Abstract
Purpose
To explore the risk and protective factors for developing ovarian cancer and construct a risk prediction model.
Methods
Information related to patients diagnosed with ovarian cancer on the electronic medical record data platform of three tertiary hospitals in Guangdong Province from May 2018 to September 2023 was collected as the case group. Patients with non-ovarian cancer who attended the clinic during the same period were included in the control group. Logistic regression analysis was used to screen the independent variables and explore the factors associated with the development of ovarian cancer. An ovarian cancer risk prediction model was constructed using a decision tree C4.5 algorithm. The ROC and calibration curves were plotted, and the model was validated.
Results
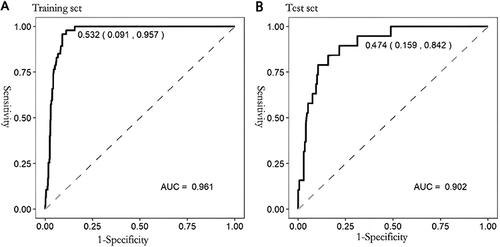
Logistic regression analysis identified independent risk and protective factors for ovarian cancer. The sample size was divided into training and test sets in a ratio of 7:3 for model construction and validation. The AUC of the training and test sets of the decision tree model were 0.961 (95% CI:0.944–0.978) and 0.902 (95% CI:0.840–0.964), respectively, and the optimal cut-off values and their coordinates were 0.532 (0.091, 0.957), and 0.474 (0.159, 0.842) respectively. The accuracies of the training and test sets were 93.3% and 84.2%, respectively, and their sensitivities were 95.7% and 84.2%, respectively.
Conclusion
The constructed ovarian cancer risk prediction model has good predictive ability, which is conducive to improving the efficiency of early warning of ovarian cancer in high-risk groups.
Introduction
Ovarian cancer (OC) is a common malignant tumor of the female reproductive system, ranking 3rd among gynecological malignancies.Citation1 90% of ovarian cancers are of an epithelial cell type and comprise multiple histologic types, with various specific molecular changes, clinical behaviors, and treatment outcomes. The remaining 10% are non-epithelial ovarian cancers, which include mainly germ cell tumors, sex cord-stromal tumors, and some sporadic tumors such as small cell carcinomas. Germ cell tumors are the most common ovarian neoplasms in women up to 30 years of age, and most of the patients are diagnosed with early-stage disease (60–70%).Citation2 There are approximately 200,000 new cases of ovarian cancer globally every year, with a mortality rate as high as 125,000 cases/year. Interestingly, the prevalence of asymptomatic ovarian masses has risen alongside the widespread use of prenatal ultrasonography. Approximately 5% of ovarian tumors complicating pregnancies, are malignant, contributing to the global increase in new cases of ovarian cancer. Currently, surgical intervention is indicated for an ovarian mass > 6 cm in diameter or when symptomatic.Citation3 Incidence trends differ between countries and regions;Citation4 the incidence rate of ovarian cancer in developed countries is decreasing by 2.8% annually on average.Citation5 In China, however, the incidence of ovarian cancer is increasing, and the number of new cases and deaths from ovarian cancer is expected to more than triple between 2019 and 2049.Citation6 Survey data from Shanghai, China, show that the incidence of ovarian cancer is increasing at a rate of 1.8% annually, and the annual mortality rate is expected to increase by 0.6%.Citation7 The onset of ovarian cancer is insidious, with atypical initial symptoms, making detection by patients or diagnosis by doctors difficult. Over 50% of patients are diagnosed at advanced stages,Citation8 often miss the best time for intervention,Citation9,Citation10 and the 3-year survival rate is lower than 30%.Citation11 The prevention and treatment of ovarian cancer have become major social and public health issues.
