Abstract
Objective
As a common endocrine and metabolic disorder, polycystic ovary syndrome (PCOS) is mostly associated with an obese phenotype. The present research focuses on the clinical significance of miR-379 in obesity-PCOS and attempts to elucidate its potential mechanisms.
Methods
Healthy individuals (n = 46), obesity-PCOS (n = 92), and non-obesity PCOS (n = 31) subjects were enrolled. Quantitative real-time polymerase chain reaction (qRT-PCR) was conducted to examine the level of serum miR-379. The receiver operating characteristic (ROC) curve and logistic regressions were applied to reveal the diagnostic significance. Dual luciferase reporters were performed to validate the targeting relationships. And cell count kit (CCK-8) assay was used to detect cell proliferation.
Results
Serum miR-379 was highly expressed in PCOS patients (P < 0.05), in especially obesity-PCOS patients. Higher miR-379 was associated with greater body mass index (BMI), higher bioavailable testosterone (bT), and greater insulin resistance (IR). Additionally, miR-379 was an independent risk factor for the development of obesity-PCOS. The sensitivity of miR-379 in identifying patients with obesity-PCOS from healthy or non-obesity-PCSO patients was 81.52% and 72.83%, and the specificity was 86.96% and 80.65%. Semaphorin 3 A (SEMA3A) was identified as a target of miR-379 and was reduced in the patients with obesity PCOS (P < 0.05). Inhibition of miR-375 reduced KGN proliferation, but this reduction was partially restored by silencing of SEMA3A (P < 0.05).
Conclusion
Elevated miR-379 assists the diagnosis of obesity-PCOS and regulates the proliferation of KGN by targeting SEMA3A engaged in disease development.
Keywords:
Introduction
Polycystic ovarian syndrome (PCOS) is a heterogeneous disease, characterized by the symptoms of polycystic ovary, hyperandrogenemia, menorrhagia, amenorrhea, and other manifestations. PCOS could last the entire life of a woman and frequently occurs at childbearing age. Women with PCOS are prone to obesity, over half of PCOS patients show signs of obesity, and obesity in turn affects the onset and development of PCOS. Additionally, there is a vicious cycle between fat deposition and hyperandrogenemia in PCOS patients, which induces endocrine disturbance.Citation1 It was also believed that obesity could influence leptin levels and lead to corresponding resistance, which inhibits ovulation by affecting the interaction of insulin-like growth factors, follicle-stimulating hormone, and other factors.Citation2 Therefore, obesity PCOS has become an important research hot point. However, the diagnosis criteria of obesity PCOS have been constantly revised in controversy and there are no definite conclusions yet, due to the unclear etiology, and pathogenesis. The diagnosis of obesity PCOS is always confirmed after the exclusion of hyperandrogenemia- or ovarian dysfunction-related diseases. Therefore, it is an urgent need to explore reliable and effective diagnostic biomarkers for obesity PCOS.
MicroRNAs (miRNAs) could bind with proteins and therefore negatively regulate the transcription and translation of target mRNAs. The function of miRNAs has been widely reported, especially for their roles in human diseases, miRNAs have been considered critical regulators in disease development. Previous studies speculated miRNAs were involved in the onset and regulate the progression of PCOS, where several potential biomarkers were identified, such as miR-199a-5p,Citation3 miR-98-3p,Citation4 and miR-130b-3p.Citation5 The identified candidate miRNAs always showed abnormal expression and could regulate cellular processes during PCOS development. Recently, a number of studies have been devoted to constructing expression profiling in PCOS aiming to explore more potential biomarkers, which lacked data confirmation. For example, Xiao et al established a miRNA expression profile in 75 PCOS women. The differentially expressed miRNAs were demonstrated to be correlated with the menstrual cycle, antral follicle count, and hormone level and showed significance in discriminating PCOS patients.Citation6 A previous sequencing study identified several hyperandrogenism-related abnormally expressed miRNAs in the follicular fluid of PCOS women, where miR-379 was included.Citation7 miR-379 has been demonstrated to be involved in various endocrine diseases, such as insulin resistance,Citation8 and was correlated with fat deposition and obesity-induced kidney injury and liver injury.Citation9–11 Therefore, miR-379 was speculated to also play a role in screening obesity PCOS and serve as a biomarker.
