Abstract
Purpose
Struma ovarii is a highly specialized teratoma consisting primarily of mature thyroid tissue. However, malignant struma ovarii coexisting with thyroid carcinoma, not to mention autoimmune disease, is uncommon. Malignant struma ovarii complicated with papillary thyroid carcinoma, Hashimoto’s thyroiditis and polycystic ovarian syndrome has never been reported in literature.
Patients and Methods
A 32-year-old female was admitted to our hospital due to a history of abdominal distension and menolipsis over the past half a year. Physical examination touched a 6 × 6 cm mass with a clear boundary, normal movement, and no pressing pain in the right adnexal area, Imaging revealed a cystic solid mass of 6 × 7 cm in the right ovary and the level of tumor markers including CA125, CA199, CA153, CEA, AFP were normal, but with low TSH and increased TPOAb, TGAb, TRAb. Laparoscopic right ovary tumor resection was performed, followed by comprehensive staging surgery, as well as thyroidectomy after pathologic diagnosis. The patient was diagnosed with a combination of follicular thyroid cancer from struma ovarii, papillary thyroid carcinoma and Hashimoto’s thyroiditis, along with polycystic ovarian syndrome. Immunohistochemical staining showed positivity for Ag, CK-pan, CK7, PAX8 and TTF-1 in the right ovarian mass, and the left thyroid was positive for the BRAF V600E mutation.
Results
The patient underwent thyroxine suppression therapy and radioactive iodine 131I therapy after operation. Serum thyroglobulin was undetectable, and no signs of recurrence or metastasis were detected in the imaging examination at the 2-year follow-up.
Conclusion
Malignant struma ovarii coexisting with thyroid carcinoma is rare. No report has been identified in literature review on the rare malignant struma ovarii coexisting with thyroid carcinoma, Hashimoto’s thyroiditis and polycystic ovarian syndrome. Our case can offer experience of diagnosis and treatment to some extent for such rare case. Therefore, it is essential to consider the association between ovarian tumors and the endocrine system. This case is valuable in understanding the diagnosis and management of such an unusual complicated disease.
Introduction
Teratomas are the most common ovarian germ cell tumors that contain tissues from three germ layers, including hair, skin, teeth, bone, and thyroid.Citation1 The ectoderm is the most common germ layer involved in malignant transformation.Citation2 Approximately 20% of teratomas contain thyroid tissue, and struma ovarii (SO) refers to teratomas that are covered with more than 50% thyroid tissue, accounting for 3% of mature teratomas and 1% of all ovarian tumors.Citation1,Citation3 Additionally, malignant struma ovarii (MSO), which accounts for 5% of all SO, refers to SO that contains malignant thyroid tissue, even if the amount of malignant tissue comprises less than 50% of the thyroid, and is also known as thyroid cancer from struma ovarii.Citation1 The pathological classification of MSO is usually based on the same criteria as that of thyroid carcinoma. The most common are papillary thyroid carcinoma and others, such as follicular variant papillary thyroid carcinoma, follicular carcinoma, and highly differentiated follicular carcinoma that is characterized by extra-ovarian dissemination.Citation4
Hashimoto’s thyroiditis (HT) is the most common autoimmune thyroid disease and initially manifests as euthyroidism and even hyperthyroidism with the elevation of thyroid peroxidase antibody and antithyroglobulin antibody. In a particular situation, the disease can manifest with Hashitoxicosis, which involves hyperthyroidism first, and then hypothyroidism. When thyroid function is impaired, subclinical hypothyroidism may occur, and may ultimately develop into hypothyroidism and enlargement of the thyroid gland.Citation5 Polycystic ovary syndrome (PCOS) is characterized by oligo- or anovulation, hyperandrogenemia, hirsutism and polycystic ovaries. In addition to the classical manifestations of PCOS, teratomas can lead to androgen secretion and a special type of polycystic ovary syndrome called HAIR-AN.Citation6 There are studies on thyroid function and PCOS in infertility, but not in ovarian tumors.
There is a lack of unified diagnostic criteria, treatment guidelines, and survival prognosis due to the rarity of MSO complicated with thyroid carcinoma. Struma ovarii with thyroid carcinoma has been reported in a series of cases in recent years, but rarely reported struma ovarii with Hashimoto’s thyroiditis, we searched 44 cases from 1965 to 2024 in PubMed, MEDLINE and Embase, using the search terms struma ovarii and Hashimoto’s thyroiditis’, “struma ovarii and autoimmune disease”, “struma ovarii and hypothyroidism”, “struma ovarii and Hashimoto’s thyroiditis and thyroid carcinoma”, “thyroid carcinoma from struma ovarii and Hashimoto’s thyroiditis’, ‘malignant struma ovarii and Hashimoto’s thyroiditis”, included all reports of struma ovarii combined with Hashimoto's thyroiditis, and excluded Italian language, Grave’s disease, unrelated theme and simple hypothyroidism, only one case of HT with PCOS,Citation7 and 14 cases of struma ovarii with Hashimoto’s thyroiditis have been reported, in literature reviews (), and most are benign, without mention of malignant struma ovarii complicated with autoimmune disease, primary thyroid cancer, and PCOS. Herein, we report a case of follicular thyroid carcinoma from struma ovarii coexisting with thyroid papillary carcinoma with polycystic ovary syndrome and Hashimoto’s thyroiditis. To analyze the pathogenesis, diagnosis and management of this rare and complicated disease, the association between struma ovarii and endocrinological abnormalities is reviewed and discussed.