There are two types of epithelial ovarian cancer. Type I epithelial ovarian cancers are relatively indolent and genetically stable tumors that typically arise from recognizable precursor lesions, such as endometriosis or borderline tumors with low malignant potential. In contrast, type II epithelial ovarian cancers are thought to be biologically aggressive tumors from the outset, with a propensity for metastasis from small-volume primary lesions. High-grade serous carcinoma, the most common type of epithelial ovarian cancer, accounting for approximately 75% of epithelial ovarian cancers, develops according to the type II pathway and present p53 and BRCA mutations.Citation12 The development of ovarian cancer may be associated with hormone use, disease, reproduction, lifestyle, family history, and genetic factors.Citation13 Effective screening tools are lacking because the cause of the disease remains unclear. For people at a high risk of ovarian cancer, multimodal screening (blood test for CA 125 combined with transvaginal ultrasound) has been shown to detect ovarian cancer earlier. However, it has not reduced the overall mortality rate of ovarian cancer and can lead to overmedication and complications.Citation14 Currently, CA 125 and HE4 are the only approved biomarkers for epithelial ovarian cancer. However, they are inadequate for early detection.
Multivariate index assays have been developed to mitigate the limitations of single serum biomarkers, particularly during the pre-surgical evaluation of adnexal masses. The Risk of malignancy algorithm (ROMA) integrates menopausal status, CA 125, and HE4 concentrations to diagnose women with pelvic masses. miRNAs (microRNAs) may have remarkable potential in various aspects of epithelial ovarian cancer prediction. However, further research is warranted regarding its characterization as a biomarker. In particular, before miRNAs can be utilized as reliable biomarkers for clinical use, the steps involved in processing samples must be standardized, and the platforms for detecting miRNA in tumors and blood must be refined.Citation15 For the general population, it is even more challenging to perform multimodal screening because of medical, economic, social, and cultural factors and limited clinical promotion. Currently, scientific management, early warning of high-risk groups, and promotion of healthy lifestyle choices are among the best ways to prevent ovarian cancer.
Therefore, a deeper understanding of ovarian cancer and the search for possible risk and protective factors for developing it is essential for its prevention. Currently, in China, there is a lack of accurate prediction models for the risk of developing ovarian cancer based on individuals’ easily accessible information. In this study, we explored new risks and evaluated known protective factors for ovarian cancer incidence through retrospective analysis. We constructed a relevant prediction model based on a machine-learning approach, providing a reference for early warning of this disease and clinical decision-making.
Materials and Methods
Objectives of the Study
This was a retrospective study, and data were obtained from the electronic medical record data platforms of three tertiary hospitals in Guangzhou City. Data were collected from 67 hospitalized ovarian cancer patients on these platforms from May 2018 to September 2023, forming the ovarian cancer case group and 567 patients in the non-ovarian cancer control group. The inclusion criteria for the case group were: 1) age ≥18 years; 2) hospitalized surgical patients diagnosed with ovarian cancer by histopathology, without preoperative chemotherapy, radiation therapy, and immunotherapy; and 3) complete case information. The exclusion criteria were concurrent malignant tumors of other organs or tissues and other serious systemic diseases (abnormalities of liver and kidney functions, blood diseases, infections, and inflammation). The inclusion criteria for the control group were members of the general population who had undergone physical examinations or consultations during the same period. The research department collected the data used in this study; it has standardized protocols and principles to follow to prevent the leakage of patient personal information. The study complied with medical ethics standards and was reviewed by the Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine (review approval number: ZE2023-291). When the patient agreed to be hospitalized, the doctor signed an admission notice after informing them of their condition. There is an entry in the patient’s bill of rights, which states that the patient’s disease-related information will be entered into an electronic data information platform. When medical research requires it, relevant data may be retrieved for statistical analysis while ensuring patient confidentiality. Owing to the retrospective nature of this study, an ethical review was conducted based on informed consent.
Research Methodology
In accordance with previous publications and other related studies, each patient’s dataset included general information (age, education, height, weight, BMI, occupation, blood type, marital status, ethnicity, family history, history of previous illnesses, and medication use), menstrual status (age at menarche, age at menopause, regular menstruation in the past, dysmenorrhea, and others), pregnancy (number of pregnancies and births, age at first pregnancy, and age at first childbirth), and lifestyle (contraceptive methods, history of smoking, alcohol consumption, coffee consumption, bowel movements, and experience of adverse life events), excluding more than 30% of the missing important medical history data.
