Abstract
Objective
To explore the correlation of female vaginal microbiota and immune factors with cervical cancer.
Methods
The distribution pattern difference of vaginal microbiota of four groups of women (cervical cancer, HPV-positive CIN, HPV-positive non-CIN, and HPV-negative groups) were compared by microbial 16S rDNA sequencing. The protein chip was used to detect the composition and changes of the immune factors in the four groups.
Results
Alpha diversity analysis demonstrated that the diversity of the vaginal microbiota was increased as the disease develops. Among those bacteria abundant in the vaginal microbiota, Lactobacillus, Prevotella, and Gardnerella dominate at the genus level of vaginal flora. Compared with the HPV-negative group, the differentially dominant bacteria, such as Prevotella, Ralstonia, Gardnerella and Sneathia, are enriched in the cervical cancer group. Likewise, Gardnerella, Prevotella, and Sneathia are more in the HPV-positive CIN group, while Gardnerella and Prevotella in the HPV-positive non-CIN group, respectively. In contrast, Lactobacillus and Atopobium are dominant in the HPV-negative group (LDA>4log10). The concentration of inflammatory immune factors IP-10 and VEGF-A were increased in the cervical cancer group (P < 0.05), compared with other groups.
Conclusion
The occurrence of cervical cancer is related to an increase of vaginal microbiota diversity and up-regulation of inflammatory immune factor proteins. The abundance of Lactobacillus was decreased while the one of Prevotella and Gardnerella were increased in the cervical cancer group, compared with other three groups. Moreover, the IP-10 and VEGF-A were also increased in the cervical cancer group. Thus, evaluation of changes in the vaginal microbiota and these two immune factor levels might be a potential non-invasive and simple method to predict cervical cancer. Furthermore, it is significant to adjust and restore the balance of vaginal microbiota and maintain normal immune function in preventing and treating cervical cancer.
Introduction
Cervical cancer (CC) is the fourth most common cancer globally and the fourth leading cause of cancer death in women, with an estimated 604,000 new cases and 342,000 deaths worldwide in 2020,Citation1 about 28% of the globally new cases occur in China.Citation2 Persistent infection with high-risk human papillomavirus (HR-HPV) is the major cause of cervical cancer.Citation3–7
Recent studies have shown that the vaginal microbiota plays a key role in the persistent infection of HPV and the development of cervical cancer. HPV infection can lead to a disorder of the vaginal microbiota,Citation8–10 increasing the risk of cervical cancer.Citation11,Citation12
Other data indicate that vaginal microbiota also plays a vital role in regulating immune response,Citation13,Citation14 and the imbalance of vaginal microbiota can easily cause the disorder of the vaginal and cervical cell cycle and the destruction of immune defense function.Citation14–16 Over time, this immune disorder may develop into a chronic inflammatory state, increasing proinflammatory cytokines and accelerating the occurrence and development of cervical lesions and cervical cancer.Citation17–19 Various inflammatory cytokines, including IFN-γ,Citation20 TGF-β1,Citation21 IL-2,Citation22 IL-6,Citation23 IL-10,Citation23,Citation24 and IL-12,Citation25 have different trends in different degrees of cervical lesions and have certain predictive significance for disease progression and prognosis. However, no single factor with significant change and clear guiding significance for treatment exists among these various factors.
Therefore, we hope to find the vaginal microbiota and immune factors closely related to the occurrence and development of cervical cancer by exploring the changes in vaginal microbiota and immune factors in different populations and analyzing the influence of vaginal microbiota and immune factors in the vaginal microecology, so as to provide a reference for the prevention and treatment of cervical cancer.
Materials and Methods
Subjects
A total of 320 female patients who underwent gynecological examination in the First Affiliated Hospital of Chongqing Medical University in China from January 2021 to May 2021 were selected as subjects. Inclusion criteria: (1) over 20 years old, with sexual behavior; (2) patients did not use antibiotics and vaginal flushing within one month; (3) patients had no sex life in the last week; (4) signed written informed consent. Exclusion criteria: (1) in pregnancy or lactation; (2) in the menstrual period; (3) with long-term use of gonadal hormone; (4) history of cervical surgery; (5) long-term use of immunosuppressants or corticosteroid hormones. According to the cervical cancer screening tests (HPV testing and ThinPrep liquid-based cytology test (TCT)) and biopsy pathology, the subjects were divided into the cervical cancer group (CC, n = 80), the HPV-positive CIN group (CIN, n = 80), the HPV-positive non-CIN group (Ctrl HPV (+), n = 80) and the HPV-negative group (HPV negative with normal cytology, Ctrl HPV (-), n=80). However, the construction of the sample library has failed in 3 cases, one case in the CC, one case in the CIN, and one case of Ctrl HPV(-). Cell samples were collected in 2.5 mL of cell preserve solution (Tellgen Corporation, Shanghai, China) for HPV DNA testing. The TellgenplexTM HPV DNA Test can identify 27 HPV types, including 14 HR-HPV (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68). This trial was registered at the Chinese Clinical Trial Registry (www.chictr.org.cn) (trial registration number ChiCTR2200060479, First registration time: 03 / 06 / 2022).
Vaginal Secretion Samples
The vagina is exposed with a sterile speculum and a sterile swab is placed on the lateral wall of the vagina in the posterior vaginal fornix and rotated repeatedly to fully absorb the secretions. The sterile swab was carefully removed, placed back into the collection tube, and quickly transferred to −80°C for subsequent detection of the vaginal microbiota. After collecting the vaginal secretions, 16S rDNA sequencing was performed by Longsee biomedical corporation, China.
