Abstract
Vulvar intraepithelial neoplasia (VIN), the precursor lesion of vulvar squamous cell carcinoma (VSCC), may present as pruritic or asymptomatic lichenified plaques surrounded by single or multiple discrete or confluent macules or papules. VIN is divided into high-grade squamous intraepithelial lesion (HSIL), which is human papillomavirus (HPV)-driven, and differentiated VIN (DVIN), which develops independently of HPV. Histopathological examination and HPV genotyping polymerase chain reaction (PCR) tests should be performed to distinguish between HSIL and DVIN. Lichenified plaques surrounded by multiple papules are found not only in VIN but also in vulvar lichen simplex chronicus (LSC). This chronic inflammatory skin disease mostly appears in labia majora and is triggered by sweating, rubbing, and mental stress. IHC staining of p16 and p53 are recommended as the most commonly used biomarkers for VIN in diagnostically challenging cases. IHC staining is also beneficial to confirm the accuracy of the HPV detection technique, as p16-negative staining may also represent a false-positive result. We report a case of the importance of p16 and p53 IHC staining in diagnosing vulvar LSC mimicking VIN with false-positive HPV-66. The patient was previously diagnosed with VIN based on clinical examination. HPV-66 was detected by PCR from a vulvar biopsy sample. Histopathological examination revealed stromal lymphocytic infiltration with non-specific chronic dermatitis; neither atypia nor koilocyte was observed. Both p16 and p53 IHC staining were negative. The patient was diagnosed and treated as vulvar LSC with 10 mg cetirizine tablet, emollient, and 0.1% mometasone furoate cream. Clinical improvement was observed as the lesions became asymptomatic hyperpigmented macules in the 4 weeks of follow-up, without recurrence after 3 years of follow-up. Both p16 and p53 IHC staining might help distinguish HSIL and DVIN mutually and from other vulvar mimics in diagnostically challenging cases.
Introduction
In 1982, the term vulvar intraepithelial neoplasia (VIN) was introduced by Crum et al,Citation1 replacing the confusing array of terms used at the time to describe precursor lesions of vulvar squamous cell carcinoma (VSCC).Citation2 The World Health Organization (WHO) classified VIN into high-grade squamous intraepithelial lesions (HSIL) and differentiated VIN (DVIN) according to the different pathogenesis of VSCC. HSIL covers all human papillomavirus (HPV)-associated intraepithelial lesions. In contrast, DVIN refers to HPV-independent lesions that generally develop in the context of dermatoses.Citation3 Therefore, histopathological examination and HPV genotyping polymerase chain reaction (PCR) are required to distinguish between HSIL and DVIN.Citation4
Human papillomaviruses (HPVs) are small, non-enveloped, epitheliotropic, double-stranded deoxyribose nucleic acid (DNA) viruses and are primarily found in sexually active women and men. The virus consists of several genotypes, which are further categorized as low-risk HPV (LR-HPV) and high-risk HPV (HR-HPV).Citation5,Citation6 LR-HPV genotypes, including HPV-6, 11, 40, 42, 43, 44, 54, 61, 70, 72, and 81, cause genital warts and low-grade squamous intraepithelial lesions (LSIL) that seldom progress to cancerous lesions. HR-HPV, including HPV-16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 66, play a crucial role in the pathophysiology of either precancerous HSIL type of VIN 5 or malignancies on the cervix, anus, oropharynx, vulva, and penis.Citation6 However, some literatures report that the inclusion of HR-HPV-66 in the HPV Array Test is an ongoing source of false-positive results.Citation7,Citation8 Additionally, in areas other than the cervix, such as the vulva, head, or neck, HPV infection cannot be reliably diagnosed by the detection of HPV DNA alone. Even de Sanjose et alCitation9 reported that HPV-66 was not detected in among 509 cases of HPV-positive VIN from 39 countries. Thus, further test such as p16 immunohistochemistry (IHC) is requiredCitation10 as a surrogate marker for HR-HPV E7 transforming gene activity to confirm HSIL diagnosis.Citation11