Quality Control
Study participants were strictly selected according to the inclusion and exclusion criteria to reduce selection bias. Two experienced researchers extracted and output data from the medical history records of the hospital’s HIS medical record information management system. We further supplemented the partial data for each medical record using statistical software to ensure data integrity. Subsequently, we entered the data using the Epidta tool to double-check, and correct logic errors. Ensure the reliability of the sample in the end.
Statistical Methods
SPSS 23.0 and R 4.2.3 statistical packages were used for statistical analysis. Logistic regression analysis was used to screen the independent variables and statistically analyze the relevant factors, with P<0.05 considered statistically significant. Seventy percent of the cases in the dataset were randomly selected as the training set for modeling, and the remaining 30% were used as the test set. The ovarian cancer risk prediction model was constructed based on the decision tree C4.5 algorithm, and receiver operating characteristic (ROC) and calibration curves were plotted for both the training and test sets to assess the differentiation and calibration of the model.
Results
Sample Size
The sample size was calculated using the PASS 15.0 software. The sample size was calculated by selecting the allowable error and overall standard deviation based on the data from previous studies and considering 20% of invalid questionnaires.
Logistic Regression Analysis of Risk Factors for Ovarian Cancer
According to the logistic regression analysis of the influencing factors that may be associated with ovarian cancer in the reported literature at home and abroad, a family history of oncologic disease, being unmarried, having uterine fibroids, the number of abortions, and having received hormone replacement therapy (HRT) were independent risk factors for developing ovarian cancer; occupation as a farmer, and irregular menstruation were protective factors for ovarian cancer ().
Table 1 Ovarian Cancer Risk Factors - Logistic Regression Analysis
Construction of Decision Tree Model
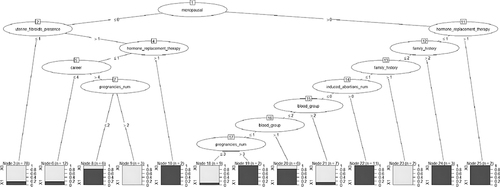
The samples were divided into training and test sets in a ratio of 7:3. Because of the imbalance between the case and control groups (67 vs 567), the samples were re-divided in the training set using an under-sampling method at a case-to-control ratio of 1:2 to train the model and entire training set. Fifteen variables were screened using logistic regression and used to construct a decision tree model using the C4.5 algorithm. The relevant factors of the input node include occupation, blood type, number of cesarean sections, number of induced abortions, marital status, family history, age at menarche, menopause history, cumulative menstrual years, regularity of menstruation, number of natural abortions, contraceptive methods, history of uterine fibroids, and whether hormone replacement therapy had been used. The factors included in the model were menopausal status, presence of uterine fibroids, use of hormone replacement therapy, career, number of pregnancies, family history, number of induced abortions, and blood group. The results showed that menopause was the root node of the decision tree, and several sub-variables, such as uterine fibroids, hormone replacement therapy, occupation, and pregnancy frequency, subsequently entered the decision tree as sub-nodes. ()
Validation of the Decision Tree Prediction Model
ROC Curve
The AUC of the training and test sets of the decision tree model were 0.961 (95% CI:0.944–0.978) and 0.902 (95% CI:0.840–0.964), respectively, and the optimal truncation values and their coordinates were 0.532 (0.091, 0.957) and 0.474 (0.159, 0.842), respectively. The accuracies of the training and test sets were 93.3% and 84.2%, respectively, and the sensitivities were 95.7% and 84.2%, respectively, suggesting that the model predicted the risk of ovarian cancer with reasonable discrimination ().
Calibration Curves
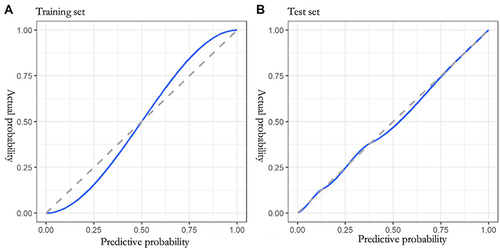
The calibration curve graphs show that the calibration curves of the training and test sets fit well and deviate less from the ideal curve, indicating that the calibration of the model constructed in this study was good ().