DNA Extraction and 16S rDNA Sequencing
DNA was extracted by using DNA extraction kits (Longsee biomedical corporation, China). Extracted DNA was amplified by PCR to construct a sequencing library targeting the V3–V4 region of the bacterial 16S rRNA gene. PCR reactions were performed using 11.5μL of DNA template, 12.5μL of 2X Kapa HiFi Hotstart ready mix, 0.5μL of 10μM primers 341F (5′-CCTACGGGNGGCWGCAG-3′) and 785R (5′-GACTACHVGGGTATCTAATCC-3′). The PCR products were sequenced using the Illumina® MiSeq platform.
Sequencing Data Analysis
Bioinformatic analysis of the bacterial 16S rDNA amplicon data was conducted using a custom QIIME2 software pipeline (https://qiime2.org). The main analyses include Alpha diversity analysis, analysis of the relative abundance of the vaginal microbiota, and LEfSe (Linear discriminant analysis Effect Size) analysis.
Serum Samples
A 5mL blood of peripheral blood of the subjects was extracted using blood vessels without anticoagulant. After resting the collecting vessels vertically for half an hour, the serum was isolated using an overspeed centrifuge. Subsequent serum immune factors detection was performed.
Immune Factor Detection
The amount of immune factors in serum was quantitatively measured using a protein chip (Human Cytokine Factor Panel 1 kit, ThermoFisher). A total of 320 patients’ serum samples were divided into 4 groups, with 80 samples each. In each group, every 20 samples were pooled as a larger group sample and used for the detection of the content of immune factors comprehensively. A total of 45 immune factors were detected, including nuclear factor (NF)-κB, tumor necrosis factor, interleukin (IL-6), IL-8, macrophage inflammatory protein 3, IL-1α, IL-1β, interferon, etc. The detection steps included antigen curing, antigen–antibody reaction, marker color development, reading reader, and result determination.
Statistical Analysis
Since microbial abundance data are usually not normal statistics, we analyzed using the nonparametric test (Kruskal–Wallis H-test). Two independent sample t-test was used to compare the measurement data satisfying normality, and Kruskal–Wallis Rank Sum Test and ANOVA test was used for multigroup continuous variables. Statistical significance was indicated by p values < 0.05.
Results
The Cervical Cancer Group Has the Highest Microbial Diversity
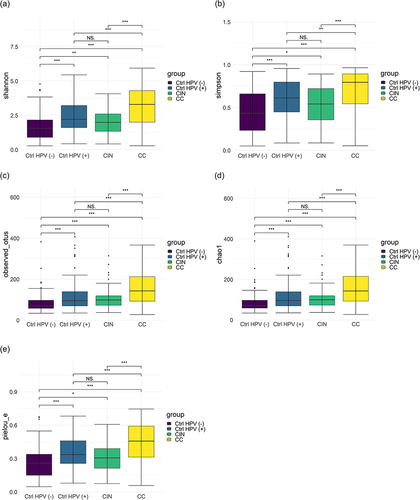
The patient characteristics and demographic information are summarized in Table S1. The mean age of each group was 54.1 ± 10.5 years in the CC group and 39.3 ± 9.6 years in the CIN group, the Ctrl HPV (+) group was 43.3 ± 11.2 years, and in the Ctrl HPV (-) group 40.4 ± 10.2 years. Specifically, the age distribution was significantly different between the CC and the Ctrl HPV (-) groups (P<0.001). BMI did not significantly differ among the groups (P>0.05). Microbiota diversities in the samples were characterized using α diversity analysis, represented by different diversity indexes including Shannon, Simpson, Observed OTUs, Chao1, and Pielou evenness (). Compared with the ones of Ctrl HPV (-) group, these 5 indexes in the CC, the CIN, and the Ctrl HPV (+) groups are significantly higher (P<0.05), and the ones in the CC group are the highest, which suggests that the CC group carries the highest microbial diversity and the lowest dominant species. There is minimally, if any, difference of these values between the CIN group and the Ctrl HPV (+) group (). In addition, the diversity of vaginal microbiota is increased upon the disease stages, while the diversity of dominant species is decreased with the severity of the disease.
Table 1 The Statistics of the α-Diversity Analyses
Figure 1 The α-diversity of vaginal microbiota in the four groups. (a) Shannon; (b) Simpson; (c) Observed OTUs; (d) Chao1; (e) Pielou_e. Larger values indicate greater sample diversity; *p < 0.05, **p < 0.01, ***p < 0.001, NSp > 0.05.
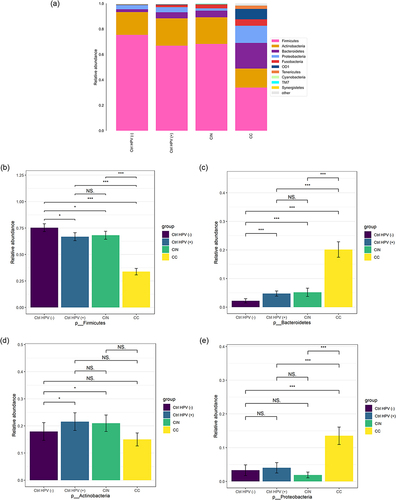
With the Severity of Cervical Lesions, the Abundance of Firmicutes Decreased and the Abundance of Bacteroidetes Increased
To specify composition of the microbial colonies of each group, we analyzed the relative abundance of the vaginal microbiota in the four groups. shows that Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria are dominant in these four groups at the phyla level. Among them, the relative abundance of Firmicutes was the lowest in the CC but the highest in the Ctrl HPV (-), and inbetween for the CIN and the Ctrl HPV (+) group. Of note, the relative abundance of Firmicutes is decreased as the disease stage goes by. Besides, the differences between the CIN and the Ctrl HPV (+) are not statistically significant (). On the contrary, the relative abundance of Bacteroidetes is increased gradually with the aggravation of lesions (). Different from Firmicutes and Bacteroidetes, the relative abundance of Actinobacteria is higher in the CIN and the Ctrl HPV (+) than the Ctrl HPV (-) (), suggesting that HPV infection might be responsible for this change in Actinobacteria. Lastly, Proteobacteria were the only dominant in CC when compared to Ctrl HPV (-) (), which indicates that Proteobacteria has an upward trend in the later stage of lesions.