The clinical manifestation of VIN, which presents as multiple raised papules or plaques that tend to coalesceCitation12 overlying the lichenified plaque, might share a similar pattern with that of lichen simplex chronicus (LSC).Citation13,Citation14 LSC is a chronic inflammatory disorder that can involve the vulva.Citation14 Vulvar LSC can be identified as papules, plaques, and lichenified plaques that are commonly present in labia majora but can also involve labia minora and perineum.Citation14,Citation15 Vulvar LSC is closely associated with a poorer quality of life, declined mental well-being, sexual function, and damage to the skin due to repeated scratching.Citation16 Moreover, rather than sharing similar clinical pattern,Citation13,Citation14 vulvar LSC might also progress into DVIN, an HPV-independent form of VIN.Citation3 Therefore, in diagnosing vulvar lesions suspicious of VIN, in addition to histopathological examination,Citation11 p53 IHC is also recommended as a biomarker of DVIN.Citation3,Citation11
Distinguishing vulvar lesions between HPV-related and HPV-independent precursors have important implications for treatment and prognosis,Citation3 as DVIN is more likely than HSIL to progress to cancer.Citation2,Citation4 Treatment options for HSIL include imiquimod, laser, and surgical excision,Citation2,Citation12 whereas surgical excision is the only treatment for DVIN.Citation2,Citation17 Underdiagnosed VIN may harbor an occult life-threatening invasive carcinoma,Citation17 while overdiagnosed VIN might cause various harms, including psychological stress, anxiety, and physical injuries due to unnecessary procedures and follow-up.Citation18 Histopathological examination is the most crucial step in diagnosing vulvar lesions but may not be representative in some cases.Citation17 In diagnostically challenging cases, IHC staining of p16 and p53 are recommended as the most commonly used biomarkers for VIN.Citation19,Citation20 This case report presents the importance of p16 and p53 IHC staining in diagnosing vulvar LSC mimicking VIN with false-positive HPV-66.
Clinical Case
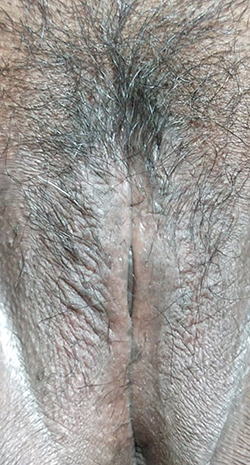
A 40-year-old Indonesian female presented with intensely itchy and coalescing papules and plaques overlying lichenified plaques on the labia majora. The itching worsened when she was angry or anxious. Her vulvar skin condition started 2 months before consultation, initially presenting as dark spots in the labia majora (). One month before the consultation, the spots became thickened plaques and soon felt more itchy, which prompted consultation with a physician at a primary health-care facility, for which she was prescribed vaginal nystatin and oral antibiotics. Two weeks before consultation, coalescing papules and plaques began to appear. The patient was therefore referred to secondary and tertiary dermatology and venereology health-care facilities. The patient had a history of hormonal contraceptive use, frequent wearing of tight clothing, and smoking. She also had a personal and familial history of allergic rhinitis from her mother. Her ex-husband was a promiscuous man with some histories of warts and urethral discharge in his genitals.
Figure 1 Clinical findings as pruritic multiple papules overlying the lichenified plaque prior to treatment.