Discussion
Ovarian cancer risk factors are among the most challenging issues for researchers and gynecologists. In addition to well-known genetic factors,Citation16 gynecologic and lifestyle-related risk factors have also been identified.Citation17 In the past decade, cost-effective strategies for early detection and prevention of OC in the general population have been investigated. Public health strategies for OC prevention and early treatment are paramount to improving disease outcomes.Citation18 However, many patients in general populations are not diagnosed early due to ineffective screening tools and treatment programs. Prophylaxis is effective at reducing OC incidence and mortality.
Furthermore, the literature suggests a clear economic benefit of prophylactic measures within high-risk groups. The cost of treatment per patient with OC remains the highest among all cancer types. For example, the average initial cost in the first year can amount to around USD 80,000, whereas the final year cost may increase to USD 100,000.Citation19 In this study, we retrospectively analyzed the influencing factors and clinical data of patients with ovarian cancer in three hospitals in Guangzhou City over the past five years. Logistic regression analysis showed that seven variables were significantly associated with ovarian cancer, of which two were protective factors and five were risk factors. A family history of oncological disease, being unmarried, a history of uterine fibroids, having a number of abortions, and a history of hormone replacement therapy were risk factors for developing ovarian cancer. Simultaneously, previous irregular menstruation and occupation as a farmer were protective factors for its occurrence. The construction of risk factor prediction models is expected to improve the efficiency of prevention and early diagnosis and reduce the incidence and mortality of ovarian cancer.Citation20
The present study found that previous irregular menstruation was a protective factor against ovarian cancer, which is inconsistent with the results of a prospective cohort study by Cirillo et al.Citation21 Furthermore, Nash et alCitation22 showed that the relationship between menstrual cycle characteristics and the risk of epithelial ovarian cancer varies by race. The difference between the results of this study and those of other studies may be related to the sample included; the control group had a sample of non-ovarian cancer patients who were hospitalized in the gynecology department during the same period, where most of the diagnoses were abnormal uterine bleeding, uterine fibroids, and ovarian cysts. Patients with abnormal uterine bleeding and fibroids tend to have previous clinical manifestations of irregular menstruation. It is also possible that the incidence of ovarian cancer is reduced because patients with menstrual abnormalities receive early outpatient treatment and interventions to improve their lifestyle.
Previous studiesCitation17 found that sedentary work is a risk factor for ovarian cancer. ChangCitation23 reviewed the relationship between shift work and the risk of ovarian cancer. However, no study has predicted the risk of ovarian cancer for a particular occupation. A study in ChinaCitation24 showed that women working as dry cleaners, telephone operators, porters, painters, and printing factories had an increased risk of ovarian cancer compared to women working in other occupations, which may be because their occupations are often exposed to organic dust and aromatic chemicals. In the present study, occupation as a farmer was found to be a protective factor against ovarian cancer development, which may be related to the living environment and mental and psychological state of farmers living in rural areas, where industrialization is relatively light and environmental conditions are comparatively good. Wenhua’s studyCitation24 showed that environmental factors affect ovarian cancer risk. In some industrialized and developed areas, the incidence of ovarian cancer is higher, which is possibly associated with increased environmental carcinogens due to industrial pollution. This may also be related to farmers being generally less educated and mentally stressed. Some studies have shown that women with higher education have more work and mental stress and a higher risk of ovarian cancer.