This study focused on the expression and significance of miR-379 in obesity PCOS aiming to identify a novel biomarker candidate for the early detection and risk prediction of obesity PCOS.
Patients and Methods
Patients
A total of 123 PCOS patients were enrolled in this study, including 92 obesity patients (BMI ≥ 25 kg/m2) and 31 non-obesity patients (BMI < 25 kg/m2). Additionally, another 46 healthy individuals were enrolled as the control group.
The diagnosis criteria of PCOSCitation12,Citation13 were: 1) dilute or anovulation; 2) hyperandrogenemia; 3) ultrasound results showed that the number of follicles with a diameter of 2–9 mm ≥ 12 in one or both sides of ovarian or the volume of ovarian ≥ 10 mL; 4) two of the above 3 terms and other hyperandrogenic causes were excluded.
The healthy individuals were non-pregnant women with regular menstrual cycles, normal ovulation, and normal endocrine hormone levels. Patients with ovarian tumors, uterine fibroids, endometriosis, abnormal uterine bleeding, thyroid and adrenal diseases, hypertension, diabetes, hyperprolactinemia, androgen tumors, and other diseases were excluded. Healthy individuals had no pregnancy or hormone use for at least three months before enrollment. All study subjects had signed informed consent and the study had obtained approval from the Ethics Committee of Beijing Tongren Hospital Affiliated to Capital Medical University.
Sample Collection
Fasting venous blood samples were collected on the 2nd-4th day of the menstrual day from all participants. Samples of amenorrhea patients were collected during the period when B ultrasonography showed no dominant follicles. Collected samples were centrifugated at 3000 r/min for 10 min after standing at room temperature for 1 h to obtain the serum. Isolated serum samples were stored at −80°C for the following analyses.
Cell Culture
KGN cell line, a nonluteinized human granulosa cell, was obtained from the Cell Bank of Chinese Academy of Science (Shanghai, China) and cultured in the DMEM culture medium. The DMEM culture medium was supplemented with 10% FBS, 100 μg/mL streptomycin, and 100 IU/mL penicillin. Cell culture was performed at 37°C.
Cell Transfection
Cultured cells were transfected with miR-379 inhibitor for its knockdown using Lipofectamine 2000 (Invitrogen, USA). Cell transfection was performed at room temperature and transfected cells were available for the following experiments after 48 h of transfection.
Real-Time Quantitative PCR
Total RNA of serum samples and cell lines was isolated with TRIzol reagent (Invitrogen, USA) and its concentration and purity were evaluated. Then, cDNA was reversed from extracted RNA with the cDNA synthesis kit (Invitrogen, USA). PCR quantification was performed on the 7500 PCR system (Applied Biosystem, USA) with the help of the SYBR Green master mix according to the manufacturer’s protocols. The relative expression of miR-379 was calculated with the 2–ΔΔCt method with miR-39 (for miR-379) and GAPDH (for SEMA3A) as the internal references.
Cell Proliferation Assay
Transfected cells were seeded into 96-well plates triplicate supplied with a completed culture medium and incubated for a certain period of time. CCK-8 reagent was added to each well at time intervals of 12, 24, 48, 72, and 96 h. Absorbance at 450 nm was measured after 2 h of adding CCK-8 and the OD450-time curve was plotted to evaluate cell growth.
Dual-Luciferase Reporter Assay
Wild-type and mutant-type Semaphorin 3 A (SEMA3A) luciferase reporter vectors were established by cloning corresponding sites predicted by the online database into the pGL3 vector (Promega). Established vectors were co-transfected with miR-379 mimic or inhibitor into KGN cells. The luciferase activity of SEMA3A was detected with Dual-Luciferase Assay System (Promega, USA) with Renilla as the internal reference.
Statistical Analysis
Pairwise comparison was performed on the basic information of study subjects using Student’s t-test (P < 0.05) and ROC analysis was employed to evaluate the potential of miR-379 in the early diagnosis of PCOS. The association of miR-379 with the clinicopathological features of PCOS patients were evaluated by the Chi-square test. The logistic regression analysis was conducted to assess the risk factors for obesity-PCOS. Experimental data were expressed as mean ± SD and analyzed by one-way ANOVA (P < 0.05).
