Abstract
Purpose
The objective of this study was to assess reproductive outcomes of D6 blastocysts transferred on day 6 in comparison to those transferred on day 7 of progesterone exposure in frozen-thawed embryo transfer cycles.
Patients and Methods
This retrospective cohort study included 2029 D6 single blastocysts from the first frozen-thawed embryo transfer cycles of patients at the Hospital for Reproductive Medicine Affiliated to Shandong University from February 2017 to January 2020. Participants were divided into Group A (blastocyst transferred on the 6th day of progesterone exposure, n=1634) and Group B (blastocyst transferred on the 7th day of progesterone exposure, n=395).
Results
The live birth rate was comparable between Group A and Group B (38.7% versus 38.7%, P=0.999). Subgroup analysis revealed a significantly higher preterm birth rate in D6 single blastocysts transferred on the 7th day than in those transferred on the 6th day of progesterone exposure for natural cycle frozen-thawed embryo transfer (5.2% versus 11.3%, P=0.020). After adjustment for potential confounders, the differences in the preterm birth rate in natural cycles persisted (adjusted odds ratio 2.347, 95% confidence interval 1.129–4.877, P=0.022).
Conclusion
In frozen-thawed embryo transfer cycles, transferring on the 6th or 7th day of progesterone exposure of D6 blastocysts did not affect the live birth rate; however, when a natural cycle protocol is adopted, the possible preterm risk of transferring D6 blastocysts on the 7th day of progesterone exposure should be noted.
Introduction
Over the last 40 years, in vitro fertilization (IVF) has become increasingly common. In the past, frozen-thawed embryo transfer (FET) has been associated with lower pregnancy rates compared with fresh transfers, likely due to suboptimal embryo survival after slow freezing.Citation1 Refinement of vitrification techniques for vitrified-warmed embryos has made it easier to conserve embryos for further use.Citation2 A small randomized controlled trial showed a higher clinical pregnancy rate with frozen-thawed embryo transfer than with fresh embryo transfer.Citation3 A multicenter, randomized trial demonstrated that the live birth rate did not differ significantly between fresh and frozen embryo transfers among ovulating women with infertility, but frozen embryo transfer resulted in a lower risk of ovarian hyperstimulation syndrome.Citation4 Thus, FET has enabled the more widespread use of embryo transfer.
Embryos cultured in vitro usually develop to the blastocyst stage by the 5th day (D5 blastocyst) after fertilization, but slower embryos can reach the blastocyst stage by day 6 (D6 blastocyst) or later. Previous studies have demonstrated that the clinical pregnancy rate (CPR) and live birth rate (LBR) are better with D5 blastocyst transfer than with D6 blastocyst transfer.Citation5 The hypothesized reasons for D6 blastocysts resulting in decreased reproductive outcomes include the quality of D6 blastocysts, which is lower than that of D5 blastocysts, and embryo–endometrium asynchrony.Citation6
It is well known that synchronization between the embryonic stage and the endometrial window of implantation (WOI) is crucial for the success of FET cycles.Citation7 The optimal window for embryo transfer has been shown to be narrow, with the highest rates occurring during a 2-day window.Citation8 Considering embryo–endometrium synchronization, D6 blastocysts are selected for transfer on the 6th or 7th day of progesterone exposure in FET. The reported pregnancy outcomes for D6 blastocysts transferred on the 6th day compared with those transferred on the 7th day remain controversial.