Table 1 Reported Cases of Hashimoto’s Thyroiditis Within or Concomitant with Struma Ovarii
Case Presentation
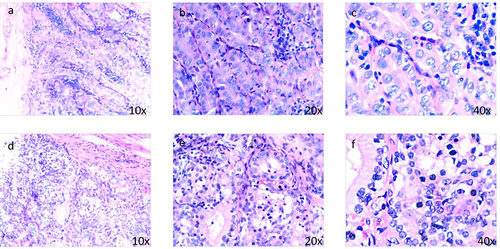
A 32-year-old gravida 3 woman (P2 by Caesarean section and experienced artificial abortion once) presented in June 2020 with a half-year history of abdominal distension and menolipsis. She had been diagnosed with PCOS at 18 years of age and received irregular Chinese medicine for PCOS. Additionally, her mother had a history of PCOS, but there was no prior diagnosis or family history of thyroid or autoimmune disorders. An abdominal ultrasound scan indicated a 6.9 × 6.0 cm cystic-solid mass in the right ovary (), and abdominal computed tomography showed a 7.1 × 5.6 ×7.5 cm heterogeneous dense mass, in the right annex, containing multiple irregular high-density calcification shadows and low-density areas in the interior. This patient had no symptoms of abnormal thyroid function or other concerning symptoms. Physical examination revealed a 6 × 6 cm mass in the right adnexal area, with a clear boundary, normal movement, and no pressing pain. The patient then underwent laparoscopic exploration and a right ovarian cystectomy. The mass was comprised of a smooth, thick-walled multilocular cyst of 6 cm diameter, filled with straw-colored fluid, a yellow-brown pasty material and a few solid components. Although intraoperative frozen pathology suggested ovarian malignancy, the patient and her family requested a postoperative pathology report before deciding on further surgery. Postoperative histological examination showed a tumor, with atypical and ground glass cellular nuclei, lacking nuclear grooves, nuclear pseudo-inclusion bodies or overlap, and infiltrating into ovarian tissue, forming hyperplastic follicles similar to those found in follicular thyroid cancer (). Immunohistochemistry analysis demonstrated positivity for TTF1 (), Ag, pan-CK, CK7, PAX8 and 5% Ki67, but negativity for CR, ER, WT1, MelanA, inhibin and SALL4. Reticular fiber staining showed a nest-like distribution of cancer cells. Based on the above pathology and immunohistochemistry, the patient was eventually diagnosed with follicular thyroid carcinoma arising from struma ovarii. The FIGO stage was IC2 and the AJCC stage was pT1c2N0M0.
Figure 1 Abdominal ultrasound indicating a mixed echogenic mass in the right enlarged ovary without clear boundary. The dotted line surrounds blood flow signals detected in the interior and surrounding tissue.
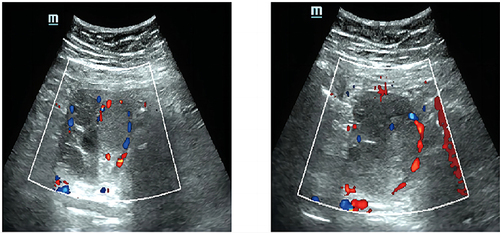
Figure 2 (a–c) Papillary structures are visible, with ground-glass nuclei, nuclear groove, and overlapping nuclei, including pseudo-inclusion bodies; (d-f) Malignant area of struma ovarii. Cellular nuclei show atypia and the tumor has infiltrated into ovarian tissues, which form hyperplastic follicles similar to follicular thyroid cancer, without nuclear grooves, nuclear pseudo-inclusion bodies or nuclear overlap and ground glass nuclei.