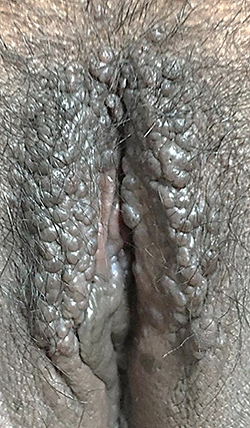
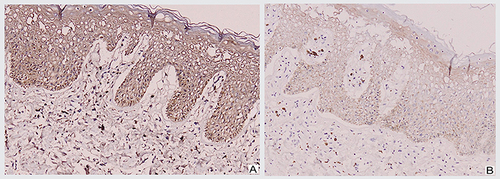
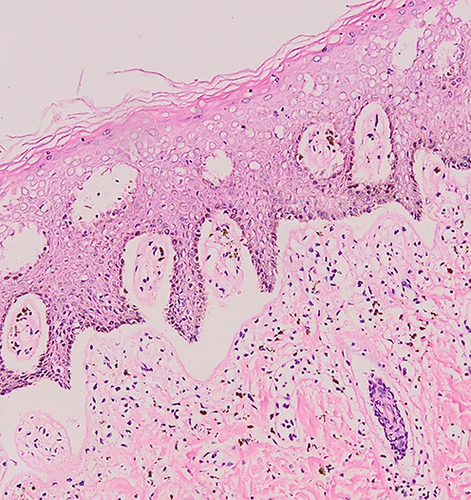
An excision biopsy was performed on the papules overlying the lichenified plaque on her vulva for histopathological examination and HPV genotyping. HPV-66 was detected by PCR. However, histopathological examination revealed stromal lymphocytic infiltration with non-specific chronic dermatitis, and neither atypia nor koilocytes was observed (). IHC of both p16 () and p53 () showed negative results. The patient was treated as vulvar LSC and the HPV-66 found in HPV genotyping PCR was considered a false-positive result. She received a 10 mg cetirizine tablet, emollient, and 0.1% mometasone furoate cream. In the 4 weeks of follow-up, clinical improvement was observed as the lesions became hyperpigmented macules without itching (). There was no recurrence after 3 years of follow-up.
Figure 2 Histopathological examination from the labia majora revealed stromal lymphocytic infiltration.
Discussion
In cases where papules and plaques tend to coalesce overlying a lichenified plaque on the vulva, the differential diagnoses of VIN or vulvar LSC should be considered.Citation4,Citation14 VIN is further classified as HPV-associated HSIL and HPV-negative DVIN.Citation2–4 The incidence of VIN registered in Nordic countries is between 2.5 and 3.1 cases per 100,000 women/year, increasing after the age of 40.Citation20 Risk factors include smoking, immunosuppression, promiscuity, hormonal contraceptives, and early coital age.Citation15 The patient is a 40-year-old female with a smoking habit, a history of hormonal contraceptive usage, and a promiscuous ex-husband.
Approximately 50% of patients with VIN are asymptomatic.Citation21 When symptomatic, the main complaints include itching, pruritus, pain, and dyspareunia.Citation12,Citation21 VIN presents as multiple plaques and papules that tend to coalesce.Citation12 The current patient’s skin lesions present as coalescing multiple raised plaques and papules on the vulva.
The histopathological features of VIN include hyperkeratosis, parakeratosis, hypergranulosis, and elongation of the rete ridges. Cell atypia might be seen as high nuclear-to-cytoplasm ratio,Citation19 hyperchromasia, pleomorphism, and mitoses.Citation4,Citation19 Apoptotic bodies are also common.Citation19 Cell atypia occurring in more than two-thirds of the epithelial thickness is suggestive of HSIL.Citation12 DVIN uniformly demonstrated spongiosis, abnormal maturation in the form of abnormal keratinization close to the base, and basal atypia.Citation19 The histopathological findings in the current patient were hyperkeratosis and spongiosis without atypia.
Distinguishing vulvar lesions between HPV-related and HPV-independent precursors is important, as it is essential in determining treatment and prognosis.Citation3 Many medical treatments, such as imiquimod and photodynamic therapy, have been attempted to avoid surgery in patients with HSIL. Imiquimod is an immune response-modifying drug with antiviral and antitumor activity that induces innate and cell-mediated immunity. In HSIL, the effectiveness of imiquimod depends on an induced immune response to HPV.Citation12,Citation17 Photodynamic therapy (PDT), which brought about 40–60% clearanceCitation22,Citation23 and 48% recurrence rate,Citation24 uses a tumor-localizing photosensitizer, 5-aminolevulinic acid (ALA), in combination with non-thermal light to generate oxygen-induced cell death.Citation12,Citation17 Extensive surgery, such as vulvectomy, is no longer advisable for HSIL.Citation12 The standard of radical vulvectomy has evolved, promoting a conservative and personalized approach.Citation25,Citation26 Surgeons began to implement a less invasive surgery, trying to ensure better aesthetic results without compromising cancer-related outcome.Citation26 Local excisions are surgical options that preserve women’s quality of life, reducing side effects like lymphedema, sexual dysfunction, urinary complications, and psychological compromission.Citation25 Oncologic safety does not seem to be significantly different.Citation25,Citation27 Moreover, Milliken et alCitation27 stated that surgical resection margin of 2–3 mm does not appear to be associated with a