Results
Subject’s Demographic Characteristics and Hormonal Indicators Comparisons
As illustrated in , BMI and LH were significantly higher in the obesity-POCS group than in the healthy group (P < 0.05). Compared to the healthy group, LH, bT, FINS, and HOMA-IR were markedly elevated in the obesity-POCS group and the non-obesity POCS group, and FSH was typically abrogated (P < 0.05). Among them, the levels of LH, FINS, and HOMA-IR were significantly higher in the obesity-POCS group than in the non-obesity-PCOS group (P < 0.05). In contrast, E2, bP, and Glu were non-statistically different in the three groups (P > 0.05).
Table 1 Clinical Characteristics and Sociodemographic Characteristics of PCOS Patients and Healthy People
miR-379 Was Remarkably Elevated in the Patients with Obesity-PCOS
To explore the clinical significance of miR-379 in obesity-PCOS patients, the levels of miR-379 were first examined. Compared with the control group, miR-379 was significantly higher in PCOS patients, with miR-379 significantly higher in the obesity-PCOS group than in the non-obesity-PCOS group (P < 0.001, ). Subsequently, based on the mean value of miR-379 in obesity-PCOS patients were divided into miR-379 high expression group (n = 49) and miR-379 low expression group (n = 44). Additionally, patients with increased miR-379 expression exhibited higher BMI (P = 0.024), bT (P < 0.001), and HOMA-IR (P = 0.014, ) levels, which are of greater concern. The findings suggest that elevated miR-379 may be a function miRNA in obesity-PCOS.
Table 2 Correlation Between miR-379 Levels and Clinical Features in Obesity Patients
Figure 1 Elevated miR-379 is a potential diagnostic biomarker for obesity-PCOS. (A) The expression of miR-379 in three groups was detected by RT-qPCR. (B) ROC was conducted to analyze the diagnostic value of miR-379 in identifying obesity-PCOS patients from the healthy or non-obesity-PCOS group. ****P < 0.0001.
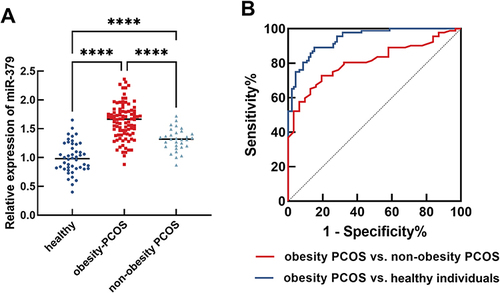
Elevated miR-379 is a Potential Diagnostic Biomarker for Obesity-PCOS
miR-379, BMI, FSH, bT, and HOMA-IR were included in logistic regression to explore potential risk factors for obesity-PCOS. As illustrated in , in addition to BMI (HR=7.362, 95% CI: 2.441–22.202, P < 0.001), miR-379 (HR=7.172, 95% CI: 2.415–21.300, P < 0.001) was also a potential risk factor for the development of obesity-PCOS. ROC curves were employed to assess the diagnostic significance. The AUC of ROC was 0.9414 and the sensitivity and specificity of miR-379 levels to identify obesity-PCOS patients from the healthy group was 81.52% and 86.96% at a cut-off value of 1.295. More importantly, miR-379 levels could significantly differentiate obesity-PCOS patients from non-obesity PCOS patients when the cut-off value was 1.465, and the sensitivity was 72.83% and 80.65% with AUC of 0.8084 (). Overall, miR-379 may be a potential diagnostic biomarker for obesity-PCOS patients.