Figure 2 Column charts show the relative abundances of species at the phylum level. (a) Relative abundance of the top ten species at the phylum level. (b–e) Comparison of relative abundance of (b) Firmicutes, (c) Bacteroidetes, (d) Actinobacteria, (e) Proteobacteria in four groups. *p < 0.05, ***p < 0.001, NSp > 0.05.
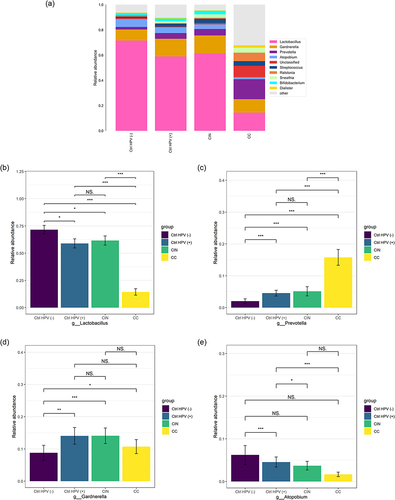
Abundance Changes of Lactobacillus, Prevotella and Gardnerella Upon the Severity of Cervical Lesions
The main strains of the vaginal microbiota were mainly Lactobacillus, Prevotella, and Gardnerella at the genus level for the four tested groups (). The features of the relative abundance of Lactobacillus were largely consistent with its behavior at the phylum level (Firmicutes), eg the lowest in the CC, the highest in the Ctrl HPV (-) (). The relative abundance of Prevotella was also reflected by its phylum level of Bacteroidetes. This relative abundance is increased gradually with the aggravation of the lesion degree (). For Gardnerella, it is similar to Actinobacteria. The Gardnerella abundance levels in the CC, the CIN, and the Ctrl HPV (+) are higher than the ones in the Ctrl HPV (-), with a statistical significance of P < 0.05. We found that the relative abundance of Gardnerella in the CC is lower than in the CIN (). In addition, Atopobium, as a genus of Actinobacteria, has a higher presence in the Ctrl HPV (-) than in the other three groups. Only the comparison between the Ctrl HPV (+) and the Ctrl HPV (-) was statistically significant, but not others (). This might be related to transient changes in the vaginal microecological environment caused by HPV infection, insufficient sample size, or individual differences.
Figure 3 Column charts show the relative abundances of species in the 4 groups at the genus level. (a)Relative abundance of the top ten species at the genus level. (b–e) Comparison of relative abundance of (b) Lactobacillus, (c) Prevotella, (d) Gardnerella, (e) Atopobium in the four groups. *p < 0.05, **p < 0.01, ***p < 0.001, NSp > 0.05.
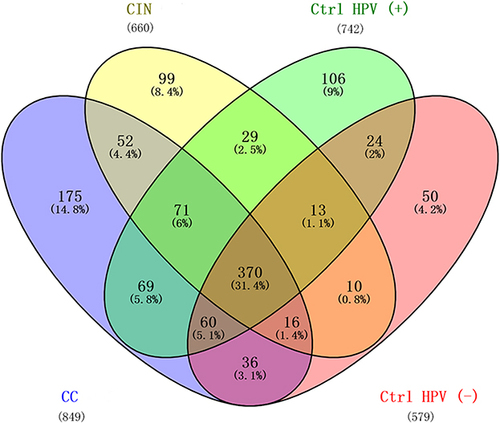
The Cervical Cancer Group Has the Most Significant Number of Unique Microbiota
To evaluate the similarities in the datasets, the number of shared or unique microbiota was compared among the groups. The four groups share 31.4% (370/1200) of the identified microbiota, and the cervical cancer group has the most significant number of unique microbiota (14.8%, n=175) (). This result supports the result of α diversity analysis.
The Differential Dominant Bacteria Features in the Four Groups
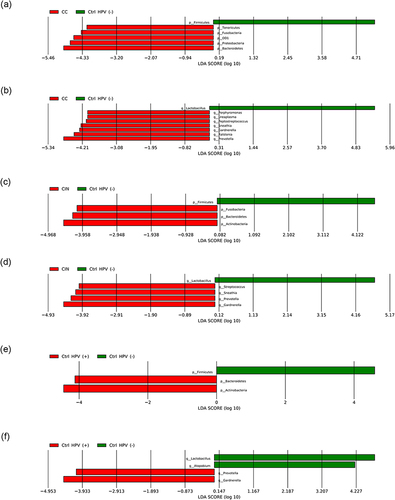
Linear discriminant analysis Effect Size (LEfSe) analysis was used to characterize the potential microbial markers with specific disease phenotypes. Firstly, a large number of differential bacteria were identified by setting the threshold for the logarithmic LDA model score of the discriminating features to 2.0 (Figures S1 and S2). To improve the significance of our analysis, LDA cut-off score was reset to 4 to select differential bacteria for further discussion at phylum or genus levels. When compared with the Ctrl HPV (-), the differential dominant bacteria in the CC mainly include Bacteroidetes, Proteobacteria, OD1, Fusobacteria, and Tenericutes at the phylum level (); and Prevotella, Ralstonia, Gardnerella, Sneathia, Peptostreptococcus, Ureaplasma and Porphyromonas at the genus level (). Differently, the differential dominant bacteria in the CIN mainly include phylum-level Actinobacteria, Bacteroidetes, and Fusobacteria (); and genus-level Gardnerella, Prevotella, Sneathia and Streptococcus (). As the control, the Ctrl HPV (-) carries Firmicutes at the phylum level ( and ) and Lactobacillus at the genus level ( and ). For the Ctrl HPV (+), the differential dominant ones are mainly Actinobacteria and Bacteroidetes at the phylum level (); and Gardnerella and Prevotella at the genus level (). The Ctrl HPV (-) is still represented by Firmicutes (), or Lactobacillus and Atopobium (). In summary, our data shows that the CC has the most differential dominant bacteria and the most unique microbiota the CIN and the Ctrl HPV (+) have similar differential dominant bacteria, with no obvious differences between the two groups, in consistent with the results of α diversity analysis.
