Abstract
Purpose
Female infertility is a global health concern. The aim of this study was to investigate the relationship between regulatory T (Treg) cells and helper T cells 17 (Th17) in peripheral blood and unexplained infertility (UI). In addition, we explored potential valuable diagnostic biomarkers for patients with UI and ascertained whether Treg and Th17 cells are associated with primary and secondary UI.
Patients and Methods
The patients underwent standard fertility evaluation test, including blood tests, ultrasound examination, fallopian tube tests, ovulation assessment, and male partner’s semen analysis. According to the inclusion and exclusion criteria, this study enrolled 37 patients with UI (30 with primary UI and 7 with secondary UI) and 26 age-matched healthy volunteers as the control group. Flow cytometry was used to detect the frequency of Treg and Th17 cells. The area under the receiver operating characteristic curve (AUC) with a 95% confidence interval (CI) was used to assess the diagnostic performance. An AUC > 0.800 indicated good diagnostic performance.
Results
The percentage of Treg decreased significantly, whereas the percentage and absolute count of Th17 cells increased. Moreover, the Th17/Treg ratio in patients with UI increased significantly. As a diagnostic biomarker for UI, the Th17/Treg ratio exhibited remarkable diagnostic performance (AUC: 0.813 (95% CI = 0.709–0.917)). However, the percentages and absolute counts of Treg and Th17 cells in the peripheral blood of women with primary and secondary UI, as well as their Th17/Treg ratios, did not differ significantly.
Conclusion
The distribution of Treg and Th17 cells is imbalanced in patients with UI. Therefore, the Th17/Treg ratio may be a promising indicator of UI. However, there were no significant differences in the distribution of Treg and Th17 cells between women with primary and secondary UI.
Introduction
Female infertility is a global reproductive health issue affecting individuals, families, and society.Citation1 Developed countries have a higher incidence of infertility.Citation2 Therefore, with economic development, the issue of infertility among Chinese women of childbearing age warrants continued attention. Research shows that the infertility rate among Chinese couples of childbearing age is between 15% and 25%.Citation3–5 Patients with unexplained infertility (UI) account for 10–17% of infertile women; UI is defined as a condition where individuals have not achieved clinical pregnancy after unprotected sexual intercourse for more than 12 months without any clear reason.Citation6
Although the exact cause of UI is unclear, an imbalance in maternal immune mechanisms seems to play an important role. Previous studies have suggested that female infertility is an adaptive immune imbalance disease.Citation7–10 The fetus carries foreign (paternal) antigens, which serve as semi-allogeneic antigens and require the mother to establish immune tolerance. Adaptive immune imbalance may disrupt tolerance and lead to UI. T helper 17 cells (Th17) and regulatory T (Treg) cells are important subgroups of CD4+ T cells that differ in development and function.Citation11–13 Maintaining a balance between Th17 and Treg is crucial for controlling the immune status of the body. Previous studies have shown that an imbalance between Treg and Th17 cells is associated with immune disorders in patients with pregnancy disorders, such as recurrent spontaneous abortion (RSA) and polycystic ovary syndrome.Citation14,Citation15 However, studies on Treg and Th17 cells in patients with UI are limited.
Currently, pre-pregnancy care and pregnancy-related counseling have garnered attention.Citation16,Citation17 Providing an early diagnosis for patients with pregnancy disorders is the basis for subsequent active treatment. Recent studies have detected circulating immune cell subsets for the diagnosis of pregnancy disorders, including RSA and repeated implantation failures (RIF).Citation18,Citation19 The aim of this study was to investigate the percentage and absolute count of Treg and Th17 cells in the peripheral blood of women with UI, as well as the Th17/Treg ratio. Additionally, we explored potential valuable diagnostic biomarkers for patients with UI and ascertained whether Treg and Th17 cells are associated with primary and secondary UI.
