Abstract
Minimally invasive treatment options are an important part of the uterine fibroid-treatment arsenal, especially among younger patients and in those who plan future pregnancies. This article provides an overview of the currently available minimally invasive therapy options, with a special emphasis on a completely noninvasive option: magnetic resonance-guided focused ultrasound (MRgFUS). In this review, we describe the background of MRgFUS, the patient-selection criteria for MRgFUS, and how the procedure is performed. We summarize the published clinical trial results, and review the literature on pregnancy post-MRgFUS and on the cost-effectiveness of MRgFUS.
Introduction
In women of reproductive age, uterine fibroids (leiomyomas or myomas) are the most common single indication for hysterectomy,Citation1 and account for significant health care costs.Citation2 Current medical treatment options of uterine fibroids range from medical management (gonadotropin-releasing hormone [GnRH] therapy) to myomectomy, or to complete hysterectomy.Citation3–Citation5 The noninvasive option of magnetic resonance-guided focused ultrasound (MRgFUS) is becoming increasingly accepted, with over 9,880 patients treated to date worldwide with one system (ExAblate 2100, InSightec, Tirat Carmel, Israel). This treatment option is important in light of the fact that women are increasingly seeking less invasive alternatives to a classic surgical intervention and seeking procedures that offer shorter recovery times with the possibility of fertility preservation.Citation6,Citation7 Another important driving force in the development and widespread application of less invasive fibroid therapy is the reduction in treatment-associated health care cost.Citation8,Citation9 The purpose of this article is to summarize the literature to date on MRgFUS as a promising option for uterine fibroid treatment.
Uterine fibroids
The estimated incidence of uterine fibroids is approximately 70% in Caucasian females over 50 years of age, and is over 80% in African American females.Citation2,Citation10,Citation11 While some patients are completely asymptomatic, approximately 25% are symptomatic and experience pelvic pain and/or pressure, menorrhagia or dysmenorrhagia, increased frequency of urination, or reproductive dysfunction.Citation12,Citation13 Fibroids are classified by their location in the uterus as subserosal, intramural, or submucosal. They can be pedunculated from either the serosal surface or from the mucosal surface. Histologically, uterine fibroids are benign, hormone-sensitive smooth-muscle tumors mixed with fibrous connective tissue, demarcated from the surrounding tissue by a pseudocapsule. Fibroids can undergo hyaline degeneration or hemorrhagic infarction, and they may calcify as they regress after menopause. The goal of fibroid treatment is relief of symptoms, such as bleeding, pelvic pressure, or pain.Citation6,Citation13 Historically, the first-line treatment of uterine fibroids has been surgical intervention (hysterectomy or myomectomy). For women who desire fertility preservation, myomectomy has been a preferable option. Less invasive treatment options are available, such as uterine artery embolization (UAE), MRgFUS, or less common thermal ablation therapies using laser, radiofrequency, and cryoablation.Citation6,Citation14 Medical therapy using hormone-based treatment is also available,Citation15 but may have a rebound effect once discontinued.Citation16 Hormone therapy is often used in patients who present with heavy menstruation or as a presurgical adjunct for those with large fibroids, as they reduce the endometrial hyperplasia associated with the fibroid (progestogens) and induce the shrinkage of the fibroid (GnRH-antagonist effect).Citation16
Surgical therapeutic options – hysterectomy and myomectomy