Figure 5 LEfSe histogram (LDA > 4). (a, c and e) Differential dominant bacteria between the groups at the phylum level. (b, d and f) Differential dominant bacteria between the groups at the genus level.
Comparison of Vaginal Immune Factors in Different Groups
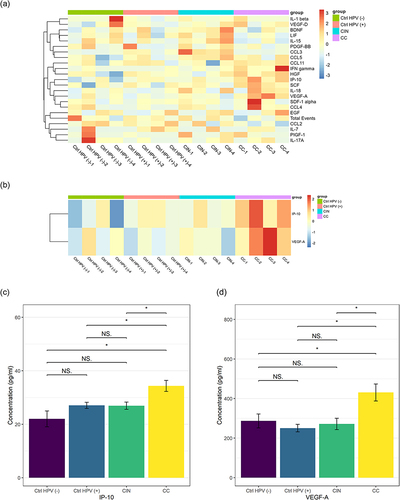
For each tested group, 80 patients’ blood samples were assayed by protein chip analysis. To simplify the process, every 20 samples were pooled as a mixed new sample for the detection of the immune factors comprehensively (). We found that the most significant immune factors are IP-10 and VEGF-A ( and ). The differential comparison of IP-10 and VEGF-A in the CC versus the CIN, the Ctrl HPV (+), and the Ctrl HPV (-) were illustrated, respectively, in . Of note, the concentration of IP-10 and VEGF-A are significantly higher in the CC than in any other group. However, no significant immune factors were found in the CIN or the Ctrl HPV (+) versus the Ctrl HPV (-), and the CIN versus the Ctrl HPV (+) ( and ). This indicates that the immune factors in the body change little before the stage of cervical cancer, while the overall immune state of the body is significantly changed at cervical cancer stage, during which IP-10 and VEGF-A are significantly upregulated. Current studies have not denied that HPV infection and precancerous lesions may cause changes in local immune status.
Table 2 Comparison of Vaginal Immune Factors in Different Groups
Table 3 Statistics for the Comparison of IP-10 and VEGF-A Between the Groups
Figure 6 IP-10 and VEGF-A upregulated in the cervical cancer group. (a) Heat maps of the concentrations of 22 immune factors in the four groups. (b) Heat maps of the concentrations of IP-10 and VEGF-A in the four groups. (c) The concentration of IP-10 in the four groups. (d) The concentration VEGF-A in the four groups. *p < 0.05, NSp > 0.05.
Discussion
Homeostasis of vaginal microbiota and local immune systematic changes play a vital role in cervical cancer development. Our study demonstrated that the microbial diversity increases along with the progression of cervical lesions. We determined the relative abundance of bacteria at different stages of cervical cancer. The cancer stage has the highest microbiota diversity and that Lactobacillus, Prevotella, and Gardnerella dominate the cervix and vagina flora of women with different health statuses. Of them, cervical cancer favors Prevotella but not Lactobacillus. Meanwhile, high abudance of Gardnerella is observed in cervical cancer. This finding is supported by previous literature that the vaginal microbial diversity is closely related to HPV persistent infectionCitation26,Citation27 and increased severity of lesions.Citation20,Citation28 In addition, high levels of immune factors IP-10 and VEGF-A are shown to be tightly associated with cervical cancer progression.
Prevotella peaked in the cervical cancer group. This raised the question whether changes of immune factor expression is induced by Prevotella. Previous studies have linked the relative abundance of Prevotella to various inflammatory disorders such as bacterial vaginosis, periodontitis and rheumatoid arthritis.Citation29–31 It is suggested that Prevotella-rich environments stimulate dendritic cells (DC) to release interleukin-1b (IL-1b), IL-6, and IL-23.Citation32 Another factor to impact immune factors is Lactobacillus, which plays a protective role in the outcome of cervical cancer.Citation33–35 In vitro studies have shown that certain Lactobacillus species can temper inflammation by reducing IL-6, IL-8, and TNF-α secretion.Citation13 Our protein chip analysis did not identify those cytokines. Instead, we have shown that IP-10 and VEGF-A are correlated with cervical cancer progression. The relation of these two with Prevotella or Lactobacillus will be tested in our future studies using bacteria-treated cervical cancer cells.
The abundance of Gardnerella was the highest in the HPV-positive CIN group. Although Gardnerella did not reach the peak in the cervical cancer group, the abundance of Gardnerella increased significantly after HPV infection, and Gardnerella is closely related to the occurrence and progression of BV,Citation36,Citation37 so it is undeniable that Gardnerella still plays a vital role in the progression of cervical cancer. The increase in Gardnerella is strongly associated with HPV infection. We suggest that taxa related to HPV infection status could be a biomarker to help forecast the risk of developing a persistent HPV infection.
LEfSe analysis showed that the dominant bacteria in the HPV-negative group were mainly Firmicutes at the phylum level and Lactobacillus and Atopobium at the genus level. Related studies have found that the internal vaginal bacteria of healthy women are mainly dominated not only by Lactobacillus and a low abundance of bacteria (such as Gardnerella, Atopobium, etc.).Citation38,Citation39 Di Paola et alCitation33 also found that the signature microbe of HPV persistent infection was Atopobium, while our study showed that Atopobium was the differential dominant bacteria in the HPV-negative group. It may be related to transient changes in the vaginal microecological environment caused by HPV infection, or it may be caused by insufficient sample size and individual differences. We will verify this in subsequent experimental studies.