Materials and Methods
Study Population
All participants were enrolled from the Reproductive Center of the Affiliated Dongyang Hospital of Wenzhou Medical University between July 2021 and October 2023. The inclusion criteria for the UI group were as follows: (1) age between 21 and 40 years; (2) failure to achieve clinical pregnancy after unprotected sexual intercourse for more than one year; (3) regular menstrual cycle and normal levels of serum anti-Müllerian, luteinizing, and follicle stimulating hormones, as well as progesterone levels and thyroid function; (4) normal results of uterine ultrasound and hysterosalpingography; and (5) normal male partner’s semen analysis. The exclusion criteria were as follows: (1) clear infection, (2) assisted reproductive therapy within three months, and (3) other known diseases that may affect fertility. According to the clinical pregnancy conditions, UI is divided into primary and secondary UI. Primary UI refers to women who have never been diagnosed with a clinical pregnancy and meet the UI criteria, whereas secondary UI refers to women who have previously been diagnosed with a clinical pregnancy but are currently unable to establish a clinical pregnancy that meets the UI criteria.Citation20 The study also included healthy women who have given birth, as controls. The inclusion criteria for the control group were as follows: (1) age range from 21 to 40 years old; (2) given birth to one or more live babies within two years; (3) more than six months after the last delivery and in non-lactation period; and (4) no history of adverse pregnancy.
This study has been performed in accordance with the principles stated in the Declaration of Helsinki. This study was reviewed and approved by the Ethics Committee of the Affiliated Dongyang Hospital of Wenzhou Medical University. All participants provided written informed consent.
Sample Collection and Flow Cytometry
Fresh peripheral whole blood samples were collected from participants during the mid-luteal phase of their menstrual cycle. Using a ten color flow cytometer (Navios; Beckman Coulter), two staining plates were designed for monoclonal fluorescent antibody labeling of Treg and Th17 cells (Supplementary Table 1). The Kaluza software (version 2.0, Beckman Coulter) was used to analyze the dataset and obtain the proportion of Treg and Th17 cells to CD4+T cells as well as the proportion of CD4+T to total lymphocytes. Treg cells are defined as CD4+CD25+CD127low/- lymphocytes, whereas Th17 cells are defined as CD4+CD45RO+CXCR5−CCR6+CXCR3− lymphocytes.Citation21,Citation22 The gate control strategy is shown in Supplementary Figure 1.
The total lymphocyte count was measured using a blood analyzer (XN-9000; SYSMEX, Japan), and the absolute count of Treg and Th17 cells was calculated based on the proportion.
Statistical Analysis
The STATA (version 14.0) and R (version 4.1.0) software were used for statistical analysis. The Shapiro–Wilk test was used for normality testing of continuous variables. The t-test and Wilcoxon rank-sum test were used to compare the intergroup differences in normally and non-normally distributed variables, respectively. The area under the receiver operating characteristic (ROC) curve (AUC) with a 95% confidence interval (CI) was used to assess the diagnostic performance. An AUC of > 0.800 indicated good diagnostic performance. Statistical significance was set at P-value < 0.05.
Results
This study included 37 patients with UI (30 with primary UI and 7 with secondary UI) and 26 age-matched healthy volunteers as the control group. The demographic and clinical data of the study population are shown in Supplemental Table 2.
Comparison Between the Control and UI Groups
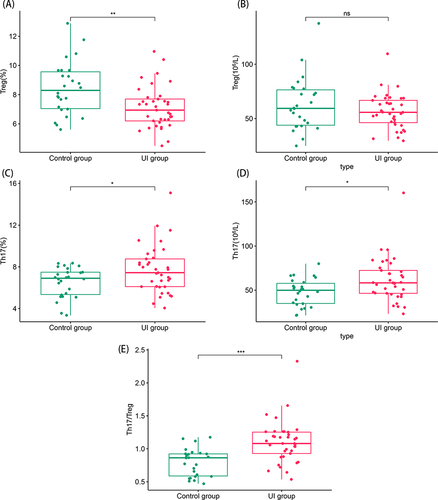
Compared with that of the control group, the percentage of Treg in the UI group was significantly low (8.4 ± 1.8 vs 7.1 ± 1.4, P<0.01, ), while there was no significant difference in the absolute count of Treg in the UI group (P>0.05, ). However, the percentage and absolute count of Th17 cells (6.9 (5.3–7.5) vs 7.4 (6.1–8.8), P<0.05; 49.9 (34.8–58.8) vs 58.3 (46.5–72.4), P<0.05; and , respectively), as well as the Th17/Treg ratio (0.86 (0.58–0.92) vs 1.08 (0.93–1.25), P<0.001, ), were significantly higher than those in the control group.