Commonly used protocols for FET in ovulatory women are natural cycles (NC), stimulated cycles (SC), and artificial cycles (AC). NC treatment has a higher chance of live birth and lower risks of pregnancy-induced hypertension (PIH), postpartum hemorrhage (PPH), and very preterm birth (VPTB) than AC for endometrial preparation in women receiving FET cycles.Citation9 The corpus luteum can produce not only estradiol (E2) and progesterone but also vasoactive products, such as relaxin and vascular endothelial growth factor, which are important for initial placentation. It is possible that the absence of a corpus luteum during the AC may contribute to these differences.Citation10 A previous retrospective study reported that D6 blastocysts transferred on the 6th day had higher CPRs and LBRs than those transferred on the 7th day of progesterone exposure.Citation11 However, another study suggested that the CPR and LBR of frozen-thawed D6 blastocysts transferred on the 6th day were not statistically different from those with blastocysts transferred on the 7th day of progesterone exposure in hormone replacement cycles (HRC).Citation12 A subgroup analysis in another study of D6 blastocysts showed that D6 blastocysts transferred on the 6th day were associated with higher miscarriage rates than those transferred on the 7th day of progesterone exposure in HRC with FET.Citation13 However, there is no consensus on the optimal transfer time for frozen-thawed D6 blastocysts. Therefore, we designed this retrospective cohort study to assess reproductive outcomes of D6 blastocysts transferred on day 6 in comparison to those transferred on day 7 of progesterone exposure in frozen-thawed embryo transfer cycles.
Materials and Methods
Study Population
This retrospective study included D6 single-blastocyst transfers in their first frozen-thawed embryo transfer cycle of IVF or intracytoplasmic sperm injection (ICSI) at the Reproductive Hospital Affiliated to Shandong University from February 2017 to January 2020. Although most of the D6 vitrified-warmed blastocysts were transferred on the 6th day of progesterone exposure, some D6 vitrified-warmed blastocysts were transferred on the 7th day of progesterone exposure. Supernumerary embryos after fresh-embryo transfer and embryos from whole-embryo freezing cycles were included in the analysis. Patients were excluded if they: (i) were ≥38 years old, (ii) were diagnosed with a double uterus with or without a double vagina and double cervix, (iii) had undergone preimplantation genetic testing, (iv) had transferred two blastocysts at a time, and (v) were oocyte recipients, defined as patients receiving oocytes from a donor. Finally, 2029 D6 single blastocysts in FET cycles were included in the study, with 1634 on the 6th day of progesterone exposure (Group A) and 395 on the 7th day of progesterone exposure (Group B).
Measures
Ovarian Stimulation and Embryo Scoring
Individualized protocols for controlled ovarian hyperstimulation were determined by experienced clinicians and were initiated using either recombinant follicle-stimulating hormone or human menopausal gonadotropin, as previously described.Citation14 When at least two follicles were 18 mm or greater in mean diameter, human chorionic gonadotropin (hCG) at a dose of 4000–10,000 IU was administered to induce the final maturation of oocytes. Oocyte retrieval was performed 34–36 h after hCG injection by experienced physicians. Fertilization was performed using conventional IVF or ICSI according to sperm quality. All oocyte retrieval and fertilization procedures were implemented in accordance with our hospital standards, as previously described.Citation15 According to morphologic criteria, embryos with scores of 6–8C at cleavage stage ≥2 were defined as excellent embryos. The degree of expansion, quality of the internal cell mass, and quality of trophectoderm cells were considered in the assessment of the quality of blastocysts according to the Gardner scoring system.Citation16 Blastocysts were divided into three groups according to morphologic quality score: Excellent (3–6 AA/AB/BA), Good (2–6 BB), and Poor (3–6 BC/CB/CC). The choice of fresh embryo transfer or frozen embryo transfer was determined based on the patient risk for ovarian hyperstimulation syndrome. The procedure for supernumerary blastocyst vitrification in our center has been previously described.Citation17
Endometrial Preparation Protocols for FET Cycles
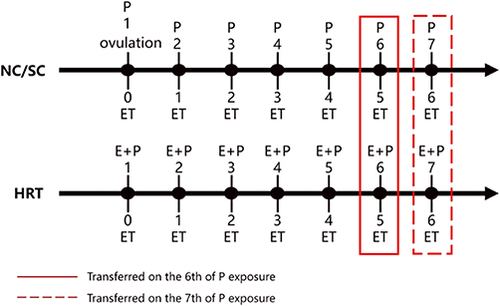
FET was performed using NC, SC, or HRC. The choice of endometrial preparation protocol was based on patient characteristics and the physician’s preference. In general, patients with regular ovulation were allocated to NC, whereas those with irregular ovulation were allocated to modified NC or HRC. For conventional NC, the development of ovarian follicles was monitored using transvaginal ultrasound. Tests for serum E2, luteinizing hormone (LH) and progesterone were carried out to ascertain the timing of ovulation and the start time of progesterone administration. For SC, if the dominant follicle was not monitored using transvaginal ultrasound when the patient chose NC, human menopausal gonadotropin was administered to promote follicle growth. The monitoring of ultrasound and serum hormones was the same as in NC. Oral dydrogesterone (20–40 mg daily) was administered for luteal-phase support after ovulation in NC and SC. In HRC, oral E2 valerate, at a dose of 4–8 mg daily, was initiated on day 1–3 of the menstrual cycle; vaginal progesterone gel (90 mg per day) and oral dydrogesterone (10 mg twice daily) were added when the endometrial thickness reached 7 mm or more.Citation18 Transfer was considered for planning when the endometrium had a tri-laminar appearance and a thickness of at least 8 mm. FET protocols are illustrated in .