higher rate of local recurrence than the widely used limit of 8 mm. Thus, standard therapy for patients with HSIL consists of surgical removal of only the visible lesions to relieve symptoms and prevent the development of invasive disease.Citation12 Surgical treatment can be performed using a cold knife or CO2 laser vaporization as a single technique or in combination. Differentiated VIN is more likely to be associated with invasive disease, and as a precursor of VSCC, it should be detected and treated with surgical excision as soon as possible.Citation12,Citation17 It is essential to obtain a specimen for histological evaluation in order to evaluate stromal invasion. Medical therapies are avoided in DVIN.Citation17
The role of HPV genotyping PCR is worth considering, as partial distinctions between HSIL and DVIN might be helpful to maintain sensitivity to precursor lesions while minimizing overtreatment.Citation7 Human papillomavirus-66 is a rare type of papillomavirus.Citation28 Although the prevalence and distribution of HPV-66 in most studies have depended highly upon the origin of the population involved, in a meta-analysis of carcinomas and intra-epithelial neoplasia of the vulva, vagina, and anus, HPV-66, among other rare types, was found in no more than 0.5% of the anogenital carcinomas tested.Citation28,Citation29 However, some literatures report that the inclusion of HPV-66 in the HPV Array Test is an ongoing source of false-positive results.Citation7,Citation8 HPV-66 was detected on the vulvar biopsy of the patient in this case report.
Characteristically, p16 is diffusely overexpressed in HSIL and carcinomas driven by HR-HPV,Citation3,Citation10,Citation11 and is induced by HPV E6 and E7 of HR-HPV types.Citation4,Citation11 The HPV E6 and E7 proteins bind to and inactivate retinoblastoma protein and p53, which leads to increased proliferation and a compensatory increase in cellular expression of p16.Citation4 Lesions showing strong and diffuse cytoplasmic and nuclear or only cytoplasmic block-like staining were considered as p16-positive, whereas patchy or complete absence of p16 staining was considered as p16-negative.Citation3,Citation10 IHC staining is also beneficial to confirm the accuracy of the HPV detection technique, as p16-negative staining may also represent a false-positive result.Citation10 False-positive tests do not mean that HPV is not present, which has not been reported to be a problem for HPV genotyping PCR. Instead, they refer to the detection of HPV infections that are not associated with the development of VSCC and its precursors.Citation7 Based on the absence of atypia in histopathological findings and p16-negative IHC, the HPV-66 detected in HPV genotyping PCR was considered a false-positive result.
Basal overexpression of p53 is useful for the diagnosis of DVINCitation2,Citation20,Citation30 as a reflection of a missense mutation of the TP53 geneCitation2,Citation30,Citation31 with 97% accuracy.Citation31,Citation32 TP53 is a tumor suppressor gene responsible for identifying DNA damage and preventing damaged cells from progressing through the cell cycle. Missense mutations in TP53 lead to a build-up of the mutated protein, seen as overexpression by IHC. TP53 mutations are found in up to two-thirds of VSCC; thus, p53 IHC has been proposed as a surrogate diagnostic marker for DVIN as its precursor lesion.Citation30 DVIN also typically develops in association with vulvar LSC and lichen sclerosus (LS).Citation2,Citation3,Citation33 Moreover, IHC overexpression of p53 has been reported in not only DVIN but also vulvar LSC and LS.Citation30,Citation33 p53 IHC with definite, usually strong, staining in almost all tumor cell nuclei, or involving more than a single layer of basal-type cellsCitation31,Citation32 is interpreted as p53-positive and commonly found in DVIN.Citation30,Citation33 In contrast, a variable, weak, patchy positive pattern of staining or involving an unexpanded layer of basal cellsCitation33,Citation34 is interpreted as p53-negative and might be observed in vulvar LSC and LS.Citation30,Citation33 The absence of atypia in histopathological examination with negative results for both p16 and p53 IHC contributed to ruling out the diagnosis of VIN and its subtypes, HSIL and DVIN.