Figure 1 Comparison of the percentage and absolute count of Treg and Th17 cells and the Th17/Treg ratio between the unexplained infertility (UI) group and the control group. ns, P > 0.05, * P < 0.05, **P < 0.01, ***P < 0.001. (A) Comparison of the percentage of Treg cells (P < 0.01). (B) Comparison of the absolute count of Treg cells (P > 0.05). (C) Comparison of the percentage of Th17 cells (P < 0.05). (D) Comparison of the absolute count of Th17 cells (P < 0.05). (E) Comparison of the ratio of Th17/Treg cells (P < 0.001).
Supplementary Figure 2 shows the typical performance of Treg and Th17 cells in flow cytometry analysis between the UI and control groups.
Diagnostic Value of Treg Cell Percentage, Th17 Cell Percentage, and Th17/Treg Ratio as Biomarkers
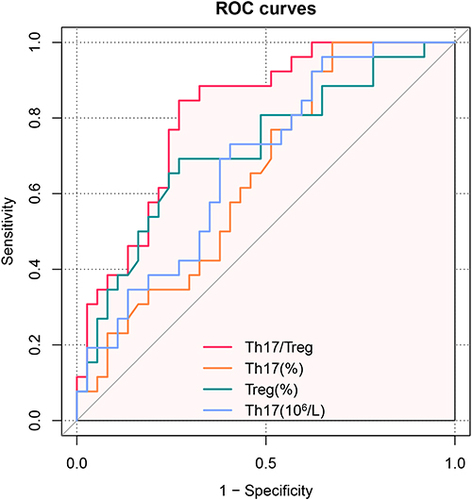
To identify potential biomarkers for UI diagnosis, ROC curves were used to evaluate the diagnostic value of Tregs percentage, Th17 cell percentage, absolute count of Th17 cells, and the Th17/Treg ratio, which showed significant differences between the UI and control groups (). The results showed that the AUCs (95% CI) for Tregs, Th17 cell percentage, absolute count of Th17 cells, and Th17/Treg ratio were 0.717 (95% CI=0.596–0.848), 0.648 (95% CI=0.513–0.782), 0.681 (95% CI=0.550–0.812), and 0.813 (95% CI=0.709–0.917), respectively. The Th17/Treg ratio demonstrated remarkable diagnostic value and was found to be superior to the use of Treg and Th17 cell percentages or absolute counts of Th17 cells alone in distinguishing patients with UI from the control group.
Comparison Between Primary and Secondary Infertilities
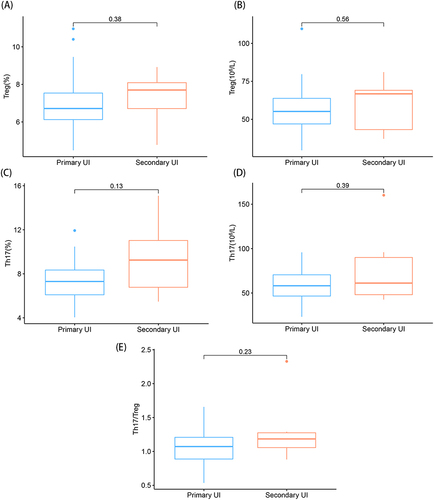
As shown in , there was no significant difference between the percentages or absolute counts of Treg and Th17 cells, or Th17/Treg ratios in the peripheral blood of women with primary and secondary UI.
Figure 3 Comparison among the percentages and absolute counts of Treg and Th17 cells and the Th17/Treg ratios for primary and secondary unexplained infertility. (A) Comparison of the percentages of Treg cells; (B) Comparison of the absolute counts of Treg cells; (C) Comparison of the percentages of Th17 cells; (D) Comparison of the absolute counts of Th17 cells; (E) Comparison of the ratios of Th17/Treg cells.
Discussion
The establishment of pregnancy is a challenge for the maternal immune system, as it must retain the ability to resist pathogen immune responses and improve immune tolerance to help the fetus evade immune attacks.Citation23 This research explored the distribution percentages and absolute counts of Treg and Th17 cells in the peripheral blood of patients with UI from the perspective of peripheral circulatory immunity and reported, for the first time, changes in the Th17/Treg ratio in patients with UI.