Table 3 Logsitic Employed to Analyze Risk Factors for the Development of Obesity PCOS
SEMA3A is a Target Gene of miR-379 in Obesity-PCOS
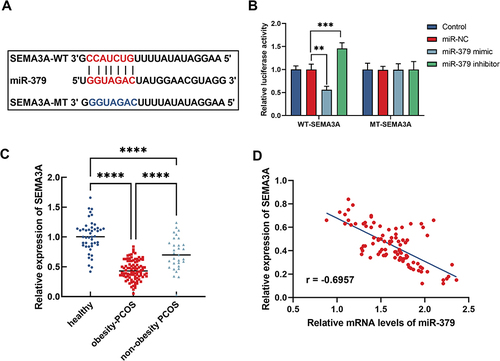
To further explore the molecular mechanism of miR-379 in obesity-PCOS, downstream targets were attempted to be searched. Bioinformatics revealed a partial binding sequence of SEMA3A to miR-379 (). The dual-luciferase report assay confirmed that miR-379 mimic significantly inhibited the luciferase activity of WT-SEMA3A, while miR-379 inhibitor significantly promoted the luciferase activity of WT-SEMA3A (P < 0.05), but neither of them had a significant effect on the luciferase activity of MT-SEMA2A (P > 0.05, ). What’s more, SEMA3A was weakly-expressed in obesity-PCOS patients than in non-obesity-PCOS patients and healthy (P < 0.05, ). SEMA3A expression was also negatively correlated with SEMA3A levels in obesity-PCOS patients (r = −0.6957, ).
Figure 2 SEMA3A is a target gene of miR-379 in obesity-PCOS. (A) Sequence fragment of SEMA3A bound to miR-379. (B) Dual luciferase reporter validates targeted binding between miR-379 and SEMA3A. (C) Expression of SEMA5A in the three groups was detected by RT-qPCR. (D) Correlation analysis between miR-379 and SEMA3A in obesity-PCOS patients. ****P < 0.0001; ***P < 0.001; **P < 0.01.
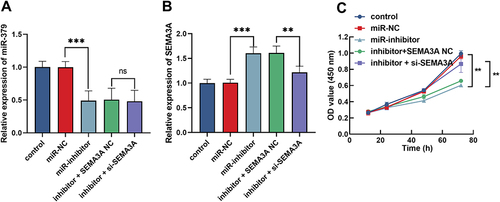
The Inhibition of KGN Proliferation by Reduced miR-379 Was Typically Attenuated by Silencing SEMA3A
Co-transfection of si-SEMA3A with miR-379 inhibitor was performed in the KGN cell. As illustrated in , miR-379 levels were typically suppressed by miR-379 inhibitor (P < 0.05) but had no effect on SEMA3A expression (P > 0.05). Furthermore, miR-379 inhibitor typically increased the level of SEMA3A, but this increase was attenuated by silencing of SEMA3A (P < 0.05, ). Meanwhile, CCK-8 confirmed that inhibition of miR-379 significantly impaired KGN proliferation, but this impairment was partially restored by silencing of SEMA3A (P < 0.05, ).
Figure 3 miR-379 affects the proliferation of KGN by regulating SEMA3A. (A) Effect of miR-379 inhibitor and si-SEMA3A on miR-379 levels in KGN detected by RT-qPCR. (B) Effect of miR-379 inhibitor and si-SEMA3A on SEMA3A levels in KGN detected by RT-qPCR. (C) CCK-8 assay was employed to examine the proliferation of KGN cells function of miR-375 and SEMA3A. ***P < 0.001,**P < 0.01.
Discussion
More than half of patients with PCOS are reported to have concomitant signs of obesity. The co-morbidity between obesity as a risk factor for the development of PCOS has long been observed in the clinical setting.Citation14 Moderate weight loss (5%) has been reported to significantly improve the reproductive, hyperandrogenic, and metabolic characteristics of PCOS.Citation15 The pathological changes of PCOS are complex, and the exact pathogenesis and etiology remain unclear, while genetic mutations and epigenetic alterations are among the main cause of PCOS pathogenesis. IR is reported to be the pathological basis of obese PCOS, where reduced insulin sensitivity leads to islet dysfunction and excessive estrogen secretion by the ovaries. Additionally, obesity affects the production of hormones and metabolites associated with follicular development, which in turn affects egg quality.Citation16 Therefore, obesity plays an integral role in the pathogenesis of PCOS. In the current study, we enrolled healthy control, non-obesity-PCOS, and obesity-PCOS. The results showed that androgen levels and glucose metabolism index levels were significantly higher in the patients with obesity-PCOS than in the non-obesity-PCOS and controls, and they showed more serious endocrine disorders and metabolic abnormalities. And this is consistent with the findings of Yang et al.Citation17