Luteal Phase Support
Luteal support started from the day of ovulation and continued until the day of serum hCG testing. Biochemical pregnancy was defined as a β-hCG level >10 U/L. For women with a positive result, progesterone was continued until 10 weeks of gestation. If conception occurred and an intrauterine gestational sac was detected using transvaginal ultrasound at 7 weeks of gestational age, clinical pregnancy was confirmed. Information on pregnancy, obstetric, and perinatal outcomes was obtained through a review of obstetric and neonatal medical records.
Outcome Measures
The primary outcome was live birth rate. Live birth rate was defined as the delivery of any surviving newborn at ≥24 weeks of gestation. Secondary efficacy outcomes included the biochemical pregnancy rate (biochemical pregnancy was defined as hCG >10 mIU/mL, measured 14 days after embryo transfer), the clinical pregnancy rate (the presence of a gestational sac in uterine cavity 35 days after embryo transfer), miscarriage rate (spontaneous loss of clinical pregnancy before 24 completed weeks of gestational age), and preterm birth rate (preterm birth was defined as births occurring after 22 weeks and before 37 completed weeks of gestational age). These definitions were in accordance with the latest revision of The International Glossary on Infertility and Fertility Care (2017).Citation19
Analysis
All statistical analyses were performed using SPSS 26.0 (IBM Corp., Armonk, NY, USA), and two-sided P-values <0.05 were considered statistically significant. The chi-squared test was used to compare categorical data. The normal distribution of continuous data was evaluated. Continuous variables are presented as mean and standard deviation. The data were compared using a t-test when they were normally distributed and the Mann–Whitney U-test when they were not normally distributed. Multivariate logistic regression was performed to identify the factors influencing preterm birth in NC; adjusted odds ratios (aOR) and 95% confidence intervals (CI) are presented. The candidate variables for multivariate logistic regression were those with P < 0.05 in a univariate analysis as well as those known to affect preterm birth.
Results
In total, 2029 FET cycles of D6 single blastocysts were performed; the number of D6 single-blastocysts transferred on the 6th and 7th days of progesterone exposure were 1634 (Group A) and 395 (Group B), respectively.
Characteristics of the 2029 patients are shown in . The results showed a significant difference in antral follicle count (AFC) between the two groups (15.14±9.03 vs 14.15±8.28, P=0.048), but there were no statistically significant differences in age (31.33±3.88 vs 31.06±3.70, P=0.205), BMI (23.87±3.79 vs 23.88±3.65, P=0.972), AMH (3.76±3.10 vs 3.90±3.10, P=0.439), type of infertility (P=0.094), etiology of infertility (P=0.274), FSH (6.82±2.32 vs 6.62±2.18, P=0.125), LH (5.60±3.87 vs 5.52±3.71, P=0.702), E2 (45.12±49.93 vs 44.64±51.34, P=0.865), the number of retrieved oocytes (10.35±6.10 vs 10.51±6.25, P=0.649), number of excellent embryos (4.38±3.16 vs 4.46±3.279, P=0.692), and fresh embryo transfer rate (42.8% vs 40.5%, P=0.400) between Group A and Group B.