Treg account for approximately 5–10% of peripheral CD4+T cells,Citation24 and primarily regulate in vivo immune responses, which are crucial for establishing maternal immune tolerance. Luo et alCitation25 observed a decrease in the percentage of Treg in the peripheral blood of patients with unexplained RSA, and their immune regulatory deficiency appeared to be mainly related to Treg impairment. Cai et alCitation19 observed a significant reduction in Treg levels in the peripheral blood of patients with RIF. Previously, impairment of the Treg phenotype in the endometrial tissue was reported to affect female fertility in patients with UI.Citation26 In this study, flow cytometry was used to determine a reduction in the percentage of Treg in the peripheral blood of patients with UI; however, it was not related to secondary or primary UI.
Th17 cells are a subgroup of CD4+ T cells that mediate inflammatory responses and play an important role in acute and chronic allograft rejection reactions.Citation27–29 The inability of the mother to establish pregnancy may be caused by immune rejection of the fetus by semi-allogeneic antigens, where Th17 cells play an important role. Recent studies have shown that the percentage of Th17 cells increases in patients with unexplained RSA, which may be related to its pathogenesis.Citation30,Citation31 In addition, Ghaebi et alCitation32 detected a higher proportion of Th17 cells in women with RIF. The findings of the current study suggest a relationship between Th17 cells and UI and that the increased percentage and absolute count of Th17 cells may affect pregnancy outcomes of patients with UI. However, no significant differences were found in the distribution of Th17 cells between women with primary and secondary UI.
Both Treg and Th17 cells exhibit synergistic effects and mutual constraints.Citation33 The imbalanced distribution of Treg and Th17 cells, leading to an increase in the Th17/Treg ratio, is believed to cause various pregnancy-related diseases, including RSA, intrahepatic cholestasis of pregnancy, and preeclampsia.Citation34–36 To the best of our knowledge, this study is the first to investigate the Th17/Treg ratio in patients with UI and find that the Th17/Treg ratio in the UI group shifted toward a direction favorable for Th17 cells, disrupting the balance between Treg and Th17 cells. Moreover, as a diagnostic biomarker for UI, the Th17/Treg ratio was found to be superior to the use of Treg or Th17 cells alone.
In the past, research samples for unexplained pregnancy disorders were diverse and included endometrial and menstrual blood.Citation37,Citation38 However, owing to its non-invasive sampling procedure and non-easily contaminated nature, peripheral blood samples have attracted increasing interest from researchers. In this study, we found that the peripheral blood Th17/Treg ratio has potential applicability in the diagnosis of UI; however, there are certain limitations. First, the patients participating in the study were from a single center and patient numbers were limited. Therefore, future multicenter studies with relatively large sample sizes are required to confirm this hypothesis. Second, combining subsequent treatment plans was difficult, thereby complicating follow up on outcomes. Moreover, the limitations of current diagnostic methods in detecting early adenomyosis and endometriosis make it difficult to rule out their potential causes of infertility. Finally, the study lacked an analysis of cellular functions, such as cytokine secretion, which constitutes scope for further research.
Conclusion
The distribution of Treg and Th17 cells is unbalanced in patients with UI. The percentage of Treg decreased significantly, whereas the percentage and absolute count of Th17 cells increased. Moreover, the Th17/Treg ratio in patients with UI increased significantly. Moreover, the Th17/Treg ratio may be a promising diagnostic biomarker of UI. However, the distribution of Treg and Th17 cells did not differ significantly between women with primary and secondary UI.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Funding
References
- Nik Hazlina NH, Norhayati MN, Shaiful Bahari I, Nik Muhammad Arif NA. Worldwide prevalence, risk factors and psychological impact of infertility among women: a systematic review and meta-analysis. BMJ open. 2022;12(3):e057132. doi:10.1136/bmjopen-2021-057132
- Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reproduct. 2007;22(6):1506–1512. doi:10.1093/humrep/dem046
- Zhou Z, Zheng D, Wu H, et al. Epidemiology of infertility in China: a population-based study. BJOG. 2018;125(4):432–441. doi:10.1111/1471-0528.14966
- Zhu C, Yan L, He C, et al. Incidence and risk factors of infertility among couples who desire a first and second child in Shanghai, China: a facility-based prospective cohort study. Reproductive Health. 2022;19(1):155. doi:10.1186/s12978-022-01459-x
- Liang S, Chen Y, Wang Q, et al. Prevalence and associated factors of infertility among 20-49 year old women in Henan Province, China. Reproductive Health. 2021;18(1):254.