The mean age of patients with vulvar LSC in the studies conducted by O’Keefe et alCitation35 was 42 years (with a range of 22–76 years). Vulvar LSC is considered a variant of atopic dermatitis, and patients who are affected often have an atopic diathesis.Citation13–15 In a study conducted by Rajalakshmi et al,Citation13 personal and/or familial history of atopy, consisting of allergic rhinoconjunctivitis, asthma, and atopic dermatitis, was present in 25.7% of patients (n=27). The patient was a 40-year-old female with a personal and familial history of allergic rhinitis originating from her mother.
Lichen simplex chronicus is a manifestation of an itch–scratch cycle.Citation13–16 The common triggering factors for itching were sweating (41.9%), rubbing of thighs while walking for long distances (9.5%), and mental stress (5.7%).Citation13 The triggering factors identified in this patient are the rubbing of thighs due to tight clothing and mental stress.
Lichenified plaque is a key feature for identifying cases of LSC. These lesions were localized plaques in 61.9% of patients and were diffuse in 40% of patients, involving the entire labia majora, labia minora, perineum, or scrotum. Lichenified plaques may also present as lesions surrounded by multiple papules in the margins in 20% of patients, multiple papules overlying the lichenified plaque in 11.4% of patients, and excoriations in the lichenified plaque in 10.4% patients.Citation13 Lichenified plaque lesions were present in this patient.
Histopathologically, vulvar LSC lesions exhibit epidermal thickening, hyperkeratosis, spongiosis, and acanthosis.Citation14,Citation15 LSC also showed irregular elongation of the rete ridges with vertically oriented collagen and mild perivascular lymphocytic infiltration.Citation36 The histopathological features of LSC might also be non-specific, showing only chronic dermatitis.Citation37 Histopathological findings in the current patient revealed stromal lymphocytic infiltration with non-specific chronic dermatitis, supporting the diagnosis of vulvar LSC.
The itch–scratch cycle that perpetuates the symptoms of vulvar LSC should be interrupted by antihistamines. This was also accomplished by applying emollient and high-potency topical corticosteroids.Citation15,Citation16,Citation36 The patient was diagnosed and treated as vulvar LSC and was given 10 mg cetirizine tablet, emollient, and 0.1% mometasone furoate cream. In the 4 weeks of follow-up, clinical improvement was observed, as the patient’s lesion became hyperpigmented macules without itching. There was no recurrence after 3 years of follow-up.
Conclusion
In suspected vulvar precancerous lesions in which clinical, histopathological, and HPV genotyping PCR findings remain ambiguous, p16 and p53 IHC staining might help confirm whether they are VIN or other vulvar mimics.
Ethics Statement
Publication of images was included in the patient’s consent for case publication. Institutional approval from the Research Ethics Committee of Dr. Hasan Sadikin General Hospital Bandung, Indonesia, has been obtained to publish the case details (approval number: LB.02.01/X.6.5/256/2023).
Consent Statement
The authors certify that they have obtained all appropriate patient consent forms. The patient signed a consent form for the publication of case details and images.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The authors would like to thank the staff of the Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran, Bandung, West Java, Indonesia.
References
- Crum CP, Fu YS, Levine RU, Richart RM, Townsend DE, Fenoglio CM. Intraepithelial squamous lesions of the vulva: biologic and histologic criteria for the distinction of condylomas from vulvar intraepithelial neoplasia. Am J Obstet Gynecol. 1982;144(1):77–83. doi:10.1016/0002-9378(82)90398-2
- Heller DS, Day T, Allbritton JI, et al. Diagnostic criteria for differentiated vulvar intraepithelial neoplasia and vulvar aberrant maturation. J Lower Genit Tract Dis. 2021;25(1):57–70. doi:10.1097/LGT.0000000000000572
- Rakislova N, Alemany L, Clavero O, et al. HPV-independent precursors mimicking high-grade squamous intraepithelial lesions (HSIL) of the vulva. Am J Surg Pathol. 2020;44(11):1506–1514. doi:10.1097/PAS.0000000000001540
- Singh N, Gilks CB. Vulval squamous cell carcinoma and its precursors. Histopathol. 2020;76:128–138. doi:10.1111/his.13989
- Mousa M, Al-amri SS, Degnah AA, et al. Prevalence of human papillomavirus in women in Jeddah, Saudi Arabia. Ann Saudi Med. 2019;39(6):403–409. doi:10.5144/0256-4947.2019.403
- Hewavisenti RV, Arena J, Ahlenstiel CL, Sasson SC. Human papillomavirus in the setting of immunodeficiency: pathogenesis and the emergence of the next-generation therapies to reduce the high associated cancer risk. Front Immunol. 2023;14:1–24 doi:10.3389/fimmu.2023.1112513.