Because of these findings, further examination for thyroid hormones and serum auto-antibodies was considered necessary. The patient’s TSH level was below 0.01µIU/mL, but FT3, FT4, TT3 and TT4 levels were normal. Thyrotropin receptor antibody, thyroid peroxidase antibody and antithyroglobulin antibodies were positive at a titer of 1.71, 843 and 57.8 IU/mL, respectively. Thyroid ultrasound showed a 15 × 12 mm hypo-echogenic node in the left lobe, and enlargement in the right lobe. The patient exhibited typical symptoms, such as obesity (BMI 32.42), hirsutism, brittle hair and alopecia areata, indicating possible abnormal endocrine function. The patient was without reproductive requirements and subsequently underwent a total abdominal hysterectomy, bilateral salpingo-oophorectomy, pelvic and para-aortic lymphadenectomy and omentectomy. Total thyroidectomy was performed one month after abdominal surgery. Pathological examination revealed that no tumor cells were found in the uterus, bilateral annex, abdominal and para-aortic lymph nodes, omentum and peritoneal washes, but papillary thyroid carcinoma was found in the left lobe of the thyroid gland. According to the American Joint Committee on Cancer 8th edition staging, considering diagnosis with pT1bN0M0. Evidence of HT was found in the bilateral lobe and isthmus of the thyroid gland. No neoplastic involvement was discovered in the thyroid capsule, perineurium and vasculature. The detection of BRAF V600E mutation was positive in the left lobe of the thyroid gland.
The patient started thyroxine suppression therapy after a total thyroidectomy and five months later radioactive 131I therapy with a 160 mCi regimen. The reason that the patient underwent total thyroidectomy and high-dose 131I therapy is that it would be helpful to evaluate the post-therapeutic RAI images. The other reason is that the distant metastasis was considered before the pathology diagnosis. Currently, the patient has been followed up for 2 years (follow-up results are shown in ), at which time the serum TG was persistently undetectable, and TGAb had become normal. Hirsutism did not improve considerably, but testosterone levels exhibited a decreasing trend. Pelvic and cervical ultrasound and a systemic whole-body 131I scan indicated no signs of recurrence or metastasis to date. The patient is still being followed up.
Table 2 Patient Laboratory Results
Discussion
Struma ovarii is a monodermal teratoma of the ovaries and consists of more than 50% thyroid tissue. The rate of malignant transformation and the possibility of metastasis are about 4–23%,Citation3,Citation18 generally with metastasis to the pelvis and abdomen, but uncommonly to the lung, bone, brain and liver.Citation20 The onset of MSO generally occurs between 40 and 60 years, but recently a case reported the youngest age is 11 years,Citation21 which implies that MSO can occur at any age, it is important to raise awareness of the increasing incidence and detection of MSO at different ages. And predominantly involves the left ovary unilaterally (94%). Malignant struma ovarii is commonly asymptomatic, although occasionally a pelvic mass, lower abdominal pain, abnormal vaginal bleeding, or ascites can appear.Citation22–24 Changes in thyroid function are uncommon, with hyperthyroidism reported in only 5–15% of cases.Citation1 Struma ovarii with Hashimoto’s thyroiditis is even rarer. Interestingly, Hashimoto’s thyroiditis can occur either in the neck or within the struma ovarii,Citation2,Citation8,Citation18 and only one case combined with polycystic ovary syndrome has been reported.Citation7
In 1965, Eres et al first reported that lymphocytic infiltration could be found in bilateral struma ovarii.Citation8 Nevertheless, until 1998, Doldi et al reported HT combined with MSO and found lymphocytes in the malignant thyroid elements.Citation2 The patient complained of abdominal swelling and discomfort but had normal thyroid function, although increased TPOAb and TGAb. After right ovarian cyst resection, pathology and fine-needle aspiration biopsy, she was eventually diagnosed with papillary thyroid carcinoma from struma ovarii with Hashimoto’s thyroiditis. No secondary surgery or adjuvant therapy was mentioned. Our patient had similar symptoms but ultimately received more radical treatment, including comprehensive staging surgery, total thyroidectomy and 131I radiation therapy. In 2016, Russo et al again reported the coexistence of MSO with Hashimoto’s thyroiditis.Citation18 However, the patient was ultimately identified because of metastasis in the right adnexa and Douglas-pouch several years after the resection of the left struma ovarii, and presented with subclinical hyperthyroidism and elevated TGAb and TPOAb. Subsequently, comprehensive staging surgery, thyroidectomy and 131I radiotherapy were carried out. The patient was diagnosed with follicular variant papillary thyroid carcinoma from bilateral struma ovarii with metastasis and cervical Hashimoto’s thyroiditis. To date, the struma ovarii combined with Hashimoto’s thyroiditis that have been reported are mainly benign, and no report on MSO with HT has appeared since 2016. To our knowledge, the current case is the third reported case of malignant struma ovarii with Hashimoto’s thyroiditis, coexisting with primary thyroid cancer. We summarized the 14 reported cases of SO with HTCitation2,Citation7–19 (), and found that most patients complained of abdominal discomfort (36%). Four cases had HT occurring within the SO, and 6 cases occurring in the thyroid gland, with the other 4 cases displaying HT in both the SO and thyroid gland. Due to the rarity of the combination disease, thyroid function of most of the patients was examined after surgery. Most of the masses are presented in the right adnexa. Nine cases (64%) had positive thyroid antibodies. Tumor markers such as CA 19–9 and CA-125 antigen are widely used for prediction of the characteristics of ovarian mass,Citation25 especially CA-125 is a useful tool to distinguish between benign and malignant ovarian masses, but lack specificity and sensitivity, since elevation can be presented in endometriosis, pelvic inflammatory, menstruation and adenomyosis.Citation26,Citation27 However, these cases were reported above, and our patients all had normal CA-125 and CA 19–9. Therefore, significant tumor markers still need to be explored in MSO’s diagnosis and follow-up after operation. The treatment adopted was based on the struma ovarii feature. However, in most cases, cystectomy was performed alone.