- Ehsani M, Mohammadnia-Afrouzi M, Mirzakhani M, Esmaeilzadeh S, Shahbazi M. Female unexplained infertility: a disease with imbalanced adaptive immunity. J Hum Reproduc Sci. 2019;12(4):274–282. doi:10.4103/jhrs.JHRS_30_19
- An LF, Zhang XH, Sun XT, Zhao LH, Li S, Wang WH. Unexplained infertility patients have increased serum IL-2, IL-4, IL-6, IL-8, IL-21, TNFα, IFNγ and increased Tfh/CD4 T cell ratio: increased Tfh and IL-21 strongly correlate with presence of autoantibodies. Immunol Invest. 2015;44(2):164–173. doi:10.3109/08820139.2014.932377
- Azargoon A, Mirrasouli Y, Shokrollahi Barough M, Barati M, Kokhaei P. The state of peripheral blood natural killer cells and cytotoxicity in women with recurrent pregnancy loss and unexplained infertility. Internat J Fertilit Steril. 2019;13(1):12–17.
- Ehsani M, Mohammadnia-Afrouzi M, Esmaeilzadeh S, Tajalli Z, Jafari M, Shahbazi M. Decreased frequency of CD8(+)HLA-G(+) T cell in the peripheral blood of primary unexplained infertile females. Rep Sci. 2021;28(7):1939–1944. doi:10.1007/s43032-020-00431-z
- Ozkan ZS, Deveci D, Kumbak B, et al. What is the impact of Th1/Th2 ratio, SOCS3, IL17, and IL35 levels in unexplained infertility? J Reprod Immunol. 2014;103:53–58. doi:10.1016/j.jri.2013.11.002
- Lan YT, Wang ZL, Tian P, Gong XN, Fan YC, Wang K. Treg/Th17 imbalance and its clinical significance in patients with hepatitis B-associated liver cirrhosis. Diagn Pathol. 2019;14(1):114. doi:10.1186/s13000-019-0891-4
- Gautam S, Kumar R, Kumar U, Kumar S, Luthra K, Dada R. Yoga maintains Th17/Treg cell homeostasis and reduces the rate of T cell aging in rheumatoid arthritis: a randomized controlled trial. Sci Rep. 2023;13(1):14924. doi:10.1038/s41598-023-42231-w
- Rao J, Li S, Wang X, et al. Comparison of peripheral blood regulatory T cells and functional subsets between ocular and generalized myasthenia gravis. Front Med. 2022;9:851808. doi:10.3389/fmed.2022.851808
- Farshchi M, Abdollahi E, Saghafi N, et al. Evaluation of Th17 and Treg cytokines in patients with unexplained recurrent pregnancy loss. J Clin Translat Res. 2022;8(3):256–265.
- Nasri F, Doroudchi M, Namavar Jahromi B, Gharesi-Fard B. T helper cells profile and CD4+CD25+Foxp3+Regulatory T cells in polycystic ovary syndrome. Iran J Immunol. 2018;15(3):175–185. doi:10.22034/IJI.2018.39387
- Bille C, Andersen AM. Preconception care. BMJ. 2009;338:b22.
