepare the intact ring device.Citation7 While this approach permits independent formulation of the component microbicides and control of release rates, the complexity of design and the required scaled manufacturing are obstacles to further development.
New designs of vaginal ring for microbicide delivery
Several new designs of vaginal ring device are also in development, all seeking to overcome the drug-permeability constraints associated with release of hydrophilic and/or macromolecular actives from conventional silicone elastomer and thermoplastic systems.
The Versaring® technology platform (Auritec Pharmaceuticals) is based on drug-loaded pod inserts, comprising polymer-coated solid drug, incorporated into a silicone elastomer ring and exhibiting pseudo-zero order release kinetics.Citation38–Citation40 Drug cores are coated with layers of semipermeable poly(lactic acid) polymer. Coated drug pods are incorporated into silicone rings, and the drug is released through a delivery window in the silicone ring, with the release rate determined by the window diameter. The amount of drug released from each ring can be adjusted by changing the amount and composition of the polymer coating of the drug core, the size of the drug-delivery window, and the number of drug pods in each ring. Vaginal rings containing tenofovir provide in vitro daily release rates spanning 2.5 orders of magnitude (10–4000 mcg/day for tenofovir). The modular nature of the rings permits the release of multiple drugs having very different physicochemical characteristics. To date, in vitro release of tenofovir and acyclovir from the same ring segments has been demonstrated.Citation40 In vivo studies have demonstrated sustained release of tenofovir in the micro-molar range over 14 days in rabbits and 28 days in macaques, with no local inflammation or altered vaginal flora.Citation40 In rabbits, the vaginal tissue tenofovir levels at sacrifice were 3597 ± 1678 ng/g. In the macaques, vaginal biopsies proximal and distal to the inserted rings at days 7 and 21 measured tenofovir levels at 76 ± 54 mcg/g, over 100 times the IC 50 of tenofovir (2 μM). The data demonstrates the feasibility of a 1-month tenofovir microbicide ring for women.
Similar to the pod-insert vaginal ring, rod and tablet-insert vaginal rings comprise one or more polymeric solid dosage forms (lyophilized polymer gel rods and directly compressed tablets, respectively) inserted into a silicone elastomer ring.Citation41 While the ring acts primarily as a retainer for the solid dosage inserts, it may also be used to load and deliver other microbicide candidates compatible with conventional permeation-controlled release mechanisms. Lyophilized inserts may be prepared by freeze-drying microbicide-loaded aqueous gel formulations. This technology is potentially useful for the production of solid dosage inserts containing peptide- and protein-based microbicide candidates, since it does not involve high temperatures, and the water-free insert serves to stabilize the active biomolecule. Once administered, the gel is slowly reconstituted by imbibing vaginal fluid, leading to sustained release of the active agent. Similar lyophilized tablets have recently been reported for the formulation of dapivirine.Citation42 The compressed tablet-insert strategy makes use of traditional direct-compression tableting technology to manufacture conventional sustained-release solid dosage forms.
Given the regulatory constraints, very few polymer materials apart from silicone elastomer and PEVA have been considered, to date, for the fabrication of vaginal rings. Matrix-type rings constructed from styrene-butadiene block copolymers have previously been evaluated for the sustained release of 17β-estradiol for the treatment of postmenopausal symptoms.Citation43 The use of polymers that control drug release by mechanisms other than permeation might be useful for the delivery of hydrophilic and/or large molecular weight actives. Vaginal rings constructed from biosoluble acacia gum, a nonbiodegradable hydrogel of 2-hydroxyethyl methacrylate, and sodium methacrylate, designed to provide sustained release for up to 28 days, have been reported for the simultaneous release of combination antiretroviral HIV microbicides and for combinations of microbicides with nonhormonal contraceptives.Citation44,Citation45
A microbicide-releasing diaphragm
The SILCS diaphragm has been under development by the Program for Appropriate Technology in Health since 1994 as an advanced, single-sized, cervical-barrier contraceptive device ().Citation46–Citation51 Similar in format to a reservoir-type vaginal ring device, SILCS contains a specially designed flexible polymeric spring core (made from nylon-6), which is over-molded with silicone elastomer to form the barrier sheath. In preliminary clinical studies, SILCS was found to be safe; and it performed as well as the standard latex diaphragm in preventing sperm from reaching the cervix when both devices were used with the spermicide nonoxynol-9. The contraceptive efficacy of SILCS is currently being evaluated in combination use with the contraceptive gel BufferGel®. If protection is similar to the standard diaphragm, these data will provide the basis for an application to the US Food and Drug Administration.