Table 1 Patients Characteristics Between Group A and Group B
The FET cycle parameters are presented in . Endometrial thickness (0.95±0.17 vs 0.95±0.17, P=0.654) and blastocyst morphological grade (P=0.777) were not significantly different between the groups. However, the proportion of patients receiving different protocols for endometrium preparation, including NC (58.0% vs 55.2%, respectively), SC (5.8% vs 10.1%, respectively), and HRC (36.2% vs 34.7%, respectively) differed statistically (P=0.009) between Group A and Group B.
Table 2 Frozen-Embryo Transfer Cycles Parameters and Pregnancy Outcomes Between Group A and Group B
Based on the actual treatment received by the patients, we carried out intragroup comparisons. The biochemical pregnancy rate was higher in Group A than in Group B (60.2% vs 53.2%, P=0.011). There was no significant difference in the CPR (49.8% vs 47.1%, P=0.330), miscarriage rate (21.6% vs 17.7%, P=0.240), preterm birth rate (6.8% vs 8.6%, P=0.377), or LBR (38.7% vs 38.7%, P=0.999) between the groups ().
A subgroup analysis was performed to compare pregnancy outcomes of D6 single blastocysts transferred on the 6th day with those transferred on the 7th day of progesterone exposure in different types of endometrium preparation. The preterm birth rate was higher for blastocysts transferred on the 7th day than those transferred on the 6th day of progesterone exposure in NC (, 11.3% vs 5.2%, P=0.020). The LBR did not differ significantly between groups for NC, SC, and HRC ().
Table 3 Pregnancy Outcomes of the Subgroup Analysis Comparing Group A with Group B in NC, SC and HRC Respectively
Multivariate regression of the preterm birth rate showed the same result (; aOR 2.347, 95% CI [1.129–4.877], P=0.022). Factors with significant differences in the univariate analysis () and those known to affect preterm birth were included in the multivariate logistic regression.
Table 4 Multivariable Regression Analysis of Preterm Birth Rate
Table 5 Univariable Analysis of Patients (with No Preterm Birth or Preterm Birth)’ Basic Characteristics in Natural Cycles
In total, 1528 patients with excellent and average blastocyst morphological grades were included in the multivariate analysis. The number of cycles of D6 single blastocysts transferred on the 6th and 7th day of progesterone exposure was 1228 (Group C) and 300 (Group D), respectively. The demographics of this group were similar to those of the overall study sample. Characteristics of the 1528 patients are shown in . There were no statistically significant differences in age (31.22±3.91 vs 30.97±3.67, P=0.306), BMI (23.85±3.81 vs 23.94±3.76, P=0.708), AMH (3.79±3.04 vs 3.94±3.11, P=0.453), type of infertility (P=0.169), etiology of infertility (P=0.115), FSH (6.85±2.66 vs 6.76±3.51, P=0.607), LH (5.66±3.92 vs 5.57±3.84, P=0.713), E2 (45.52±45.25 vs 46.20±57.95, P=0.589), antral follicle count (15.09±8.80 vs 14.26±8.46, P=0.140), number of retrieved oocytes (10.56±6.09 vs 10.55±6.33, P=0.973), number of excellent embryos (4.56±3.15 vs 4.61±3.40, P=0.800), and the fresh embryo transfer rate (45.8% vs 40.0%, P=0.072) between Group C and Group D.
Table 6 Characteristics of Patients with Excellent and Average Blastocyst Morphological Grades Between Group C and Group D
The FET cycle parameters are presented in . Protocols of endometrium preparation (P=0.094), endometrial thickness (0.97±0.17 vs 0.96±0.17, P=0.549), and blastocyst morphological grade (P=0.110) were not significantly different between the groups.