- Xu J, Li X, Zhou Q. Nationwide-free preconception care strategy: experience from China. Front Public Health. 2022;10:934983. doi:10.3389/fpubh.2022.934983
- Ou M, Luo L, Yang Y, et al. Decrease of peripheral natural killer cell level during early pregnancy predicts live birth in women with unexplained recurrent pregnancy loss: A prospective cohort study. Am J Clin Exp Obstet Gynecol. 2023. doi:10.1016/j.ajog.2023.10.042
- Cai JY, Tang YY, Deng XH, et al. Recurrent implantation failure may be identified by a combination of diagnostic biomarkers: an analysis of peripheral blood lymphocyte subsets. Front Endocrinol. 2022;13:865807. doi:10.3389/fendo.2022.865807
- Zegers-Hochschild F, Adamson GD, Dyer S, et al. The international glossary on infertility and fertility care, 2017. Hum Reproduct. 2017;32(9):1786–1801. doi:10.1093/humrep/dex234
- Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the human immunology project. Nat Rev Immunol. 2012;12(3):191–200. doi:10.1038/nri3158
- Pitoiset F, Cassard L, El Soufi K, et al. Deep phenotyping of immune cell populations by optimized and standardized flow cytometry analyses. Cytometry Part A. 2018;93(8):793–802. doi:10.1002/cyto.a.23570
- Boly TJ, Bermick JR. Maternal-fetal tolerance: not just a uterine affair. J Leukoc Biol. 2022;111(3):515–517. doi:10.1002/JLB.5CE1021-560
- Tang Y, Shen L, Bao JH, Xu DY. Deficiency of Tregs in hypertension-associated left ventricular hypertrophy. J Clin Hypert. 2023;25(6):562–572. doi:10.1111/jch.14660
- Luo L, Zeng X, Huang Z, Luo S, Qin L, Li S. Reduced frequency and functional defects of CD4(+)CD25(high)CD127(low/-) regulatory T cells in patients with unexplained recurrent spontaneous abortion. Reprod Biol Endocrinol. 2020;18(1):62. doi:10.1186/s12958-020-00619-7
- Jasper MJ, Tremellen KP, Robertson SA. Primary unexplained infertility is associated with reduced expression of the T-regulatory cell transcription factor Foxp3 in endometrial tissue. Mol Hum Reprod. 2006;12(5):301–308. doi:10.1093/molehr/gal032
- Benghiat FS, Charbonnier LM, Vokaer B, De Wilde V, Le Moine A. Interleukin 17-producing T helper cells in alloimmunity. Transplantat Rev. 2009;23(1):11–18. doi:10.1016/j.trre.2008.08.007
- Huang DL, He YR, Liu YJ, et al. The immunomodulation role of Th17 and Treg in renal transplantation. Front Immunol. 2023;14:1113560. doi:10.3389/fimmu.2023.1113560
- Kroemer A, Belyayev L, Khan K, et al. Rejection of intestinal allotransplants is driven by memory T helper type 17 immunity and responds to infliximab. Am J Transpl. 2021;21(3):1238–1254. doi:10.1111/ajt.16283
- Saifi B, Rezaee SA, Tajik N, et al. Th17 cells and related cytokines in unexplained recurrent spontaneous miscarriage at the implantation window. Reproduct Biomed Online. 2014;29(4):481–489. doi:10.1016/j.rbmo.2014.06.008
- Wang WJ, Hao CF, Yi L, et al. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J Reproduct Immunol. 2010;84(2):164–170. doi:10.1016/j.jri.2009.12.003
- Ghaebi M, Abdolmohammadi-Vahid S, Ahmadi M, et al. T cell subsets in peripheral blood of women with recurrent implantation failure. J Reprod Immunol. 2019;131:21–29. doi:10.1016/j.jri.2018.11.002
- Figueiredo AS, Schumacher A. The T helper type 17/regulatory T cell paradigm in pregnancy. Immunology. 2016;148(1):13–21. doi:10.1111/imm.12595
- Qian J, Zhang N, Lin J, et al. Distinct pattern of Th17/Treg cells in pregnant women with a history of unexplained recurrent spontaneous abortion. Biosci Trends. 2018;12(2):157–167. doi:10.5582/bst.2018.01012
- Kong X, Kong Y, Zhang F, Wang T, Zhu X. Expression and significance of dendritic cells and Th17/Treg in serum and placental tissues of patients with intrahepatic cholestasis of pregnancy. J Mater Fet Neonat Med. 2018;31(7):901–906. doi:10.1080/14767058.2017.1300652
- Braga A, Neves E, Guimarães J, Braga J, Vasconcelos C. Th17 / Treg ratio: a prospective study in a group of pregnant women with preeclampsia and fetal growth restriction. J Reprod Immunol. 2023;159:104122. doi:10.1016/j.jri.2023.104122
- Hosseini S, Shokri F, Ansari Pour S, et al. A shift in the balance of T17 and Treg cells in menstrual blood of women with unexplained recurrent spontaneous abortion. J Reprod Immunol. 2016;116:13–22. doi:10.1016/j.jri.2016.03.001
- Fujii M, Tanaka Y, Okimura H, et al. Decrease in activated regulatory T cell populations in the endometrium during ovulation in endometriosis. J Reproduct Immunol. 2023;156:103825. doi:10.1016/j.jri.2023.103825