- Schiffman M, de Sanjose S. False positive cervical HPV screening test results. Papillomavirus Res. 2019;7:184–187. doi:10.1016/j.pvr.2019.04.012
- Eklund C, Forslund O, Wallin KL, Zhou T, Dillner J. The 2010 global proficiency study of Human Papillomavirus genotyping in vaccinology. J Clin Microbiol. 2012;50(7):2289–2298. doi:10.1128/JCM.00840-12
- de Sanjosé S, Alemany L, Ordi J, et al. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur J Cancer. 2013;49(16):3450–3461. doi:10.1016/j.ejca.2013.06.033
- Nicolas I, Saco A, Barnadas E, et al. Prognostic implications of genotyping and p16 immunostaining in HPV-positive tumors of cervix uteri. Modern Pathol. 2020;33(1):128–137. doi:10.1038/s41379-019-0360-3
- Leeman A, Jenkins D, Marra A, et al. Grading immunohistochemical markers p16 INK4a and HPV E4 identifies productive and transforming lesions caused by low- and high-risk HPV within high-grade anal squamous intraepithelial lesions. Br J Dermatol. 2020;182(4):1026–1033. doi:10.1111/bjd.18342
- Bogliatto F. Vulvar intraepithelial neoplasia. In: Bornstein J, editor. Vulvar Disease: Breaking the Myths. 1st ed. Cham: Springer; 2019:121–128.
- Rajalakhsmi R, Thappa DM, Jaisankar TJ, Nath AK. Lichen simplex chronicus of anogenital region: a clinico-etiological study. Indian J Dermatol Venereol Leprol. 2011;77(1):28–36. doi:10.4103/0378-6323.74970
- Verma SB. Scrotal labia – an uncommon presentation of vulvar lichen simplex chronicus. Indian Dermatol Online J. 2021;12(4):590‑2. doi:10.4103/idoj.IDOJ_896_20
- Mortaki D, Mortakis A. Lichen simplex chronicus. In: Bornstein J, editor. Vulvar Disease: Breaking the Myths. 1st ed. Cham: Springer; 2019:371–378.
- Sanchez A, Gandhi K, Lee B, Garcia JG, Ventolini G. Vulvar neurodermatitis in a postmenopausal African-American patient: a case report. J Menop Med. 2022;28:42–45 doi:10.6118/jmm.21032.
- Preti M, Scurry J, Marchitelli CE, Micheletti L. Vulvar intraepithelial neoplasia. Best Pract Res Clin Obstet Gynaecol. 2014;28(7):1051–1062. doi:10.1016/j.bpobgyn.2014.07.010
- Aitken CA; de Kok IMCM. Striking a Balance: Complete Evaluation of Organised Cervical Cancer Screening Programmes is Not Possible Until Harms of Screening are Better Quantified. Rotterdam: Erasmus University; 2021:1–10.
- Watkins JC, Yang E, Crum CP, et al. Classic vulvar intraepithelial neoplasia with superimposed lichen simplex chronicus: a unique variant mimicking differentiated vulvar intraepithelial neoplasia. Int J Gynecol Pathol. 2019;38(2):175–182. doi:10.1097/PGP.0000000000000509.