Table 7 Frozen-Embryo Transfer Cycles Parameters and Pregnancy Outcomes Between Group C and Group D in Patients with Excellent and Average Blastocyst Morphological Grades
The biochemical pregnancy rate was higher in Group C than in Group D (63.4% vs 55.0%, P=0.007). There was no significant difference in the CPR (54.2% vs 49.3%, P=0.134), miscarriage rate (20.0% vs 16.2%, P=0.292), preterm birth rate (7.7% vs 8.1%, P=0.857), or LBR (42.8% vs 41.0%, P=0.565) between the groups ().
Discussion
Several studies have explored the optimal transfer time for frozen-thawed D6 blastocysts, but controversy remains.Citation11–13,Citation20 In this study, we found no difference in the LBR of D6 single-blastocyst embryos transferred on the 6th day compared with those transferred on the 7th day of progesterone exposure. Comparing subgroups of patients with excellent and average blastocyst morphological grades, there was also no difference in LBR. However, an additional detailed subgroup analysis of FET endometrium preparation protocols was performed, showing that the preterm birth rate was higher in blastocysts transferred on the 7th day than on the 6th day of progesterone exposure in NC. To our knowledge, this is currently the largest-sample study comparing D6 single blastocysts transferred on the 6th day compared with those transferred on the 7th day of progesterone exposure.
Blastocyst transfer may be advantageous in assisted reproduction techniques because the exposure of the embryo to the uterine environment is similar to the NC.Citation21 In addition, blastocyst culture improves synchronization of the uterus and embryo and the ability to self-select viable embryos, thereby increasing implantation rates.Citation22 Embryos that are cultured in vitro usually develop to D5 blastocysts after fertilization, but slower embryos can achieve blastulation on Day 6 (D6 blastocysts). The question of whether D6 blastocysts should be transferred on the 6th day of progesterone exposure poses a dilemma. There is often concern that the embryo–endometrium asynchrony when using D6 blastocysts may increase the risk of pregnancy failure.Citation23 The optimal duration of progesterone exposure in FET cycles thus assumes the utmost importance for ensuring the best FET outcomes. This information could be important to reassure couples who conceive following the transfer of a D6 blastocyst.
The outcomes of assisted reproductive technology pregnancies are influenced by endometrial receptivity and embryo–endometrium synchrony.Citation24 Endometrial receptivity is regulated by many factors, including uterine anatomical factors, immunity, and metabolism.Citation7,Citation25 With the influence of inflammation or other factors, shortening of or missing the endometrial implantation window can lead to infertility or pregnancy failures.Citation26 Estrogen and progesterone are important regulatory factors in the implantation process. Estrogen and progesterone bind to specific high-affinity receptors, which, in turn, regulate the transcription of a large number of genes that drive the endometrium to enter a period of receptivity.Citation27 The time of progesterone exposure is related to the endometrial implantation window. Whether the embryo is synchronized with the endometrium at the WOI determines whether a blastocyst can be successfully implanted.Citation28 A previous study found that D6 blastocysts transferred on the 6th day had a higher LBR compared with those transferred on the 7th day of progesterone exposure; it may be assumed that each embryo has to spend a certain amount of time in the uterus before implantation and that the WOI is more likely to have closed when the embryo is transferred on the 7th day of progesterone exposure.Citation11 However, the results of our study demonstrated similar LBRs between D6 blastocysts transferred on the 6th and 7th days of progesterone exposure.