The pathogenesis and association of SO combined with HT, PCOS and concomitant primary thyroid cancer have not been sufficiently elucidated. A series of studies have shown that patients with PCOS are more prone to HT, which might reflect genetic susceptibility.Citation28 On the one hand, the FBN3 gene of PCOS patients carries the D19S884 allele, which causes low levels of TGF-β1, which can stimulate Tregs to inhibit the occurrence of excessive immunity. On the other hand, the high estrogen/progesterone ratio caused by the anovulation cycle in PCOS patients can strengthen the immune response.Citation28 Nonetheless, Kim et al suggested that there is no correlation between PCOS and HT, although they discovered patients who have PCOS combined with HT can have higher insulin resistance and obesity than those who suffer with only PCOS.Citation29 HT is a risk factor for PTC, and patients with both PTC and HT are more likely to have multifocal tumors, but it should be noted that in differentiated thyroid cancer, HT can be a protective factor when PTC occurs.Citation30,Citation31 In other words, patients with combined PTC with HT have a better prognosis and a lower risk of recurrence than those who have PTC alone. A case report had suggested that hypothyroidism may be associated with ovarian cyst formation, because hypothyroidism increases ovarian sensitivity to gonadotropin, leading to ovarian hypertrophy and formation of multi-follicular cysts.Citation32 Most HT can eventually develop into hypothyroidism. We can speculate that patients with HT are also more likely to form ovarian cysts. Furthermore, there is a case report demonstrating that a teratoma can cause androgen secretion and an extreme subtype of polycystic ovary syndrome called HAIR-AN syndrome.Citation6 “Field cancerization” as well as early genomic instability may be responsible for the thyroid-type tissue occurring in multifocal areas,Citation33 which supports the coexistence of primary tumors in the ovary and thyroid gland. Although HT can produce autoimmune antibodies, the secreted products of tumors can lead to autoimmunity as well. Russo et al speculated that MSO causes the elevation of TGAb and occurrence of secondary Hashimoto’s thyroiditis.Citation18 Thus, we can hypothesize that patients with a decades-long history of PCOS will be more prone to HT, after which HT will become a risk factor for PTC, leading to the multifocality of thyroid-type tissue caused by field cancerization and early genomic instability, as well as the combination of HT and PTC, eventually resulting in MSO. Moreover, MSO can also further cause secondary Hashimoto’s thyroiditis and an extreme subtype of PCOS characterized by hyperandrogenism.
Due to the rarity of malignant struma ovarii, there is no unified principle for diagnosis and management. Therefore, the criteria for thyroid cancer treatment are commonly followed. Due to the absence of a capsule in the ovary, histological diagnosis of ovarian thyroid follicular carcinoma based on the following criteria is challenging: 1) the tumor infiltrates the ovarian cortex and grows on the serosal surface, as is the case reported here, 2) vascular invasion, and 3) the occurrence of spread or metastasis.Citation34 Follicular carcinoma from struma ovarii needs to be differentiated from benign struma ovarii, with up to 34% being misdiagnosed.Citation35 When thyroid carcinoma is presented, the differential diagnosis of metastatic thyroid carcinoma and malignant struma ovarii must be considered. The differential diagnosis of metastatic disease and coexisting primary tumors can be distinguished by the mode of metastasis, the ovarian site and the teratoma features. Compared with MSO, the occurrence of thyroid cancer metastasis to the ovary is more uncommon, and frequently involves bilateral metastasis.Citation33,Citation36 The different pathological types and the unilateral ovary malignant transformation may provide support for our reported case of two independent primary thyroid cancers. Unfortunately, we evaluated BRAF V600E(+) only in papillary thyroid carcinoma and did not perform further molecular analysis in malignant struma ovarii tissue.