Figure 5 The SILCS diaphragm. (A) The thermoplastic spring core consists of nylon-6 in the original SILCS device and polyoxymethylene in the dapivirine-releasing device. (B) Spring core with over-molded silicone elastomer spacers, for positioning the spring core within the injection molds during the final overmolding step. (C) Finished diaphragm device, formed by overmolding the spring core + spacers with silicone elastomer. (D) Design features of the SILCS diaphragm. (E) Photograph of the SILCS diaphragm.
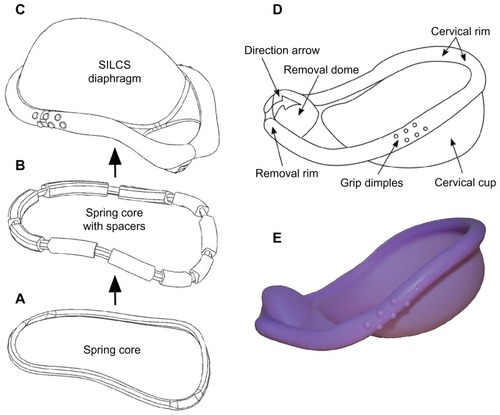
Given the similarity of structure between the nonmedicated SILCS device and a reservoir-type, drug-releasing vaginal ring (ie, both contain a central polymeric core and an over-molded polymer sheath), and the newly emerging emphasis on the development of multipurpose prevention strategies,Citation52–Citation54 the incorporation of a microbicide delivery function into SILCS is currently being actively pursued.Citation55 A modified SILCS device comprising an injection-molded polyoxymethylene spring core, loaded with up to 20% dapivirine and over-molded with the standard silicone elastomer material, has been shown to provide constant in vitro daily release rates during continuous testing over 6 months.Citation56
Manufacturing and scale-up
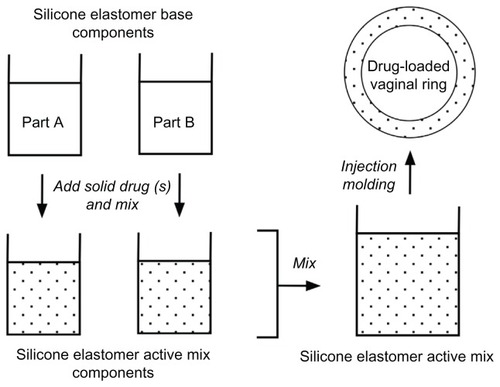
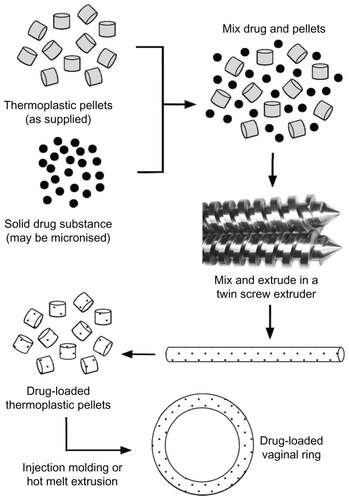
The general methods for the manufacture of silicone elastomer and thermoplastic vaginal rings, outlined in and respectively, involve the incorporation of the solid drug active(s) into the polymer, followed by injection-molding or extrusion into precision-machined ring-holds to form the final device. Depending on the format and complexity of the ring product design, there may be several distinct steps in the manufacturing process. Although the polymer-processing techniques of injection molding and extrusion are ubiquitous in the manufacturing of plastic parts for a wide variety of everyday applications (including various medical devices), the availability of contract manufacturers capable of handling the regulatory issues, manufacturing complexities, and raw material supplies associated with drug-delivery devices is very limited. These issues have been discussed previously,Citation57 and are particularly pertinent, given the progress of the 25 mg dapivirine ring to Phase III clinical testing. If the MTN 020 trial () successfully demonstrates the efficacy of the 25 mg dapivirine matrix ring in reducing the incidence of HIV transmission, then several hurdles will need to be overcome before commercial launch, including accurate projected forecast demand and significant expansion of manufacturing capacity to meet global needs.