With the aggravation of cervical lesions, immune factors also change. We found that IP-10 and VEGF-A increased in the cervical cancer group, suggesting that the changes in IP-10 and VEGF-A play a vital role in the progression of cervical cancer.
Interferon-γ (IFN-γ)-induced protein 10 (IP-10 or CXCL-10) is a chemokine involved in the transport of immune cells to inflammatory sites and belongs to the CXC family of chemokines.Citation40 Chemokines can selectively guide the directional migration and intracellular aggregation of leukocyte subsets.Citation41 IP-10 was associated with changes in the vaginal microbiota. Previous studies showed that Prevotella positively correlated with IP-10.Citation42 Lactobacillus can degrade IP-10 or inhibit IP-10 secretion, thus reducing the inflammatory response.Citation43,Citation44 IP-10 are biomarkers for detecting asymptomatic STIs and vaginal dysbiosis (bacterial vaginosis (BV) or intermediate microbiota).Citation45 A possible mechanism for vaginal dysbiosis is the increased production of pro-inflammatory cytokines and chemokines, associated with the increase in pathogenic microbial diversity, contributing to the further recruitment of immune cells and amplifying the inflammatory response.Citation46 Chronic vaginal inflammation and changes in the immune response are related to carcinogenesis.Citation46–48 Elevated IP-10 levels are associated with cancer,Citation49 including breast cancer,Citation50 pancreatic cancer,Citation51 colon cancer,Citation52 and gastric cancer.Citation53 Interestingly, IP-10 may be a pathogenic factor in some diseases and may also play a role in tissue repair.Citation54–56 IP-10 is rarely studied in cervical diseases, and its dual role also guides us to explore it further.
VEGF-A can promote angiogenesis, which is critical for the occurrence, growth, and development of solid tumors.Citation57,Citation58 It can also be involved in tumor immune evasion. VEGF in tumor tissues can avoid tumor immune function by inhibiting the differentiation of antigen-presenting cell-dendritic cells and downregulating its anti-tumor immune system.Citation59 Metabolite secretions of Lactobacillus plantarum may inhibit cancer cell metastasis by suppressing the VEGF-MMP2/9 signaling pathway. VEGF inhibitors can prolong the survival of patients with cervical cancer,Citation60,Citation61 so the development of drugs targeting VEGF and VEGFR has important practical significance for treating cervical cancer.
We also found that during the development of cervical cancer, microbiota changes were earlier than the changes in immune factors. There were obvious changes in the microbiota after HPV infection, especially in the cervical cancer group. However, there was no significant difference in immune factors between the HPV-positive, the CIN group, the HPV-positive non-CIN group, and the HPV-negative group, and only the cervical cancer group IP-10 and VEGF-A were significantly upregulated compared with the other three groups. In other words, the changes in immune factors are mainly reflected in the later stage of cervical cancer, while the microbiota has noticeable changes after HPV infection. Previous studiesCitation11,Citation62,Citation63 only showed a specific correlation between vaginal microbiota and immune status. Our study can largely explain that the change in microbiota is earlier than the change in immune factors. It is likely that HPV infection causes the imbalance of vaginal microbiota and increases microbiota diversity, thus affecting the change of immune factors.
A healthy female vaginal environment is generally associated with low microbial diversity and Lactobacillus colonization.Citation64 Lactobacillus can not only maintain the ecological balance of the vagina but also prevent the growth and proliferation of other pathogenic bacteria by producing lactic acid, hydrogen peroxide (H2O2), and bacteriocin in the vagina, to maintain the integrity of the vaginal mucosal barrier, resist the invasion of viruses and harmful bacteria, and enhance the local anti-infection and anti-tumor effects of the vagina.Citation35,Citation65,Citation66 On the contrary, if the content of dominant bacteria to maintain the vaginal microecological balance decreases, it can cause the pH value to increase, immune function to decline, and then reduce the resistance to HPV, and the risk of HPV invasion will increase. At the same time, self-clearance of HPV was significantly reduced in patients already infected with HPV. The normal immune system plays an immune surveillance role for foreign invading pathogens. Once an abnormal immune system occurs, the pathogenic microbiota cannot be removed in time, which leads to the breakdown of the balance of the patient’s vaginal internal environment and may accelerate the development of cervical cancer.Citation67
Limitations
As the study included only patients from China and the sample size was not large enough, it may not be representative. However, we still believe that analysis of geographically tailored microbiomes and immune factors is warranted. It is believed that as more such studies become available, we will soon be able to pool the potential carcinogenic mechanisms of vaginal microbiota and immune factors worldwide into a more representative conclusion. This will provide a biological basis for the prevention, diagnosis and treatment of cervical cancer.
Conclusions
In conclusion, the occurrence of cervical cancer is related to an increase of vaginal microbiota diversity and up-regulation of inflammatory immune factor proteins. Increasing age may be a risk factor for cervical cancer. With the aggravation of the disease, the content of Lactobacillus decreased, and the content of inflammatory immune factors IP-10 and VEGF-A increased, indicating that the balance of vaginal microbiota is destroyed, the immune function is disordered, and the body’s immune inflammatory factors are increased, thus creating a favorable inflammatory environment for the occurrence of cancer. In addition, with the aggravation of cervical lesion severity, the abundance of Prevotella gradually increased, and the abundance of Prevotella was the highest in cervical cancer, indicating that Prevotella plays a crucial role in the development of cervical cancer. Moreover, the IP-10 and VEGF-A were increased in the cervical cancer group compared with other groups. Thus, the vaginal microbiota and specific immune factors may be a potential non-invasive and simple method for predicting cervical cancer. Furthermore, it is of great significance to adjust and restore the balance of vaginal microbiota and maintain normal immune function in preventing and treating cervical cancer.