Epidemiological investigationsCitation25 have shown that a family history of breast, cervical, and ovarian cancer is a risk factor for ovarian cancer, and this study further confirms this finding. Moreover, other studiesCitation26,Citation27 have shown that most ovarian cancer patients have mutations in the hereditary breast and ovarian cancer susceptibility genes, BRCA1 and BRCA2. Unmarried women who have never been pregnant have an increased risk of ovarian cancer due to ovarian epithelial stimulation caused by persistent ovulation, which induces malignant transformation of epithelial cells.Citation28 In the present study, a history of uterine fibroids was a risk factor for ovarian cancer. Park et al and Herreros-Villanueva et alCitation29,Citation30 showed no association with ovarian cancer in women with a history of uterine fibroids or ovarian cysts, but a history of endometriosis was associated with ovarian cancer (OR 1.78; 95% CI 1.09–2.90). These findings may have been related to the study population. However, the risk of ovarian cancer associated with previous uterine fibroids requires further investigation. This study found that abortion is also a risk factor for ovarian cancer, probably because abortion is prone to gynecological inflammation and infertility, which is in line with the findings of Chi et alCitation31 who emphasized that the risk of ovarian cancer in women with more than one abortion is 4.199 times higher than that in women with less than one abortion. Sung et alCitation32 showed that spontaneous abortion was negatively associated with Clear Cell (CLC) ovarian cancer. Still, they indicated that induced abortion is an important protective factor when considering the overall incidence of epithelial ovarian cancer. Reid et alCitation33 found that hormone replacement therapy increases the risk of ovarian cancer, especially in patients with more than five years of life, in line with the results of the present study and those of Sung et al.Citation32
Studies on ovarian cancer risk factors have focused on European and North American countries, probably because of the low incidence of ovarian cancer in Asian populations. Most studies have focused on risk factors and model construction for postoperative recurrence and complications, with few risk assessment models.Citation34 For example, Wang et alCitation35 constructed an advanced risk assessment model involving an optimal combination of 9 pathways and one clinical factor. Lee et alCitation36 constructed a multifactorial risk prediction model for epithelial duct ovarian cancer that could facilitate stratification, especially with specificity in women with cancer or a dominant family history of intermediate-risk and high-risk causative variants.
The decision tree model constructed in this study for the risk of developing ovarian cancer incorporated 15 variables: menopausal status, previous uterine fibroids, hormone replacement therapy, occupation, number of pregnancies, family history, number of abortions, and blood type. An AUC of 0.5–0.7 indicates a low diagnostic value, and the model had an AUC of 0.961 in the training set and 0.902 in the testing set, indicating the model’s good differentiation ability. The higher sensitivity of the decision tree model reflects the model’s capacity to predict the risk of ovarian cancer better and accurately differentiate between high-risk and low-risk groups. This model has high accuracy and good generalizability, which can help clinicians and community workers quickly and accurately identify high-risk groups to perform targeted interventions and health management. Furthermore, it can help patients understand their lifestyles and habits, improve their knowledge of ovarian cancer risk factors, and establish the concepts of prevention and “comprehensive risk intervention” to form better living habitsCitation17 and positive medical behaviors.
Conclusions
In summary, ovarian cancer is detected late and has a poor prognosis. Therefore, there is an urgent need to identify high-risk groups and implement interventions as early as possible. The relevant factors identified in this study and the prediction model constructed based on the decision tree can be used by community service centres for the assessment and health education of the population. The clinical staff can also use them to identify high-risk patients, conduct early interventions, to reduce the morbidity rate caused by poor lifestyles and gynecological disorders, and improve patient prognosis.
This was a retrospective analysis of ovarian cancer cases in the last five years conducted in three hospitals, with an unbalanced sample size due to the varying completeness of clinical data collected from electronic case records in different hospitals. The influencing factors included in the text are based on the domestic and international literature. However, some patients were excluded because of missing data or unavailable risk factors, which may have affected the model’s efficacy. The case data in this study were recorded over a significant period, and further prospective studies are required to verify the model’s reliability.
In future, we will incorporate studies with sub-levels, multiple hospitals, and larger sample sizes to refine the model or use other models for further research.
Abbreviations
OC, Ovarian cancer; ROMA, Risk of malignancy algorithm; miRNAs, microRNAs; ROC, receiver operating characteristic; HRT, hormone replacement therapy; CLC, Clear Cell.
Ethics Approval and Consent to Participate
The study complied with medical ethics standards and was reviewed by the Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine (review approval number: ZE2023-291). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
Yulan Guan and Jinguo Zhao are the co-corresponding authors. The paper has been edited and proofread by Medjaden Inc.