The principle of treatment for malignant struma ovarii has been controversial. Currently, the main treatment is pelvic surgery based on fertility requirements. However, whether to implement postoperative adjuvant therapy is disputed. Most experts recommend operating prophylactic thyroidectomy and 131I radiation therapy, which can reduce the risk of recurrence and is conducive to accurate follow-up,Citation37,Citation38 but Hatami et al believe that aggressive therapy should be adopted for patients with recurrence and residual disease.Citation24 Addley et al proposed that the coexistence of primary thyroid carcinoma and MSO leads to more invasiveness.Citation39 Therefore, aggressive therapy is still recommended for primary thyroid carcinoma combined with MSO. Moreover, Mulita et al demonstrated that total thyroidectomy was performed in patients with differentiated thyroid carcinoma without increasing the risk of early complications in comparison with subtotal thyroidectomy.Citation40 LigaSure vessels and harmonic scalpel were devices widely applied in thyroidectomy, A 15-year single-centre retrospective study demonstrated that harmonic scalpel was more effective in achieving haemostasis compared with LigaSure vessels and especially in the patients with thyroid carcinoma,Citation41 which means harmonic scalpel applied in thyroid carcinoma with MSO may reduce the incidence of bleeding complications and bring better prognosis in some extent. It is increasingly accepted that treatment measures should be taken according to risk stratification. Yassa et al proposed a risk stratification for malignant struma ovarii in patients whose tumor size is over 2 cm, whose spread extends to the outside of the ovary, or whose invasive histological features suggest thyroidectomy and 131I radioiodine ablation therapy are needed.Citation42 However, limitations in considering reproductive desires and precise measuring of tumor size should be taken for stratifying risk.Citation43 Chemotherapy is applied in advanced ovarian tumors or in the presence of recurrence and metastasis, but it actually has a poor effect when applied in MSO.Citation44 131I radioiodine ablation therapy should be taken for first-line therapy when MSO has spread to the outside of the ovary, specifically for metastatic struma ovarii, and lithium pretreatment before 131I radioiodine ablation may improve the effectiveness of 131I.Citation1,Citation18 Administration of recombinant human thyrotropin before radiotherapy can help to boost the absorption of 131I when endogenous thyrotropin is hard to elevate, and sorafenib can be administered in patients who respond poorly to 131I radioiodine ablation therapy.Citation44 Regardless of the type of therapy, long-term monitoring (at least 10 years), with clinical monitoring, whole-body scan and measurement of serum thyroglobulin, is recommended.Citation18,Citation43 Nevertheless, Feldt-Rasmussen et al believe that TGAb should be used as a surrogate marker for follow-up instead of TG when HT is coexisting with thyroid cancer.Citation45 Goffredo et al analyzed a cohort of 68 patients diagnosed with malignant struma ovarii from 1973 to 2011 in the Surveillance, Epidemiology, and End Results database, and discovered that the overall survival rates at 5, 10, and 20 years were 96.7%, 94.3%, and 84.9%, respectively, regardless of the treatment adopted.Citation46 In addition, some evidence suggests that HT can exert a better outcome for thyroid cancer,Citation30,Citation31,Citation45 but lacks the prognosis study in coexistence of primary thyroid cancer and malignant struma ovarii.
Here, we present a case of follicular thyroid carcinoma from struma ovarii with papillary thyroid carcinoma, HT and PCOS, which has not been reported in the literature. Our patient complained of abdominal discomfort and the elevation of TRAb, TPOAb, TGAb and testosterone were examined. Due to the rarity of this disease and without reproductive requirements, we performed more radical treatment, including comprehensive staging surgery, thyroidectomy, and 131I radioiodine ablation therapy for the patient. There is a system for reporting thyroid cytopathology called the Bethesda system, which can be divided into six categories. Our patient may belong to Bethesda II, which can be caused by Hashimoto’s disease, or Bethesda III that includes atypia of undetermined significance or follicular lesion of undetermined significance.Citation47,Citation48 One study reported that incidental malignancy was found in 1.53% of Bethesda II cases, and the most common type of malignancy was papillary thyroid carcinoma.Citation49 And the other study reported that incidental malignancy was 19.19% in 344 cases of Bethesda III, but the most common type of malignancy was the follicular variant of papillary thyroid carcinoma.Citation50
HT coexisting with two different thyroid-type carcinomas is a high-risk factor, so we recommend comprehensive staging surgery and thyroidectomy, along with 131I radioiodine ablation therapy for this unusual combined disease rather than conservative management. There is a paucity of data on synchronous primary thyroid cancer and MSO combined with endocrinological disease, so our case may contribute to the diagnosis and management of this uncommon situation.