Disclosure
The authors report no conflicts of interest in this work.
References
- FolkmanJLongDMJrRosenbaumRSilicone rubber: a new diffusion property useful for general anesthesiaScience196615437451481495922861
- FolkmanJLongDMThe use of silicone rubber as a carrier for prolonged drug therapyJ Surg Res19644313914214130164
- DziukPJCookBPassage of steroids through silicone rubberEndocrinology19667812082115948426
- DuncanGWThe Upjohn CompanyMedicated devices and methodsUnited States patentUS 35454391281970
- HoffmanASThe origins and evolution of “controlled” drug delivery systemsJ Control Release2008132315316318817820
- GuptaKMPearceSMPoursaidAEPolyurethane intravaginal ring for controlled delivery of dapivirine, a nonnucleoside reverse transcriptase inhibitor of HIV-1J Pharm Sci200897104228423918338805
- JohnsonTGuptaKFabianJAlbrightTKiserPSegmented polyurethane intravaginal rings for the sustained combined delivery of antiretroviral agents dapivirine and tenofovirEur J Pharm Sci201039420321219958831
- ClarkMRKiserPFLoxleyAMcConvilleCMalcolmRKFriendDRPharmacokinetics of UC781-loaded intravaginal ring segments in rabbits: a comparison of polymer matricesDrug Deliv Transl Res201113238246
- KaurMGuptaKMPoursaidAEEngineering a degradable polyurethane intravaginal ring for sustained delivery of dapivirineDrug Delivery Trans Res201113223237
- ColasACurtisJSilicone biomaterials: history and chemistryBiomaterials Science: An Introduction to Materials in Medicine2nd edElsevierAcademic Press20048085
- CurtisJColasAMedical applications of siliconesRatnerBDHoffmanASSchoenFJLemonsJEBiomaterials Science: An Introduction to Materials in Medicine2nd edElsevierAcademic Press2004698707
- Al-AzzawiFBucklerHMfor the United Kingdom Vaginal Ring Investigator GroupComparison of a novel vaginal ring delivering estradiol acetate versus oral estradiol for relief of vasomotor menopausal symptomsClimacteric20036211812712841882
- BarentsenRvandeWeijerPHMSchramJHNContinuous low dose estradiol released from a vaginal ring versus estriol vaginal cream for urogenital atrophyEur J Obstet Gynecol Reprod Biol199771173809031963
- BucklerHAl-AzzawiFfor the UK VR Multicentre Trial GroupThe effect of a novel vaginal ring delivering oestradiol acetate on climacteric symptoms in postmenopausal womenBrit J Obstet Gynecol20031108753759
- SperoffLEfficacy and tolerability of a novel estradiol vaginal ring for relief of menopausal symptomsObstet Gynecol2003102482383414551014
- CroxattoHBBracheVMassaiRFeasibility study of Nestorone-ethinylestradiol vaginal contraceptive ring for emergency contraceptionContraception2006731465216371294
- KumarNKoideSSTsongYSundaramKNestorone: a progestin with a unique pharmacological profileSteroids20006510–1162963611108869
- FraserISWeisbergEBracheVSerum Nestorone and ethinyl estradiol levels, and ovulation inhibition in women using three different dosage combinations of a Nestorone progestogen-ethinyl estradiol contraceptive vaginal ring on a bleeding-signaled regimenContraception2005721404515964291
- SivinIMishellDRJrAlvarezFContraceptive vaginal rings releasing Nestorone and ethinylestradiol: a 1-year dose-finding trialContraception200571212212915707562