Abbreviations
CC, cervical cancer; CIN, cervical intraepithelial neoplasia; HSIL, high-grade squamous intraepithelial lesions; LSIL, low-grade squamous intraepithelial lesion; BV, bacterial vaginosis; HPV, human papillomavirus; HIV, human immunodeficiency virus; TCT, ThinPrep cytology test; OTUs, operational taxonomic units; LEfSe, Linear discriminant analysis Effect Size; α diversity analysis, alpha diversity analysis; IP-10 or CXCL-10, interferon-γ (IFN-γ)-induced protein 10.
Data Sharing Statement
All the sequencing data have been deposited in the NIH sequence read archive (SRA, PRJNA905720). This Sequence Read Archive (SRA) submission has been released on 2023-12-31 and is available at https://dataview.ncbi.nlm.nih.gov/object/PRJNA905720?reviewer=sg60ck12kptla3s5ms01vsib7h. Other datasets are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee and with the 1964 Helsinki Declaration and its later comparable ethical standards. This study was approved by the Ethics Committee of The First Affiliated Hospital of Chongqing Medical University (Ethical number: 2020-787). Written informed consent was obtained from the participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors declare that there are no conflicts of interest in this work.
Acknowledgments
The authors would like to thank the medical staff and patients in the Department of Obstetrics and Gynecology of the First Affiliated Hospital of Chongqing Medical University for supporting this clinical specimen collection.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
- National Health Commission of The People’s Republic of China. Chinese guidelines for diagnosis and treatment of cervical cancer 2018 (English version). Chin J Cancer Res. 2019;31(2):295–305. doi:10.21147/j.issn.1000-9604.2019.02.04
- Crosbie EJ, Einstein MH, Franceschi S, Kitchener HC. Human papillomavirus and cervical cancer. Lancet. 2013;382(9895):889–899. doi:10.1016/S0140-6736(13)60022-7
- Balasubramaniam SD, Balakrishnan V, Oon CE, Kaur G. Key molecular events in cervical cancer development. Medicina. 2019;55(7):384. doi:10.3390/medicina55070384
- Hu Z, Ding M. The precision prevention and therapy of HPV-related cervical cancer: new concepts and clinical implications. Cancer Med. 2018;7(10):5217–5236. doi:10.1002/cam4.1501
- Fan Q, Ting H, Xiao S, et al. HPV-16/18 E6-induced APOBEC3B expression associates with proliferation of cervical cancer cells and hypomethylation of Cyclin D1. Mol Carcinog. 2021;60(5):313–330. doi:10.1002/mc.23292
- Graham SV. The human papillomavirus replication cycle, and its links to cancer progression: a comprehensive review. Clin Sci. 2017;131(17):2201–2221. doi:10.1042/CS20160786
- Chase D, Goulder A, Zenhausern F, Monk B, Herbst-Kralovetz M. The vaginal and gastrointestinal microbiomes in gynecologic cancers: a review of applications in etiology, symptoms and treatment. Gynecol Oncol. 2015;138(1):190–200. doi:10.1016/j.ygyno.2015.04.036
- Mitra A, MacIntyre DA, Marchesi JR, et al. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: what do we know and where are we going next? Microbiome. 2016;4(1):58. doi:10.1186/s40168-016-0203-0
- Norenhag J, Du J, Olovsson M, et al. The vaginal microbiota, human papillomavirus and cervical dysplasia: a systematic review and network meta-analysis. BJOG. 2020;127(2):171–180. doi:10.1111/1471-0528.15854
- Li Y, Yu T, Yan H, et al. Vaginal microbiota and HPV infection: novel mechanistic insights and therapeutic strategies. Infect Drug Resist. 2020;13:1213–1220. doi:10.2147/IDR.S210615
- Champer M, Wong AM, Champer J, et al. The role of the vaginal microbiome in gynaecological cancer. BJOG. 2018;125(3):309–315. doi:10.1111/1471-0528.14631
- Rose WA, McGowin CL, Spagnuolo RA, et al. Commensal bacteria modulate innate immune responses of vaginal epithelial cell multilayer cultures. PLoS One. 2012;7(3):e32728. doi:10.1371/journal.pone.0032728
- Nicolò S, Tanturli M, Mattiuz G, et al. Vaginal lactobacilli and vaginal dysbiosis-associated bacteria differently affect cervical epithelial and immune homeostasis and anti-viral defenses. Int J Mol Sci. 2021;22(12):6487. doi:10.3390/ijms22126487
- Sommer F, Bäckhed F. The gut microbiota--masters of host development and physiology. Nature Rev. 2013;11(4):227–238. doi:10.1038/nrmicro2974
- Brinkman JA, Hughes SH, Stone P, et al. Therapeutic vaccination for HPV induced cervical cancers. Dis Markers. 2007;23(4):337–352. doi:10.1155/2007/245146
- Borgdorff H, Gautam R, Armstrong SD, et al. Cervicovaginal microbiome dysbiosis is associated with proteome changes related to alterations of the cervicovaginal mucosal barrier. Mucosal Immunol. 2016;9(3):621–633. doi:10.1038/mi.2015.86
- Libby EK, Pascal KE, Mordechai E, Adelson ME, Trama JP. Atopobium vaginae triggers an innate immune response in an in vitro model of bacterial vaginosis. Microbes Infect. 2008;10(4):439–446. doi:10.1016/j.micinf.2008.01.004