Data Sharing Statement
The datasets generated and analyzed in the present study are available from the corresponding author Jinguo Zhai upon reasonable request.
References
- Lin C, Zeng Z, Lin Y, et al. Naringenin suppresses epithelial ovarian cancer by inhibiting proliferation and modulating gut microbiota. Phytomedicine. 2022;106:154401. doi:10.1016/j.phymed.2022.154401
- Saani I, Raj N, Sood R, et al. Clinical challenges in the management of malignant ovarian germ cell tumours. Int J Environ Res Public Health. 2023;20(12):6089. doi:10.3390/ijerph20126089
- Boussios S, Moschetta M, Tatsi K, et al. A review on pregnancy complicated by ovarian epithelial and non-epithelialmalignant tumors: diagnostic and therapeutic perspectives. J Adv Res. 2018;12:1–9. doi:10.1016/j.jare.2018.02.006
- Lowe KA, Chia VM, Taylor A, et al. An international assessment of ovarian cancer incidence and mortality. Gynecologic Oncol. 2013;130(1):107–114. doi:10.1016/j.ygyno.2013.03.026
- Shen L, Zang R. Progress of research on primary prevention of ovarian cancer. Tumor Contr Res. 2023;50(3):224–228.
- Feng J, Xu L, Chen Y, Lin R, Li H, He H. Trends in incidence and mortality for ovarian cancer in China from 1990 to 2019 and its forecasted levels in 30 years. Jovarian Res, 2023 16 1 139 doi: 10.1186/s13048-023-01233-y.
- Huang Z, Zheng Y, Wen W, et al. Incidence and mortality of gynaecological cancers: secular trends in urban Shanghai, China over 40 years. Eur J Cancer. 2016;63:1–10. doi:10.1016/j.ejca.2016.04.016
- Stewart C, Ralyea C, Lockwood S. Ovarian cancer: an integrated review. Semin Oncol Nurs. 2019;35(2):151–156. (). doi:10.1016/j.soncn.2019.02.001
- Menon U, Gentry-Maharaj A, Burnell M, et al. Mortality impact, risks, and benefits of general population screening for ovarian cancer: the UKCTOCS randomised controlled trial. Health Technol Assess. 2023;1:1–81.
- Xiong X, Lai X, Zhang J, et al. FBP1 knockdown decreases ovarian cancer formation and cisplatin resistance through EZH2-mediated H3K27me3. Biosci Rep. 2022;42(9):BSR20221002.
- Lee JY, Kim EY, Jung KW, et al. Trends in gynecologic cancer mortality in East Asian regions. J Gynecol Oncol. 2014;25(3):174–182. doi:10.3802/jgo.2014.25.3.174
- Pavlidis N, Rassy E, Vermorken JB, et al. The outcome of patients with serous papillary peritoneal cancer, fallopian tubecancer, and epithelial ovarian cancer by treatment eras: 27 years data from the SEER registry. Cancer Epidemiol. 2021;75:102045. doi:10.1016/j.canep.2021.102045
- Sendon-Lago J, Seoane S, Saleh F, et al. In vivo effects of conditioned medium from human uterine cervical stem cells in an ovarian cancer xenograft mouse model. Cancer Genomics Proteomics. 2022;19(5):570–575. doi:10.21873/cgp.20341
- Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening randomized controlled trial. JAMA. 2011;305(22):2295–2303. doi:10.1001/jama.2011.766
- Ghose A, McCann L, Makker S, et al. Diagnostic biomarkers in ovarian cancer: advances beyond CA125 and HE4. Ther Adv Med Oncol. 2024;29(16):17588359241233225.