Differentially expressed miRNAs are present in the serum, follicular fluid, GCs, and adipose tissue of PCOS patients.Citation18 miRNAs can be engaged in the development of PCOS by regulating insulin sensitivity, androgen synthesis, and follicular development.Citation19 Such as miR-182,Citation20 miR-664a-3p,Citation21 and miR-24.Citation22 MiR-379 is identified as an obesity-associated miRNA that regulates the development of obesity through multiple signaling pathways or metabolic modalities, thereby influencing the development of disease. Abdollahi et al found reduced obesity in miR-379 knock mice on a high-fat diet and that miR-379 regulates IR and obesity by suppressing adipogenesis in endoplasmic reticulum stress.Citation8 Additionally, miR-379 accelerates obesity-induced kidney injury through endoplasmic reticulum stress.Citation10 As an obesity-related metabolic syndrome, NAFLD can be typically ameliorated by the lack of miR-379 through the regulation of steatosis and hyperlipidemia.Citation11 Furthermore, miR-379 has been shown to be a key regulator of glucose metabolismCitation23 and lipid metabolism.Citation24
More notable was the discovery of miR-379 in an investigation of dysregulated miRNAs associated with hyperandrogenemia in the follicular fluid of PCOS.Citation7 Therefore, we suspect that miR-379 may be implicated in the progression of PCOS and may be associated with obesity-PCOS. To test our speculation, we examined the expression of miR-379 in the subjects. The results confirmed that miR-379 levels were elevated in PCOS patients compared with healthy controls. Among those, miR-379 levels were higher in obesity-PCOS patients. Hyperandrogenism and hyperinsulinemia are the main pathological causes of PCSO, and they are associated with abnormal obesity.Citation25 In the current study, we found that high miR-379 expression was associated with larger BMI, higher androgenic, and greater IR in PCOS patients. Further exploration of the potential clinical value of mIR-379 in obesity-PCOS revealed that miR-379 is a potential risk factor for obesity PCOS along with BMI. miR-379 can also identify obesity-PCOS patients from healthy individuals or non-obesity-PCOS patients, demonstrating a high diagnostic significance. It is suggested that miR-379 is expected to provide favorable assistance in the early identification and early intervention management of obesity-PCOS. The significant association of miR-379 with obesity, however, whether it could differentiate obesity-PCOS patients and obesity non-PCOS patients has not been disclosed in the present study, which needs further investigation with expanding study subjects.
SEMA3A is a secreted protein in the semaphoring family that has been found to disrupt hormone secretion through its multiple molecular mechanisms. SEMA3A and its receptor mutations were greatly enriched in 982 severely obese patients.Citation26 SEMA3A was also found that estrogen-induced the expression of SEMA3A in osteoblasts.Citation27 Additionally, the targeted binding of miR-379 to SEMA3A was demonstrated in autoimmune uveitis.Citation28 The targeted binding of SEMA3A to miR-379 was identified in our study. And SEM3A3 was abnormally reduced in obesity-PCOS and was negatively correlated with miR-379 levels. Therefore, we speculate that miR-379 may be engaged in the progression of obesity-PCOS by regulating SEMA3A. KGN was reported to be closely associated with oocytes and its survival and proliferation may be the cause of PCOS.Citation29 To further confirmed our conjecture, we validated the commonly used cell line KGNCitation30 in an in vitro PCOS model construct. We found no significant effect on SEMA3A levels when miR-379 was suppressed, but significantly promoted SEMA3A levels, and this promotion was partially eliminated by silencing of SEMA3A, suggesting that SEMA3A may be a downstream gene of miR-379. Inhibition of miR-379 significantly impaired KGN proliferation, but this impairment was partially restored by silencing SEMA3A.
All in all, current results indicate that miR-379 is typically elevated in obesity-PCOS and could function as a potential diagnostic biomarker for it. Mechanistically, miR-379 may weaken SEMA3A levels regulating PCOS progression.
Data Sharing Statement
The data used and analyzed can be obtained from the corresponding author under a reasonable request.
Ethics Approval and Consent to Participate
The experimental procedures were all in accordance with the guideline of the Ethics Committee of Beijing Tongren Hospital Affiliated to Capital Medical University and has approved by the Ethics Committee of Beijing Tongren Hospital Affiliated to Capital Medical University. This study complies with the Declaration of Helsinki. A signed written informed consent was obtained from each patient.