For perimenopausal women with symptomatic fibroids who are family-complete, hysterectomy (the surgical removal of the uterus) is an effective treatment. Uterine fibroids remain the most common indication for hysterectomy in the US.Citation6,Citation14 However, hysterectomy is a major operation, and can cause significant disability for 2 months postoperatively.Citation17,Citation18 The mortality rate is 0.38–1 per 1,000 patients, significant complications develop in 3% of patients, and minor complications are experienced by up to 30% of patients.Citation17–Citation19 The long-term consequences of hysterectomy may include urinary incontinence and early ovarian failure,Citation20,Citation21 in addition to the potential psychological consequences of the uterus removal.Citation22 There is an increased trend of women desiring pregnancies later in their reproductive age, and as such there is an increased demand for conservative treatment of fibroids. An alternative to hysterectomy is an open or laparoscopic myomectomy. Open myomectomy results in high patient satisfaction, and approximately 80% of patients report alleviation in their symptoms;Citation23 however, recovery can take 6–8 weeks.Citation24,Citation25 Patients who undergo open myomectomy are usually younger, weigh less, are of lower parity, and have smaller fibroids and uterus size compared to patients who undergo hysterectomy.Citation23 The most common side effects are fever and hemorrhage in both procedures.Citation19
When pregnancy is desired and the uterus is small (<5 cm) with a small number (fewer than three) of fibroids, laparoscopic myomectomy can be considered.Citation1,Citation26 Smaller fibroids (3–8 cm) can be removed from the uterus via a laparoscopic approach (for intramural and subserosal fibroids) or a hysteroscopic approach (for submucosal fibroids). Several studies have shown that laparoscopic myomectomy is associated with shorter hospital stay, less postoperative pain, and reduced blood loss compared to hysterectomy and open myomectomy.Citation27–Citation30 Removal of larger fibroids in this fashion is more difficult, and a common surgical strategy has been the use of morcellation. However, of recent times, the US Food and Drug Administration (FDA) has recommended against the use of this technique.Citation31,Citation32 As indicated on the FDA website,
[the] FDA discourages use of laparoscopic power morcellation for removal of uterus or uterine fibroids. The procedure poses the risk of spreading undetected cancerous tissue in women with unsuspected cancer.
If morcellation is banned, a new management strategy for larger uterine fibroids may have to be considered.Citation31,Citation32
Small (<5 cm) submucosal fibroids may be accessible by hysteroscopic removal. After cervical dilatation, the hysteroscope is inserted transvaginally into the uterine cavity, the cavity is insufflated, and after careful inspection, the fibroid can be removed.Citation33
Minimally invasive nonsurgical therapies
Uterine artery embolization
UAE was introduced in 1995, and is widely used and accepted for the treatment of uterine fibroids.Citation34–Citation36 Absolute contraindications to the procedure are pregnancy, active infection, or suspected malignancy (uterine, cervical, or adnexal). Relative contraindications are coagulopathy, severe contrast allergy, or renal impairment.Citation37 The procedure involves catheterization of a femoral artery, through which the uterine artery is accessed. Embolization is brought about through bilateral intra-arterial infusion of microparticles, producing blockage in the arterial blood flow to the uterus. UAE treatment efficacy is high, the patients’ symptoms improve in 80%–90% of cases, and the median duration of hospital stay and recovery times are shorter compared with hysterectomy.Citation38,Citation39 The major complication rate is low (1%–4%).Citation17,Citation19,Citation39 The rate of hysterectomy for major complications postprocedure is 1.1%–1.5%.Citation17,Citation40 Other complications of UAE are postprocedural pain, ovarian failure, and allergic reaction to the injected compound.Citation41–Citation45 Over time, the rate of complications after UAE has been decreasing, with greater operation experience and technical advancement.Citation39 The overall failure and recurrence rate in patients who were enrolled in the Fibroid registry, which looked at long-term outcomes after UAE, was 20% at 5 years.Citation46
Image-guided thermal therapies
Thermal ablation occurs when there is a rapid change in the local tissue temperature (>55°C for heat and <−20°C to −50°C for cold) in response to the targeted therapy. Imaging is critically important in thermal ablation. It provides information on tumor detection, characterization, and guidance for treatment targeting. Currently, MR imaging (MRI) is the only modality that is able to provide such information and guidance, including reliable real-time quantitative temperature measurements. Current minimally invasive thermal ablation techniques include laser, cryo-, and radiofrequency ablation (RFA).