Most previous studies have focused on the optimal duration of progesterone exposure before transferring frozen-thawed blastocysts in HRC.Citation12,Citation13 However, this has remained elusive for different endometrium preparation protocols of FET, especially in NC, which is generally the preferred method for preparing the endometrium. Previous studies have indicated comparable pregnancy outcomes among NC, SC, and HRC, and there has been insufficient evidence to support the use of one protocol over another.Citation29,Citation30 Reproductive clinicians should choose endometrium preparation protocols individually according to ovulation and other important factors. Thus, it is possible that the optimal duration of progesterone exposure has an effect on pregnancy outcomes in different endometrium preparation protocols. For this reason, we performed a subgroup analysis to compare pregnancy outcomes in NC, SC, and HRC regimens. We found that the LBR was not significantly different between D6 single blastocysts transferred on the 6th and 7th days of progesterone exposure in NC, SC, and HRC. However, a higher preterm birth rate was seen for blastocysts transferred on the 6th day than on the 7th day in NC. Thus, we speculated that the WOI of SC and HRC is more stable than that of NC due to the regulation of exogenous hormones. FranasiakCitation7 also proposed that, although implantation can occur in a broad window, the optimal time might be more restricted NC. Another consideration was that the histological dating may be inconsistent with the actual day after ovulation.Citation1 Further, delayed endometrial development in the luteal phase has been shown in around a quarter of women.Citation31 On the basis of this finding, when choosing NC as an endometrium preparation protocol for FET, one should note the preterm birth risk for patients transferring D6 blastocysts. Infection, cervical pathology, uterine overdistension, progesterone deficiency, stress on the mother and fetus, allograft reaction, and allergic phenomena, may lead to preterm birth. These several causes may improperly stimulate the usual pathway between the decidua and the fetal membranes, resulting in cervical ripening, membrane rupture, and uterine contractility, and finally, preterm birth occurs.Citation32
Age and the aneuploidy rate may influence the embryo–endometrium synchrony.Citation3 One study found that, if embryos were selected on the basis of morphology alone, the euploidy rate was 86% in patients 22–34 years of age and 69% in those 35–44 years of age.Citation33 In our study, there was no difference in age between the two groups, and the effects of age on the euploidy rate and age itself on synchrony can be ignore assumed negligible. One previous study also suggested that D6 embryos have poor embryo quality and high aneuploidy rates.Citation34 However, another study compared D5 and D6 blastocysts that underwent a single biopsy in FET cycles and did not demonstrate differences in euploidy, aneuploidy, or mosaicism rates.Citation35 Even if patients underwent PGT, the false-negative rate of a low-level mosaic embryo and the health risk of the fetus cannot be avoided.Citation36 In our study, we excluded patients who underwent PGT, thus preventing us from determining whether the embryos had chromosomal abnormalities that might influence pregnancy outcomes. In the future, studies including D5 and D6 embryos that have undergone PGT may provide more convincing results.
This is the largest retrospective study of the effect of progesterone exposure time on pregnancy outcomes in D6 single blastocyst frozen-thawed embryo transfer. Subgroup analysis was performed to analyze the effects of different endometrial preparation protocols on the pregnancy outcomes of D6 single blastocyst embryo transfer. This study provides an important reference and basis for the time of D6 single blastocyst embryo transfer. This study had several limitations. A matched propensity score analysis may be necessary due to differences in sample size between groups. However, there were no significant differences in group characteristics, as a result, the findings are unlikely to have been skewed by this. Further, this study was retrospective, and we were not able to completely rule out all potential confounders. In addition, we did not measure hormone levels before luteal support, and more rigorous studies on this are needed. Meanwhile, further prospective research with sufficient sample sizes and more sophisticated stratified analyses is needed to confirm these findings. In addition, our study included only D6 blastocyst transfers; pregnancy outcomes of fresh blastocyst transfers and D5 blastocyst transfers during the same period should be compared.
Conclusion
In conclusion, in FET cycles, D6 blastocyst transfer on the 6th or 7th day of progesterone exposure did not affect the LBR; however, if the NC protocol was adopted, the higher possible preterm birth risk of blastocyst transferred on the 7th day should be noted.
Ethics Approval and Informed Consent
This study complied with the Declaration of Helsinki. This study was approved by the independent ethics committee of the Center for Reproductive Medicine Shandong University. Informed consent was obtained from all the patients for this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The authors thank our patients and all participants in the data collection.
Data Sharing Statement
The data used to support the findings of this study are included in the article. Also, if readers need detailed information, he/she can Email the corresponding author.
Additional information
Funding
References
- Casper RF, Yanushpolsky EH. Optimal endometrial preparation for frozen embryo transfer cycles: window of implantation and progesterone support. Fertil Steril. 2016;105(4):867–872. doi:10.1016/j.fertnstert.2016.01.006
- Rienzi L, Gracia C, Maggiulli R, et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update. 2017;23:139–155.
- Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011;96(2):344–348. doi:10.1016/j.fertnstert.2011.05.050
- Shi Y, Sun Y, Hao C, et al. Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med. 2018;378(2):126–136. doi:10.1056/NEJMoa1705334
- Bourdon M, Pocate-Cheriet K, Finet de Bantel A, et al. Day 5 versus Day 6 blastocyst transfers: a systematic review and meta-analysis of clinical outcomes. Hum Reprod. 2019;34(10):1948–1964. doi:10.1093/humrep/dez163
- Yerushalmi GM, Shavit T, Avraham S, et al. Day 5 vitrified blastocyst transfer versus day 6 vitrified blastocyst transfer in oocyte donation program. Sci Rep. 2021;11(1):10715. doi:10.1038/s41598-021-90238-y
- Franasiak JM, Ruiz-Alonso M, Scott RT, Simón C. Both slowly developing embryos and a variable pace of luteal endometrial progression may conspire to prevent normal birth in spite of a capable embryo. Fertil Steril. 2016;105(4):861–866. doi:10.1016/j.fertnstert.2016.02.030
- Navot D, Scott RT, Droesch K, Veeck LL, Liu HC, Rosenwaks Z. The window of embryo transfer and the efficiency of human conception in vitro. Fertil Steril. 1991;55(1):114–118. doi:10.1016/S0015-0282(16)54069-2
- Wu H, Zhou P, Lin X, Wang S, Zhang S. Endometrial preparation for frozen-thawed embryo transfer cycles: a systematic review and network meta-analysis. J Assist Reprod Genet. 2021;38(8):1913–1926. doi:10.1007/s10815-021-02125-0
- Singh B, Reschke L, Segars J, Baker VL. Frozen-thawed embryo transfer: the potential importance of the corpus luteum in preventing obstetrical complications. Fertil Steril. 2020;113(2):252–257. doi:10.1016/j.fertnstert.2019.12.007
- Bilgory A, Kalma Y, Kopel R, Azem F. Transfer of day 6 frozen-thawed blastocysts on day 5 compared with day 6: catching up with the window of implantation-a retrospective study. Reprod Sci. 2021;28(8):2208–2215. doi:10.1007/s43032-021-00458-w
- Yang X, Bu Z, Hu L. Live birth rate of frozen-thawed single blastocyst transfer after 6 or 7 days of progesterone administration in hormone replacement therapy cycles: a propensity score-matched cohort study. Front Endocrinol. 2021;12:706427. doi:10.3389/fendo.2021.706427
- Roelens C, Santos-Ribeiro S, Becu L, et al. Frozen-warmed blastocyst transfer after 6 or 7 days of progesterone administration: impact on live birth rate in hormone replacement therapy cycles. Fertil Steril. 2020;114(1):125–132. doi:10.1016/j.fertnstert.2020.03.017
- Wang Z, Zhao J, Ma X, et al. Effect of orlistat on live birth rate in overweight or obese women undergoing IVF-ET: a randomized clinical trial. J Clin Endocrinol Metab. 2021;106(9):e3533–e3545. doi:10.1210/clinem/dgab340
- Chen ZJ, Shi Y, Sun Y, Zhang B, Liang X, Cao Y. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N Engl J Med. 2016;375(6):523–533. doi:10.1056/NEJMoa1513873
- Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73(6):1155–1158. doi:10.1016/S0015-0282(00)00518-5
- Li S, Ma S, Zhao J, et al. Non-assisted hatching trophectoderm biopsy does not increase the risks of most adverse maternal and neonatal outcome and may be more practical for busy clinics: evidence from China. Front Endocrinol. 2022;13:819963. doi:10.3389/fendo.2022.819963
- Wei D, Liu JY, Sun Y, et al. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet. 2019;393(10178):1310–1318. doi:10.1016/S0140-6736(18)32843-5
- Zegers-Hochschild F, Adamson GD, Dyer S, et al. The international glossary on infertility and fertility care, 2017. Hum Reprod. 2017;32(9):1786–1801. doi:10.1093/humrep/dex234
- Xu H, Qiu S, Chen X, Zhu S, Sun Y, Zheng B. D6 blastocyst transfer on day 6 in frozen-thawed cycles should be avoided: a retrospective cohort study. BMC Pregnancy Childbirth. 2020;20(1):519. doi:10.1186/s12884-020-03224-z
- Oatway C, Gunby J, Daya S. Day three versus day two embryo transfer following in vitro fertilization or intracytoplasmic sperm injection. Cochrane Database Syst Rev. 2004;2:CD004378.