Disclosure
The authors declare that they have no competing interests.
Additional information
Funding
References
- Glueck CJ, Goldenberg N. Characteristics of obesity in polycystic ovary syndrome: etiology, treatment, and genetics. Metabolism. 2019;92:108–120. doi:10.1016/j.metabol.2018.11.002
- Tosi F, Di Sarra D, Kaufman JM, et al. Total body fat and central fat mass independently predict insulin resistance but not hyperandrogenemia in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2015;100(2):661–669. doi:10.1210/jc.2014-2786
- Shao S, Wang H, Shao W, Liu N. miR-199a-5p stimulates ovarian granulosa cell apoptosis in polycystic ovary syndrome. J Mol Endocrinol. 2020;65(4):187–201. doi:10.1530/JME-20-0077
- Hu M, Gao T, Du Y. MiR-98-3p regulates ovarian granulosa cell proliferation and apoptosis in polycystic ovary syndrome by targeting YY1. Med Mol Morphol. 2022;55(1):47–59. doi:10.1007/s00795-021-00307-4
- Bao D, Li M, Zhou D, et al. miR-130b-3p is high-expressed in polycystic ovarian syndrome and promotes granulosa cell proliferation by targeting SMAD4. J Steroid Biochem Mol Biol. 2021;209:105844. doi:10.1016/j.jsbmb.2021.105844
- Jiang X, Li J, Zhang B, et al. Differential expression profile of plasma exosomal microRNAs in women with polycystic ovary syndrome. Fertil Steril. 2021;115(3):782–792. doi:10.1016/j.fertnstert.2020.08.019
- Xue Y, Lv J, Xu P, et al. Identification of microRNAs and genes associated with hyperandrogenism in the follicular fluid of women with polycystic ovary syndrome. J Cell Biochem. 2018;119(5):3913–3921. doi:10.1002/jcb.26531
- Abdollahi M, Kato M, Lanting L, et al. miR-379 mediates insulin resistance and obesity through impaired angiogenesis and adipogenesis regulated by ER stress. Mol Ther Nucleic Acids. 2022;30:115–130. doi:10.1016/j.omtn.2022.09.015
- Fei X, Jin M, Wang Y, et al. Transcriptome reveals key microRNAs involved in fat deposition between different tail sheep breeds. PLoS One. 2022;17(3):e0264804. doi:10.1371/journal.pone.0264804
- Abdollahi M, Kato M, Lanting L, Wang M, Tunduguru R, Natarajan R. Role of miR-379 in high-fat diet-induced kidney injury and dysfunction. Am J Physiol Renal Physiol. 2022;323(6):F686–F699. doi:10.1152/ajprenal.00213.2022
- Cao C, Duan P, Li W, et al. Lack of miR-379/miR-544 cluster resists high-fat diet-induced obesity and prevents hepatic triglyceride accumulation in mice. Front Cell Dev Biol. 2021;9:720900. doi:10.3389/fcell.2021.720900
- Franks S. Controversy in clinical endocrinology: diagnosis of polycystic ovarian syndrome: in defense of the Rotterdam criteria. J Clin Endocrinol Metab. 2006;91(3):786–789. doi:10.1210/jc.2005-2501
- Al Wattar BH, Fisher M, Bevington L, et al. Clinical practice guidelines on the diagnosis and management of polycystic ovary syndrome: a systematic review and quality assessment study. J Clin Endocrinol Metab. 2021;106(8):2436–2446. doi:10.1210/clinem/dgab232
- Liu Q, Zhu Z, Kraft P, Deng Q, Stener-Victorin E, Jiang X. Genomic correlation, shared loci, and causal relationship between obesity and polycystic ovary syndrome: a large-scale genome-wide cross-trait analysis. BMC Med. 2022;20(1):66. doi:10.1186/s12916-022-02238-y
- Barber TM, Hanson P, Weickert MO, Franks S. Obesity and polycystic ovary syndrome: implications for pathogenesis and novel management strategies. Clin Med Insights Reprod Health. 2019;13:1179558119874042. doi:10.1177/1179558119874042