- Jeffreys M, Jeffus SK, Herfs M, Quick CM. Accentuated p53 staining in usual type vulvar dysplasia–A potential diagnostic pitfall. Pathol Res Pract. 2018;214(1):76–79. doi:10.1016/j.prp.2017.11.009
- Leonard B, Kridelka F, Delbecque K, et al. A clinical and pathological overview of vulvar condyloma acuminatum, intraepithelial neoplasia, and squamous cell carcinoma. BioMed Res Int. 2014;10:1–11. doi:10.1155/2014/480573
- Hillemanns P, Untch M, Dannecker C, et al. Photodynamic therapy of vulvar intraepithelial neoplasia using 5 aminolevulinic acid. Int J Cancer. 2000;85(5):649–653. doi:10.1002/(SICI)1097-0215(20000301)85:5<649:AID-IJC9>3.0.CO;2-E
- Fehr MK, Hornung R, Degen A, et al. Photodynamic therapy of vulvar and vaginal condyloma and intraepithelial neoplasia using topically applied 5-aminolevulinic acid. Lasers Surg Med. 2002;30(4):273–279. doi:10.1002/lsm.10048
- Hillemanns P, Wang X, Staehle S, et al. Evaluation of different treatment modalities for vulvar intraepithe lial neoplasia (VIN): CO2 laser vaporization, photo dynamic therapy, excision and vulvectomy. Gynecol Oncol. 2006;100(2):271–275. doi:10.1016/j.ygyno.2005.08.012
- Gianini A, D’Oria O, Chiofalo B, et al. The giant steps in surgical downsizing toward personalized treatment of vulvar cancer. J Obstet Gynaecol Res. 2022;48(3):535–540. doi:10.1111/jog.15103
- Panici PB, Tomao F, Domenici L, et al. Prognostic role of inguinal lymphadenectomy in vulvar squamous carcinoma: younger and older patients should be equally treated. A prospective study and literature review. Gynecol Oncol. 2015;137(3):373–379. doi:10.1016/j.ygyno.2015.03.013
- Milliken S, May J, Sanderson PA, et al. Reducing the radicality of surgery for vulvar cancer: are smaller margins safer? Minerva Obstet Gynecol. 2021;73(2):160–165. doi:10.23736/S2724-606X.20.04743-7
- Kotsopoulos IC, Tampakoudis GP, Evaggelinos DG, et al. Implication of human papillomavirus-66 in vulvar carcinoma: a case report. J Med Case Rep. 2011;5(1):232–236. doi:10.1186/1752-1947-5-232
- Liu YA, Ji JX, Almadani N, et al. Comparison of p53 immunohistochemical staining in differentiated vulvar intraepithelial neoplasia (dVIN) to inflammatory dermatoses and benign squamous lesions in the vulva. Histopathol. 2021;78(3):424–431. doi:10.1111/his.14238
- de Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a metaanalysis. Int J Cancer. 2009;124(7):1626–1636. doi:10.1002/ijc.24116
- Kortekaas KE, Bastiaannet E, van Doorn HC, et al. Vulvar cancer subclassification by HPV and p53 status results in three clinically distinct subtypes. Gynecol Oncol. 2020;159(3):649–656. doi:10.1016/j.ygyno.2020.09.024
- Kortekaas KE, Solleveld-Westerink N, Tessier-Cloutier B, et al. Performance of the pattern based interpretation of p53 immunohistochemistry as a surrogate for TP53 mutations in vulvar squamous cell carcinoma. Histopathol. 2020;77(1):92–99. doi:10.1111/his.14109
- Pinto AP, Miron A, Yassin Y, et al. Differentiated vulvar intraepithelial neoplasia contains Tp53 mutations and is genetically linked to vulvar squamous cell carcinoma. Modern Pathol. 2010;23:404–412. doi:10.1038/modpathol.2009.179
- Barlow EL, Lambie N, Donoghoe M, Naing Z, Hacker NF. The clinical relevance of p16 and p53 status in patients with squamous cell carcinoma of the vulva. J Oncol. 2020;2020:1–8 doi:10.1155/2020/3739075.
- O’Keefe RJ, Scurry JP, Dennerstein G, Sfameni S, Brenan J. Audit of 114 non-neoplastic vulvar biopsies. Br J Obstet Gynaecol. 1995;102:780–786. doi:10.1111/j.1471-0528.1995.tb10842.x
- Ju T, Vander Does A, Mohsin N, Yosipovitch G. Lichen simplex chronicus itch: an update. Acta Dermatol Venereol. 2022;102:1–6. doi:10.2340/actadv.v102.4367
- Marks JG, Miller JJ. Eczematous rash. In: Marks JG, Miller J, editors. Lookingbill and Marks’ Principles of Dermatology. 6th ed. Philadelphia: Elsevier; 2019:95–112.