- MalcolmKWoolfsonDRussellJAndrewsCIn vitro release of nonoxynol-9 from silicone matrix intravaginal ringsJ Control Release200391335536412932713
- Van DammeLRamgeeGAlvaryMEffectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trialLancet2002360933897197712383665
- RoddyREZekengLRyanKA controlled trial of nonoxynol 9 film to reduce male-to-female transmission of sexually transmitted diseasesN Engl J Med19983395045109709043
- MalcolmRKWoolfsonADTonerCFLong-term, controlled release of the HIV microbicide TMC120 from silicone elastomer vaginal ringsJ Antimicrob Chemother200556595495616155060
- RomanoJVarianoBCoplanPVan RoeyJDouvilleKRosenbergZSafety and availability of dapivirine (TMC120) delivered from an intravaginal ringAIDS Res Hum Retroviruses200925548348819388819
- NelASmytheSYoungKSafety and pharmacokinetics of dapivirine delivery from matrix and reservoir intravaginal rings to HIV-negative womenJ Acquir Immune Defic Syndr200951441642319623693
- Di FabioSVan RoeyJGianniniGInhibition of vaginal transmission of HIV-1 in hu-SCID mice by the non-nucleoside reverse transcriptase inhibitor TMC120 in a gel formulationAIDS200317111597160412853741
- HerrewegeYVMichielsJVan RoeyJIn vitro evaluation of nonnucleoside reverse transcriptase inhibitors UC-781 and TMC120-R147681 as human immunodeficiency virus microbicidesAntimicrob Agents Chemother200448133733914693562
- WoolfsonADElliottGRGilliganCAPassmoreCMDesign of an intravaginal ring for the controlled delivery of 17 beta-estradiol as its 3-acetate esterJ Control Release199961331932810477804
- WoolfsonADMalcolmRKGallagherRJDesign of a silicone reservoir intravaginal ring for the delivery of oxybutyninJ Control Release200391346547612932723
- WoolfsonADMalcolmRKGormanSPJonesDSBrownAFMcCullaghSDSelf-lubricating silicone elastomer biomaterialsJ Mater Chem2003131024652470
- MalcolmRKMcCullaghSDWoolfsonADGormanSPJonesDSCuddyJControlled release of a model antibacterial drug from a novel self-lubricating silicone biomaterialJ Control Release200497231332015196758
- Promadej-LanierNSmithJSrinivasanPDevelopment and evaluation of a vaginal ring device for sustained delivery of HIV microbicides to non-human primatesJ Med Primatol200938426327119476564
- FetherstonSGeerLVeazeyRSPartial protection against multiple RT-SHIV162P3 vaginal challenge of rhesus macaques by a silicone elastomer vaginal ring releasing the NNRTI MC1220J Antimicrob Chemother
- MalcolmRKVeazeyRSGeerLSustained release of the CCR5 inhibitors CMPD167 and maraviroc from vaginal rings in rhesus macaquesAntimicrob Agents Chemother201256522522258
- SulakPJSmithVCoffeeAFrequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring: a randomized controlled trialObstets Gynecol20081123563571
- LoxleyAMitchnickMOkohOEthylene vinyl acetate intravaginal rings for the simultaneous delivery of the antiretroviral UC781 and contraceptive levonorgestrelDrug Delivery Trans Res201113247255
- ClarkMRJohnsonTJMcCabeRTA hot-melt extruded intravaginal ring for the sustained delivery of the antiretroviral microbicide UC781J Pharm Sci2012101257658721976110
- BaumMMButkyavicheneIGilmanJAn intravaginal ring for the simultaneous delivery of multiple drugsJ Pharm Sci201210182833284322619076