Conclusion
In conclusion, the diagnosis of malignant struma ovarii coexisting with primary thyroid cancer and Hashimoto’s thyroiditis and polycystic ovary syndrome is a significant challenge. This is largely dependent on postoperative pathology and supplementary examination, with molecular analysis playing a crucial role in differentiating the coexistence of MSO and primary thyroid carcinoma. Consequently, when patients present with ovarian masses and abnormal thyroid function, it is advisable to be alert to the possibility of struma ovarii and to undergo examination of the TG, TGAb and thyroid ultrasound, in order to reduce the incidence of secondary surgery. Multi-disciplinary participation will be conducive to the diagnosis and treatment of malignant struma ovarii complicated with thyroid lesions. This case is valuable for its rarity, as is the coexistence of Hashimoto’s thyroiditis with polycystic ovary syndrome, follicular thyroid carcinoma arising from the struma ovarii and papillary thyroid carcinoma. Patients with PCOS have a genetic predisposition to Hashimoto’s thyroiditis, which is a high-risk factor for papillary thyroid carcinoma. Furthermore, patients who suffer from Hashimoto’s thyroiditis and papillary thyroid carcinoma are more prone to have multifocal tumours. However, interestingly, these patients have a better prognosis. The exploration of the potential benefits of the coexistence of Hashimoto’s thyroiditis on the prognosis of MSO requires large sample sizes in the future. Additionally, further research is needed to determine if the “Field cancerization” resulting from papillary thyroid cancer and Hashimoto’s thyroiditis affects the development of MSO.
Ethics
Institutional approval was required for the publication of the case details. Cancer Hospital of Shantou University Medical College has approved the publication of case details.
Patient Consent
Written informed consent was obtained from the patient for publication of this Case report and any accompanying images.
Disclosure
The authors declare that there is no conflict of interest that could impair the impartiality of the paper.
Acknowledgments
We acknowledge Doctor Xiaolong Wei, from the Department of Pathology of the Cancer Hospital of Shantou University Medical College, for providing specimens for histological and immunohistochemical investigations with us.
References
- Wolff EF, Hughes M, Merino MJ. et al. Expression of benign and malignant thyroid tissue in ovarian teratomas and the importance of multimodal management as illustrated by a BRAF-positive follicular variant of papillary thyroid cancer. Thyroid. 2010;20(9):981–987. doi:10.1089/thy.2009.0458
- Doldi N, Taccagni GL, Bassan M, et al. Hashimoto’s disease in a papillary carcinoma of the thyroid originating in a teratoma of the ovary (malignant struma ovarii). Gynecological Endocrinol. 1998;12(1):41–42. doi:10.3109/09513599809024969
- Talerman A. Germ cell tumors of the ovary. In: Kurman RL. editor. Blaustein’s Pathology of the Female Genital Tract. Vol. 3, edn. New York, NY, USA: Springer-Verlag;2011:659–721
- Roth LM, Karseladze AI. Highly differentiated follicular carcinoma arising from struma ovarii: a report of 3 cases, a review of the literature, and a reassessment of so-called peritoneal strumosis. Int J Gynecol Pathol. 2008;27(2):213–222. doi:10.1097/PGP.0b013e318158e958
- Caturegli P, De Remigis A, Rose NR. Hashimoto’s thyroiditis: clinical and diagnostic criteria. Autoimmun Rev. 2014;13(4–5):391–397. doi:10.1016/j.autrev.2014.01.007
- Ho JH, Abd Wahab AV, Fung YK, et al. An adolescent girl with coexisting ovarian mature cystic teratoma and HAIR-AN syndrome, an extreme subtype of polycystic ovarian syndrome. Endocrinol Diabetes Metab Case Rep. 2021;2021. Doi:10.1530/EDM-20-0195
- Nielsen VT. A benign cystic teratoma of the ovary with chronic thyroiditis. Am J Obstet Gynecol. 1984;148(8):1142–1144. doi:10.1016/0002-9378(84)90645-8
- Erez SE, Richart RM, Shettles LB. Hashimoto’s disease in a benign cystic teratoma of the ovary. Am J Obstet Gynecol. 1965;92(2):273–274. doi:10.1016/S0002-9378(65)80023-0
- Watson AA. Histological Hashimoto’s disease in a benign cystic ovarian teratoma. J Clin Pathol. 1972;25(3):240–242. doi:10.1136/jcp.25.3.240
- Farrell DJ, Bloxham CA, Scott DJ. Hashimoto’s disease in a benign cystic teratoma of the ovary. Histopathology. 1991;19(3):283–284. doi:10.1111/j.1365-2559.1991.tb00038.x
- Carvalho JP, Carvalho FM, Lima de Oliveira FF, et al. Hypothyroidism following struma ovarii tumor resection: a case report. Rev Hosp Clin Fac Med Sao Paulo. 2002;57(3):112–114. doi:10.1590/S0041-87812002000300006
- Amareen VN, Haddad FH, Al-Kaisi NS. Hypothyroidism due to Hashimoto thyroiditis post struma ovarii excision. Saudi Med J. 2004;25(7):948–950.