- Prat J, Ribé A, Gallardo A. Hereditary ovarian cancer. Human Pathol. 2005;36(8):861–870. doi:10.1016/j.humpath.2005.06.006
- Abdulaziz G, Welc NA, Gąsiorowska E, Nowak-Markwitz E. Assessment of gynecological and lifestyle-related risk factors of ovarian cancer. Przeglad Menopauzalny. 2021;20(4):184–192. doi:10.5114/pm.2021.109847
- Ghose A, Bolina A, Mahajan I, et al. Hereditary ovarian cancer: towards a cost-effective prevention strategy. Int J Environ Res Public Health. 2022;19(19):12057. doi:10.3390/ijerph191912057
- Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020. J Nat Cancer Inst. 2011;103(2):117–128. doi:10.1093/jnci/djq495
- Jing B, Chen G, Yang M, et al. Development of prediction model to estimate future risk of ovarian lesions: a multi-center retrospective study. Prevent Med Rep. 2023;35:102296. doi:10.1016/j.pmedr.2023.102296
- Cirillo PM, Wang ET, Cedars MI, Chen LM, Cohn BA. Irregular menses predicts ovarian cancer: prospective evidence from the Child Health and Development Studies. Int J Cancer, 2016 139 5 1009–1017 doi: 10.1002/ijc.30144.
- Nash R, Johnson CE, Harris HR, et al. Race differences in the associations between menstrual cycle characteristics and epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prevent. 2022;31(8):1610–1620. doi:10.1158/1055-9965.EPI-22-0115
- Gao C, Zhang S, Wen ZY. Research progress on the relationship between shift work and ovarian cancer. Cancer Prevent Contr Res. 2021;48(3):293–298.
- Zhao WH. Progress in the study of risk factors for ovarian cancer. Int J Obstetr Gynecol. 2013;40(1):50–53.
- Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–296. doi:10.3322/caac.21456
- Kotsopoulos J, Gronwald J, Karlan B, et al. Age-specific ovarian cancer risks among women with a BRCA1 or BRCA2 mutation. Gynecologic Oncol. 2018;150(1):85–91. doi:10.1016/j.ygyno.2018.05.011
- Custódio N, Savisaar R, Carvalho C, et al. Expression profiling in ovarian cancer reveals coordinated regulation of brca1/2 and homologous recombination genes. Biomedicines. 2022;10(2):199. doi:10.3390/biomedicines10020199
- Troisi R, Bjørge T, Gissler M, et al. The role of pregnancy, perinatal factors and hormones in maternal cancer risk: a review of the evidence. J Internal Med. 2018;283(5):430–445. doi:10.1111/joim.12747
- Park HK, Schildkraut JM, Alberg AJ, et al. Benign gynecologic conditions are associated with ovarian cancer risk in African-American women: a case-control study. Cancer Causes Control. 2018;29(11):1081–1091. doi:10.1007/s10552-018-1082-4
- Herreros-Villanueva M, Chen CC, Tsai EM, Er TK. Endometriosis-associated ovarian cancer: what have we learned so far? Int J Clin Chem. 2019;493:63–72. doi:10.1016/j.cca.2019.02.016
- Chi XD, Zhou BS, Li LK. Study on factors affecting the development of ovarian cancer. Chongqing Med. 2009;38(10):1198–1199.
- Sung S, Hong Y, Kim BG, et al. Stratifying the risk of ovarian cancer incidence by histologic subtypes in the Korean Epithelial Ovarian Cancer Study (Ko-EVE). Cancer Med. 2023;12(7):8742–8753. doi:10.1002/cam4.5612
- Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med. 2017;14(1):9–32. doi:10.20892/j.issn.2095-3941.2016.0084
- Yang H, Dai H, Li L, et al. Age at menarche and epithelial ovarian cancer risk: a meta-analysis and Mendelian randomization study. Cancer Med. 2019;8(8):4012–4022. doi:10.1002/cam4.2315
- Wang X, Lin FK, Li JR, Wang HS. A comprehensive risk assessment model for ovarian cancer patients with phospho-stat3 and il-31 as immune infiltration relevant genes. Onco Targets Ther. 2020;13:5617–5628. doi:10.2147/OTT.S254494
- Lee A, Yang X, Tyrer J, et al. Comprehensive epithelial tubo-ovarian cancer risk prediction model incorporating genetic and epidemiological risk factors. J Med Genet. 2022;59(7):632–643. doi:10.1136/jmedgenet-2021-107904