- KellerMJMaloneAMCarpenterCASafety and pharmacokinetics of aciclovir in women following release from a silicone elastomer vaginal ringJ Antimicrob Chemother20126782005201222556381
- MossJAMaloneAMSmithTJSimultaneous delivery of tenofovir and acyclovir via an intravaginal ringAntimicrob Agents Chemother201256287588222123689
- MorrowRJWoolfsonADDonnellyLSustained release of proteins from a modified vaginal ring deviceEur J Pharm Biopharm201177131021055465
- WoolfsonADUmrethiaMLKettVLFreeze-dried, mucoadhesive system for vaginal delivery of the HIV microbicide, dapivirine: optimisation by an artificial neural networkInt J Pharm201038813614320045043
- VartiainenJWahlströmTNilssonCGEffects and acceptability of a new 17 beta-oestradiol-releasing vaginal ring in the treatment of postmenopausal complaintsMaturitas19931721291378231904
- HanYASinghMSaxenaBBDevelopment of vaginal rings for sustained release of nonhormonal contraceptives and anti-HIV agentsContraception200776213213817656183
- SaxenaBHanYFuDSustained release of microbicides by newly engineered vaginal ringsAIDS200923891792219381077
- YangCCMaravillaKRKilbourne-BrookMAustinGMagnetic resonance imaging of SILCS diaphragm: anatomical considerations and corroboration with clinical fitContraception200776323824417707723
- CoffeyPSKilbourne-BrookMBracheVCochónLComparative acceptability of the SILCS and Ortho ALL-FLEX® diaphragms among couples in the Dominican RepublicContraception200878541842318929740
- CoffeyPSKilbourne-BrookMWear and care of the SILCS diaphragm: experience from three countriesSex Health20107215916420465980
- CoffeyPSKilbourne-BrookMBeksinskaMThongkrajaiEShort-term acceptability of a single-size diaphragm among couples in South Africa and ThailandJ Fam Plann Reprod Health Care200834423323618854068
- SchwartzJLBallaghSACreininMDSILCS diaphragm: postcoital testing of a new single-size contraceptive deviceContraception200878323724418692615
- MoenchTRChipatoTPadianNSPreventing disease by protecting the cervix: the unexplored promise of internal vaginal barrier devicesAIDS200115131595160211546933
- FriendDIntravaginal rings: controlled release systems for contraception and prevention of transmission of sexually transmitted infectionsDrug Deliv Transl Res201113185193
- FriendDDoncelGCombining prevention of HIV-1, other sexually transmitted infections and unintended pregnancies: development of dual-protection technologiesAntiviral Res201088Suppl 1S47S5421109068
- ThurmanARClarkMRDoncelGFMultipurpose prevention technologies: biomedical tools to prevent HIV-1, HSV-2, and unintended pregnanciesInfect Dis Obstet Gynecol Epub 2011 Aug 9
- MajorILowryDMalcolmKDevelopment of a microbicide-releasing diaphragm as an HIV prevention strategyConf Proc IEEE Eng Biol Soc201010891092
- MajorIBoydPKilbourne-BrookMA modified SILCS contraceptive diaphragm for long-term controlled release of the HIV microbicide dapivirineContraception
- MalcolmRKEdwardsKLKiserPRomanoJSmithTJAdvances in microbicide vaginal ringsAntiviral Res201088Suppl 1S30S3921109066
- AravantinouMSingerRDerbyNThe nonnucleoside reverse transcription inhibitor MIV-160 delivered from an intravaginal ring, but not from a carrageenan gel, protects against simian/human immunodeficiency virus-RT infectionAIDS Res Human Retrovir2012In press