- Morrissey K, Winkel C, Hild S, et al. Struma ovarii coincident with Hashimoto’s thyroiditis: an unusual cause of hyperthyroidism. Fertil Steril. 2007;88(2):497. [ e15-7]. doi:10.1016/j.fertnstert.2006.11.095
- Bozkurt NC, Karbek B, Ozkaya EC, et al. Struma ovarii presenting with Hashimoto’s thyroiditis: a case report. J Med Case Rep. 2011;5(1):572. doi:10.1186/1752-1947-5-572
- Lupi I, Fessehatsion R, Manca A, et al. Hashimoto’s thyroiditis in a benign cystic teratoma of the ovary: case report and literature review. Gynecol Endocrinol. 2012;28(1):39–42. doi:10.3109/09513590.2011.579659
- Berendt-Obolonczyk M, Siekierska-hellmann M, Wojtylak S, et al. From struma ovarii to Hashimoto disease—an unusual diagnosis of primary hypothyroidism: case report. Gynecol Endocrinol. 2012;28(1):43–45. doi:10.3109/09513590.2011.588750
- Laganà AS, Santoro G, Triolo O, Giacobbe V, Certo R, Palmara V. Hashimoto thyroiditis onset after laparoscopic removal of struma ovarii: an overview to unravel a rare and intriguing finding. Clin Exp Obstet Gynecol. 2015;42(5):673–678. doi:10.12891/ceog1992.2015
- Russo M, Marturano I, Masucci R, et al. Metastatic malignant struma ovarii with coexistence of Hashimoto’s thyroiditis. Endocrinol Diabetes Metab Case Rep. 2016;2016:160030. doi:10.1530/EDM-16-0030
- Koehler VF, Keller P, Waldmann E, et al. An unusual case of struma ovarii. Endocrinol Diabetes Metab Case Rep. 2021;1:2021.
- Yi Z, Chang W, Guo-Nan Z, et al. Papillary thyroid cancer located in malignant struma ovarii with omentum metastasis: a case report and review of the literature. World J Surg Onco. 2016;14(1):17. doi:10.1186/s12957-016-0776-x
- Al-Shammaa M, Abdlkadir A, Al-Adhami D, et al. Thyroid carcinoma arising from struma ovarii at adolescence: a challenging case with favorable outcome. Cureus. 2023;15(10):e47163. doi:10.7759/cureus.47163
- Salman W, Singh M, Twaij Z. A case of papillary thyroid carcinoma in struma ovarii and review of the literature. Patholog Res Int. 2010;2010:352476. doi:10.4061/2010/352476
- Zhang X, Axiotis C. Thyroid-type carcinoma of struma ovarii. Arch Pathol Lab Med. 2010;134(5):786–791. doi:10.5858/134.5.786
- Hatami M, Breining D, Owers RL, et al. Malignant struma ovarii-A case report and review of the literature. Gynecol Obstet Invest. 2008;65(2):104–107. doi:10.1159/000108654
- Mulita F, Oikonomou N, Tchabashvili L, et al. A giant ovarian mucinous tumor in a 58-year-old postmenopausal patient with persistent abdominal pain and high serum levels of CA 19-9. Pan Afr Med J. 2020;37:76. doi:10.11604/pamj.2020.37.76.25932
- Mulita F, Tavlas P, Maroulis I. A giant ovarian mass in a 68-year-old female with persistent abdominal pain and elevated serum CA-125 level. Prz Menopauzalny. 2020;19(2):108–110. doi:10.5114/pm.2020.97870
- Mulita F, Liolis E, Kehagias D, et al. An enormous pelvic tumor in a 46-year-old woman with an elevated serum CA 125 level, what lies beneath it? Investigation of uterine tumors in postmenopausal women. Prz Menopauzalny. 2021;20(3):154–157. doi:10.5114/pm.2021.109773
- Gaberscek S, Zaletel K, Schwetz V, et al. Mechanisms in endocrinology: thyroid and polycystic ovary syndrome. Eur J Endocrinol. 2015;172(1):R9–21. doi:10.1530/EJE-14-0295
- Kim JJ, Yoon JW, Kim MJ, et al. Thyroid autoimmunity markers in women with polycystic ovary syndrome and controls. Hum Fertil. 2022;25(1):128–134. doi:10.1080/14647273.2019.1709668
- Hanege FM, Tuysuz O, Celik S, et al. Hashimoto’s thyroiditis in papillary thyroid carcinoma: a 22-year study. Acta Otorhinolaryngol Ital. 2021;41(2):142–145. doi:10.14639/0392-100X-N1081
- Xu S, Huang H, Qian J, et al. Prevalence of Hashimoto thyroiditis in adults with papillary thyroid cancer and its association with cancer recurrence and outcomes. JAMA Network Open. 2021;4(7):e2118526. doi:10.1001/jamanetworkopen.2021.18526