- Glujovsky D, Farquhar C, Quinteiro Retamar AM, Alvarez Sedo CR, Blake D. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2016;6:CD002118.
- Sehring J, Beltsos A, Jeelani R. Human implantation: the complex interplay between endometrial receptivity, inflammation, and the microbiome. Placenta. 2022;117:179–186.ary 2006.
- Bortoletto P, Bakkensen J, Anchan RM. Embryo transfer: timing and techniques. Minerva Endocrinol. 2018;43(1):57–68. doi:10.23736/S0391-1977.17.02649-9
- Karizbodagh MP, Rashidi B, Sahebkar A, Masoudifar A, Mirzaei H. Implantation window and angiogenesis. J Cell Biochem. 2017;118(12):4141–4151. doi:10.1002/jcb.26088
- Lessey BA, Young SL. What exactly is endometrial receptivity? Fertil Steril. 2019;111(4):611–617. doi:10.1016/j.fertnstert.2019.02.009
- Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 1999;340(23):1796–1799. doi:10.1056/NEJM199906103402304
- Ferreux L, Bourdon M, Sallem A, et al. Live birth rate following frozen-thawed blastocyst transfer is higher with blastocysts expanded on Day 5 than on Day 6. Hum Reprod. 2018;33(3):390–398. doi:10.1093/humrep/dey004
- Groenewoud ER, Cohlen BJ, Al-Oraiby A, et al. A randomized controlled, non-inferiority trial of modified natural versus artificial cycle for cryo-thawed embryo transfer. Hum Reprod. 2016;31(7):1483–1492. doi:10.1093/humrep/dew120
- Glujovsky D, Pesce R, Sueldo C, Quinteiro Retamar AM, Hart RJ, Ciapponi A. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Database Syst Rev. 2020;10(10):CD006359. doi:10.1002/14651858.CD006359.pub3
- Murray MJ, Meyer WR, Zaino RJ, et al. A critical analysis of the accuracy, reproducibility, and clinical utility of histologic endometrial dating in fertile women. Fertil Steril. 2004;81(5):1333–1343. doi:10.1016/j.fertnstert.2003.11.030
- Khandre V, Potdar J, Keerti A. Preterm birth: an overview. Cureus. 2022;14(12):e33006. doi:10.7759/cureus.33006
- Forman EJ, Upham KM, Cheng M, et al. Comprehensive chromosome screening alters traditional morphology-based embryo selection: a prospective study of 100 consecutive cycles of planned fresh euploid blastocyst transfer. Fertil Steril. 2013;100(3):718–724. doi:10.1016/j.fertnstert.2013.04.043
- Desai N, Ploskonka S, Goodman L, et al. Delayed blastulation, multinucleation, and expansion grade are independently associated with live-birth rates in frozen blastocyst transfer cycles. Fertil Steril. 2016;106(6):1370e8. doi:10.1016/j.fertnstert.2016.07.1095
- Wu TF, Chen MJ, Lee MS, et al. Comparison of clinical outcome between day 5 and day 6 single blastocyst transfers in cycles undergoing preimplantation genetic testing for aneuploidy. Taiwan J Obstet Gynecol. 2023;62(3):429–433. doi:10.1016/j.tjog.2023.03.005
- Greco E, Yakovlev P, Kornilov N, et al. Two clinical case reports of embryonic mosaicism identified with PGT-A persisting during pregnancy as true fetal mosaicism. Hum Reprod. 2023;38(2):315–323. doi:10.1093/humrep/deac263