- Vitkauskaite A, Urboniene D, Celiesiute J, et al. Circulating inflammatory markers in cervical cancer patients and healthy controls. J Immunotoxicol. 2020;17(1):105–109. doi:10.1080/1547691X.2020.1755397
- Audirac-Chalifour A, Torres-Poveda K, Bahena-Román M, et al. Cervical microbiome and cytokine profile at various stages of cervical cancer: a pilot study. PLoS One. 2016;11(4):e0153274. doi:10.1371/journal.pone.0153274
- You X, Wang Y, Meng J, et al. Exosomal miR‑663b exposed to TGF‑β1 promotes cervical cancer metastasis and epithelial‑mesenchymal transition by targeting MGAT3. Oncol Rep. 2021;45(4):12. doi:10.3892/or.2021.7963
- Valle-Mendiola A, Gutiérrez-Hoya A, Lagunas-Cruz C, et al. Pleiotropic effects of IL-2 on cancer: its role in cervical cancer. Mediators Inflamm. 2016;2016:2849523. doi:10.1155/2016/2849523
- Bonin-Jacob CM, Almeida-Lugo LZ, Puga MAM, et al. IL-6 and IL-10 in the serum and exfoliated cervical cells of patients infected with high-risk human papillomavirus. PLoS One. 2021;16(3):e0248639. doi:10.1371/journal.pone.0248639
- Brooks DG, Trifilo MJ, Edelmann KH, et al. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12(11):1301–1309. doi:10.1038/nm1492
- Chen X, Han S, Wang S, et al. Interactions of IL-12A and IL-12B polymorphisms on the risk of cervical cancer in Chinese women. Clin Cancer Res. 2009;15(1):400–405. doi:10.1158/1078-0432.CCR-08-1829
- Kyrgiou M, Mitra A, Moscicki AB. Does the vaginal microbiota play a role in the development of cervical cancer? Transl Res. 2017;179:168–182. doi:10.1016/j.trsl.2016.07.004
- Salas-Jara MJ, Ilabaca A, Vega M, García A. Biofilm forming lactobacillus: new challenges for the development of probiotics. Microorganisms. 2016;4(3):35. doi:10.3390/microorganisms4030035
- Manca S, Upadhyaya B, Mutai E, et al. Milk exosomes are bioavailable and distinct microRNA cargos have unique tissue distribution patterns. Sci Rep. 2018;8(1):11321. doi:10.1038/s41598-018-29780-1
- Anahtar MN, Byrne E, Doherty K, et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity. 2015;42(5):965–976. doi:10.1016/j.immuni.2015.04.019
- Scher JU, Sczesnak A, Longman RS, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife. 2013;2:e01202. doi:10.7554/eLife.01202
- Berezow AB, Darveau RP. Microbial shift and periodontitis. Periodontology. 2011;55(1):36–47. doi:10.1111/j.1600-0757.2010.00350.x
- Larsen JM. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology. 2017;151(4):363–374. doi:10.1111/imm.12760
- Di Paola M, Sani C, Clemente AM, et al. Characterization of cervico-vaginal microbiota in women developing persistent high-risk human papillomavirus infection. Sci Rep. 2017;7(1):10200. doi:10.1038/s41598-017-09842-6
- Wang KD, Xu DJ, Wang BY, Yan DH, Lv Z, Su JR. Inhibitory effect of vaginal lactobacillus supernatants on cervical cancer cells. Probiotics Antimicrob Proteins. 2018;10(2):236–242. doi:10.1007/s12602-017-9339-x
- Chen Y, Qiu X, Wang W, et al. Human papillomavirus infection and cervical intraepithelial neoplasia progression are associated with increased vaginal microbiome diversity in a Chinese cohort. BMC Infect Dis. 2020;20(1):629. doi:10.1186/s12879-020-05324-9
- Lev-Sagie A, Goldman-Wohl D, Cohen Y, et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat Med. 2019;25(10):1500–1504. doi:10.1038/s41591-019-0600-6
- Ling Z, Kong J, Liu F, et al. Molecular analysis of the diversity of vaginal microbiota associated with bacterial vaginosis. BMC Genom. 2010;11:488. doi:10.1186/1471-2164-11-488
- Rosca AS, Castro J, Sousa LGV, Cerca N. Gardnerella and vaginal health: the truth is out there. FEMS Microbiol Rev. 2020;44(1):73–105. doi:10.1093/femsre/fuz027
- Ravel J, Moreno I, Simón C. Bacterial vaginosis and its association with infertility, endometritis, and pelvic inflammatory disease. Am J Obstet Gynecol. 2021;224(3):251–257. doi:10.1016/j.ajog.2020.10.019
- Ruffilli I, Ferrari SM, Colaci M, et al. IP-10 in autoimmune thyroiditis. Hormone Metabol Res. 2014;46(9):597–602. doi:10.1055/s-0034-1382053
- Kunkel EJ, Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 2002;16(1):1–4. doi:10.1016/S1074-7613(01)00261-8
- Kaur US, Shet A, Rajnala N, et al. High Abundance of genus Prevotella in the gut of perinatally HIV-infected children is associated with IP-10 levels despite therapy. Sci Rep. 2018;8(1):17679. doi:10.1038/s41598-018-35877-4
- Hoermannsperger G, Clavel T, Hoffmann M, et al. Post-translational inhibition of IP-10 secretion in IEC by probiotic bacteria: impact on chronic inflammation. PLoS One. 2009;4(2):e4365. doi:10.1371/journal.pone.0004365
- von Schillde M-A, Hörmannsperger G, Weiher M. Lactocepin secreted by Lactobacillus exerts anti-inflammatory effects by selectively degrading proinflammatory chemokines. Cell Host Microbe. 2012;11(4):387–396. doi:10.1016/j.chom.2012.02.006