- Wu L, Fang Q, Wang M, et al. Effect of weight loss on pregnancy outcomes, neuronal-reproductive-metabolic hormones and gene expression profiles in granulosa cells in obese infertile PCOS patients undergoing IVF-ET. Front Endocrinol. 2022;13:954428. doi:10.3389/fendo.2022.954428
- Yang X, Wang K, Lang J, et al. Up-regulation of miR-133a-3p promotes ovary insulin resistance on granulosa cells of obese PCOS patients via inhibiting PI3K/AKT signaling. BMC Womens Health. 2022;22(1):412. doi:10.1186/s12905-022-01994-6
- Yang Y, Lang P, Zhang X, et al. Molecular characterization of extracellular vesicles derived from follicular fluid of women with and without PCOS: integrating analysis of differential miRNAs and proteins reveals vital molecules involving in PCOS. J Assist Reprod Genet. 2023;40(3):537–552. doi:10.1007/s10815-023-02724-z
- Prayitno GD, Lestari K, Sartika CR, et al. Potential of mesenchymal stem cells and their secretomes in decreasing inflammation markers in polycystic ovary syndrome treatment: a systematic review. Medicines (Basel). 2022;10(1). doi:10.3390/medicines10010003
- Xu L, Xiong F, Bai Y, et al. Circ_0043532 regulates miR-182/SGK3 axis to promote granulosa cell progression in polycystic ovary syndrome. Reprod Biol Endocrinol. 2021;19(1):167. doi:10.1186/s12958-021-00839-5
- He M, Mao G, Xiang Y, et al. MicroRNA-664a-3p inhibits the proliferation of ovarian granulosa cells in polycystic ovary syndrome and promotes apoptosis by targeting BCL2A1. Ann Transl Med. 2021;9(10):852. doi:10.21037/atm-21-1614
- Yuanyuan Z, Zeqin W, Xiaojie S, Liping L, Yun X, Jieqiong Z. Proliferation of ovarian granulosa cells in polycystic ovarian syndrome is regulated by MicroRNA-24 by targeting Wingless-Type Family Member 2B (WNT2B). Med Sci Monit. 2019;25:4553–4559. doi:10.12659/MSM.915320
- Kato M, Abdollahi M, Tunduguru R, et al. miR-379 deletion ameliorates features of diabetic kidney disease by enhancing adaptive mitophagy via FIS1. Commun Biol. 2021;4(1):30. doi:10.1038/s42003-020-01516-w
- de Guia RM, Rose AJ, Sommerfeld A, et al. microRNA-379 couples glucocorticoid hormones to dysfunctional lipid homeostasis. EMBO J. 2015;34(3):344–360. doi:10.15252/embj.201490464
- Zhang H, Wang W, Zhao J, et al. Relationship between body composition, insulin resistance, and hormonal profiles in women with polycystic ovary syndrome. Front Endocrinol. 2022;13:1085656. doi:10.3389/fendo.2022.1085656
- van der Klaauw AA, Croizier S, Mendes de Oliveira E, et al. Human semaphorin 3 variants link melanocortin circuit development and energy balance. Cell. 2019;176(4):729–42 e18. doi:10.1016/j.cell.2018.12.009
- Hayashi M, Nakashima T, Yoshimura N, Okamoto K, Tanaka S, Takayanagi H. Autoregulation of osteocyte Sema3A orchestrates estrogen action and counteracts bone aging. Cell Metab. 2019;29(3):627–37 e5. doi:10.1016/j.cmet.2018.12.021
- Li M, Gao X, Liu K, Bao N, Jiang Z. MiR-379-5p aggravates experimental autoimmune uveitis in mice via the regulation of SEMA3A. Autoimmunity. 2021;54(5):275–283. doi:10.1080/08916934.2021.1931841
- Zhong Z, Li F, Li Y, et al. Inhibition of microRNA-19b promotes ovarian granulosa cell proliferation by targeting IGF-1 in polycystic ovary syndrome. Mol Med Rep. 2018;17(4):4889–4898. doi:10.3892/mmr.2018.8463
- Deng J, Li C, Luo J, Xie J, Peng C, Deng X. MicroRNA-125b controls growth of ovarian granulosa cells in polycystic ovarian syndrome by modulating cyclin B1 expression. Arch Med Sci. 2022;18(3):746–752. doi:10.5114/aoms.2019.85809