- Fitko R, Kucharski J, Szlezyngier B. The importance of thyroid hormone in experimental ovarian cyst formation in gilts. Anim Reprod Sci. 1995;39(2):159–168. doi:10.1016/0378-4320(95)01382-A
- Leong A, Roche PJ, Paliouras M, et al. Coexistence of malignant struma ovarii and cervical papillary thyroid carcinoma. J Clin Endocrinol Metab. 2013;98(12):4599–4605. doi:10.1210/jc.2013-1782
- Limaiem F, Bouraoui S. Follicular carcinoma arising from struma ovarii. A case report. Pathologica. 2020;112(4):224–228. doi:10.32074/1591-951X-122
- McGill J, Sturgeon C, Angelos P.metastatic struma ovarii treated with total thyroidectomy and radioiodine ablation. Endocr Pract. 2009;15(2):167–173. doi:10.4158/EP.15.2.167
- Leite I, Cunha TM, Figueiredo JP, et al. Papillary carcinoma arising in struma ovarii versus ovarian metastasis from primary thyroid carcinoma: a case report and review of the literature. J Radiol Case Rep. 2013;7(10):24–33. doi:10.3941/jrcr.v7i10.1593
- Brusca N, Del Duca SC, Salvatori R, et al. A case report of thyroid carcinoma confined to ovary and concurrently occult in the thyroid: is conservative treatment always advised? Int J Endocrinol Metab. 2015;13(1):e18220. doi:10.5812/ijem.18220
- Gomes-Lima CJ, Nikiforov YE, Lee W, et al. Synchronous independent papillary thyroid carcinomas in struma ovarii and the thyroid gland with different RAS mutations. J Endocr Soc. 2018;2(8):944–948. doi:10.1210/js.2018-00132
- Addley S, Mihai R, Alazzam M, et al. Malignant struma ovarii: surgical, histopathological and survival outcomes for thyroid-type carcinoma of struma ovarii with recommendations for standardising multi-modal management. A retrospective case series sharing the experience of a single institution over 10 years. Arch Gynecol Obstet. 2021;303(4):863–870. doi:10.1007/s00404-021-05969-0
- Mulita F, Verras GI, Dafnomili VD, et al. Thyroidectomy for the management of differentiated thyroid carcinoma and their outcome on early postoperative complications: a 6-year single-centre retrospective study. Chirurgia. 2022;117(5):556–562. doi:10.21614/chirurgia.2736
- Mulita F, Theofanis G, Verras GI, et al. Comparison of postoperative bleeding using harmonic scalpel and LigaSure in thyroid surgery: a 15-year single-centre retrospective study. Med Glas. 2023;20(2):1.
- Yassa L, Sadow P, Marqusee E. Malignant struma ovarii. Nat Clin Pract Endocrinol Metab. 2008;4(469):– 472. doi:10.1038/ncpendmet0887
- Gonzalez Aguilera B, Guerrero Vazquez R, Gros Herguido N, et al. The lack of consensus in management of malignant struma ovarii. Gynecol Endocrinol. 2015;31(4):258–259. doi:10.3109/09513590.2014.995616
- Wu M, Hu F, Huang X, et al. Extensive peritoneal implant metastases of malignant struma ovarii treated by thyroidectomy and 131I therapy: a case report. Medicine. 2018;97(51):e13867. doi:10.1097/MD.0000000000013867
- Feldt-rasmussen U, Rasmussen AK. Autoimmunity in differentiated thyroid cancer: significance and related clinical problems. Hormones. 2010;9(2):109–117. doi:10.14310/horm.2002.1261
- Gofredo P, Swaka AM, Pura J, et al. Malignant struma ovarii: a population level analysis of a large series of 68 patients. Thyroid. 2015;25(2):211–215. doi:10.1089/thy.2014.0328
- Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid. 2017;27(11):1341–1346. doi:10.1089/thy.2017.0500
- Anand B, Ramdas A, Ambroise MM, Kumar NP. The Bethesda system for reporting thyroid cytopathology: a cytohistological study. J Thyroid Res. 2020;2020:8095378. doi:10.1155/2020/8095378
- Mulita F, Iliopoulos F, Tsilivigkos C, et al. Cancer rate of Bethesda category II thyroid nodules. Med Glas. 2022;19(1):1.
- Kopczynski J, Suligowska A, Niemyska K, et al. The influence of the reclassification of NIFTP as an uncertain tumour on risk of malignancy for the diagnostic categories according to the Bethesda system for reporting thyroid cytopathology. Endokrynol Pol. 2019;70(3):232–236. doi:10.5603/EP.a2019.0008