- Masson L, Barnabas S, Deese J, et al. Inflammatory cytokine biomarkers of asymptomatic sexually transmitted infections and vaginal dysbiosis: a multicentre validation study. Sex Transm Infect. 2019;95(1):5–12. doi:10.1136/sextrans-2017-053506
- Torcia MG. Interplay among vaginal microbiome, immune response and sexually transmitted viral infections. Int J Mol Sci. 2019;20(2):266. doi:10.3390/ijms20020266
- de Castro-Sobrinho JM, Rabelo-Santos SH, Fugueiredo-Alves RR. Bacterial vaginosis and inflammatory response showed association with severity of cervical neoplasia in HPV-positive women. Diagn Cytopathol. 2016;44(2):80–86. doi:10.1002/dc.23388
- Mastromarino P, Di Pietro M, Schiavoni G, et al. Effects of vaginal lactobacilli in Chlamydia trachomatis infection. Int J Med Microbiol. 2014;304(5–6):654–661. doi:10.1016/j.ijmm.2014.04.006
- Kochumon S, Al-Sayyar A, Jacob T, et al. TNF-α increases IP-10 expression in MCF-7 breast cancer cells via activation of the JNK/c-Jun pathways. Biomolecules. 2021;11(9):1355. doi:10.3390/biom11091355
- Clark AM, Heusey HL, Griffith LG, et al. IP-10 (CXCL10) can trigger emergence of dormant breast cancer cells in a metastatic liver microenvironment. Front Oncol. 2021;11:676135. doi:10.3389/fonc.2021.676135
- Lunardi S, Lim SY, Muschel RJ, et al. IP-10/CXCL10 attracts regulatory T cells: implication for pancreatic cancer. Oncoimmunology. 2015;4(9):e1027473. doi:10.1080/2162402X.2015.1027473
- Shibahara T, Wilcox JN, Couse T, Madara JL. Characterization of epithelial chemoattractants for human intestinal intraepithelial lymphocytes. Gastroenterology. 2001;120(1):60–70. doi:10.1053/gast.2001.20904
- Kim HJ, Song DE, Lim SY, et al. Loss of the promyelocytic leukemia protein in gastric cancer: implications for IP-10 expression and tumor-infiltrating lymphocytes. PLoS One. 2011;6(10):e26264. doi:10.1371/journal.pone.0026264
- Herder C, Baumert J, Thorand B, et al. Chemokines as risk factors for type 2 diabetes: results from the MONICA/KORA Augsburg study, 1984–2002. Diabetologia. 2006;49(5):921–929. doi:10.1007/s00125-006-0190-y
- Christensen JE, de Lemos C, Moos T, Christensen JP, Thomsen AR. CXCL10 is the key ligand for CXCR3 on CD8+ effector T cells involved in immune surveillance of the lymphocytic choriomeningitis virus-infected central nervous system. J Immunol. 2006;176(7):4235–4243. doi:10.4049/jimmunol.176.7.4235
- Bertolino P, Bowen DG, McCaughan GW, Fazekas de St Groth B. Antigen-specific primary activation of CD8+ T cells within the liver. J Immunol. 2001;166(9):5430–5438. doi:10.4049/jimmunol.166.9.5430
- Eichmann A, Simons M. VEGF signaling inside vascular endothelial cells and beyond. Curr Opin Cell Biol. 2012;24(2):188–193. doi:10.1016/j.ceb.2012.02.002
- Yang L, Lin Z, Huang Q, et al. Effect of vascular endothelial growth factor on remodeling of C6 glioma tissue in vivo. J Neurooncol. 2011;103(1):33–41. doi:10.1007/s11060-010-0356-9
- da Silva L, Neves BM, Moura L, Cruz MT, Carvalho E. Neurotensin downregulates the pro-inflammatory properties of skin dendritic cells and increases epidermal growth factor expression. Biochim Biophys Acta. 2011;1813(10):1863–1871. doi:10.1016/j.bbamcr.2011.06.018
- Braicu EI, Gasimli K, Richter R, et al. Role of serum VEGFA, TIMP2, MMP2 and MMP9 in monitoring response to adjuvant radiochemotherapy in patients with primary cervical cancer--results of a companion protocol of the randomized NOGGO-AGO Phase III clinical trial. Anticancer Res. 2014;34(1):385–391.
- Akaza H, Oya M, Iijima M, et al. A large-scale prospective registration study of the safety and efficacy of sorafenib tosylate in unresectable or metastatic renal cell carcinoma in Japan: results of over 3200 consecutive cases in post-marketing all-patient surveillance. Jpn J Clin Oncol. 2015;45(10):953–962. doi:10.1093/jjco/hyv099
- Mitra A, MacIntyre DA, Paraskevaidi M, et al. The vaginal microbiota and innate immunity after local excisional treatment for cervical intraepithelial neoplasia. Genome Med. 2021;13(1):176. doi:10.1186/s13073-021-00977-w
- Mei C, Yang W, Wei X, Wu K, Huang D. The unique microbiome and innate immunity during pregnancy. Front Immunol. 2019;10:2886. doi:10.3389/fimmu.2019.02886
- Zheng JJ, Miao JR, Wu Q, et al. Correlation between HPV-negative cervical lesions and cervical microenvironment. Taiwan J Obstet Gynecol. 2020;59(6):855–861. doi:10.1016/j.tjog.2020.08.002
- Mortaki D, Gkegkes ID, Psomiadou V, et al. Vaginal microbiota and human papillomavirus: a systematic review. J Turk German Gynecol Assoc. 2020;21(3):193–200. doi:10.4274/jtgga.galenos.2019.2019.0051
- Chee WJY, Chew SY, Than LTL. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb Cell Fact. 2020;19(1):203. doi:10.1186/s12934-020-01464-4
- Shannon B, Yi TJ, Perusini S, et al. Association of HPV infection and clearance with cervicovaginal immunology and the vaginal microbiota. Mucosal Immunol. 2017;10(5):1310–1319. doi:10.1038/mi.2016.129