Laser ablation of uterine fibroids
Laser ablation of the fibroids can be performed through a laparoscopic or endoscopic route (since 1989), or as a percutaneous (since 1999) treatment with the assistance of MRI guidance.Citation47–Citation49 The treatment uses interstitial laser photocoagulation, which applies a low-power laser to destroy the fibroid tissue, guided by thermal monitoring. It uses a 1,064 nm-wavelength laser that penetrates deep into tissue. Laparoscopic or hysteroscopic laser ablation has been shown to decrease fibroid volume by 50%–70%.Citation47,Citation48
Cryotherapy
Cryoablation is an ablation technique that damages tissues by rapid freezing and rapid thawing using high-pressure argon gas (temperature −20°C to −50°C). The temperature change leads to vascular stasis, thrombosis, and ultimately local tissue ischemia. As a result, the cell membranes rupture and the cells die. Cryoablation of the fibroid can take place through a laparoscopic or hysteroscopic approach. Since the early 2000s, it has been performed with MRI guidance. The reduction of the mean fibroid size ranges between 31% and 80%. The postprocedural complications can include fever and infection.Citation50–Citation52
Radiofrequency ablation
RFA was first used for fibroid treatment in 2005 through a laparoscopic approach.Citation53–Citation56 Ultrasound or CT (computerized tomography) scan is used to guide the procedure. Since RFA is incompatible with MRI, there is no reliable way to monitor the local temperature or to estimate the true ablation area. The advantage of RFA over cryoablation is that a larger ablation area (up to 6 cm in diameter) can be achieved in a reasonable time (~30 minutes). No major complications are associated with RFA; in some cases, spotting bleeding can last up to 8 weeks postablation.Citation57
Noninvasive therapeutic option for uterine fibroid treatment: magnetic resonance-guided focused ultrasound surgery
Milestones in the development of MRgFUS
Wood and Loomis, who are often called the “fathers of ultrasonics”, were the first to describe that US has a biologic effect on living tissues in the 1920s.Citation58 In 1942, Lynn et al proposed the use of FUS to induce thermal or mechanical effects at a focal location in living tissue.Citation59 Subsequently, the Fry brothers built a clinical FUS device to treat hyperkinetic diseases, such as Parkinson’s disease in the 1950s.Citation60,Citation61 In the 1980s, FUS was introduced as a treatment option for glaucoma, and the FDA approved the first FUS system (Sonocare CST-100) to treat glaucoma shortly thereafter.Citation62 Hynynen et al and others introduced MRgFUS in the 1990s.Citation63–Citation65 It con tained a single geometrically focused transducer adopted to perform in a high-field-strength MR magnet.Citation63–Citation65 In the 2000s, the first MRI-compatible phased array and its driving system were introduced to create a larger treated volume of tissue, and to deliver sonication in different locations by steering the US beam from one target to the next. In the same time period, MRI-based thermal dosimetry was developed for guidance and for confirmation of thermal delivery.Citation66–Citation70
In 2004, the ExAblate 2000 system (InSightec) received FDA clearance for fibroid treatments. Currently, there are two FDA-approved MRgFUS platforms: the ExAblate 2000 and the ExAblate 2100 (InSightec). Sonalleve MR-HIFU (Philips Healthcare, Andover, MA, USA) is another system approved in Europe. Since its introduction to the market in 2004, the ExAblate systems have treated 9,880 patients to date (10,527 uterine fibroids; information provided by InSightec). MRgFUS has also been used as a palliative treatment of bone metastases, breast fibroadenomas, breast cancer, brain tumors, and liver disease.Citation71–Citation73
Patient selection for MRgFUS
One of the first steps in selecting patients for MRgFUS treatment is to determine symptomatology and confirm the presence of uterine fibroids, as other conditions (such as adenomyosis) can cause similar symptomatology. At our institution, all suitable candidates complete the symptom-severity score (SSS) of the Uterine Fibroid Symptoms and Quality of Life (UFS-QOL) questionnaire, a validated instrument for assessing symptoms specific for quality of life due to uterine fibroids.Citation74 The SSS of the UFS-QOL is a 100-point scale composed of eight questions, assessing symptoms related to bleeding and bulk- or volume-related complaints. The score is significantly higher in women with symptomatic fibroids. This objective scoring system is used for preprocedure evaluation and postprocedure monitoring.
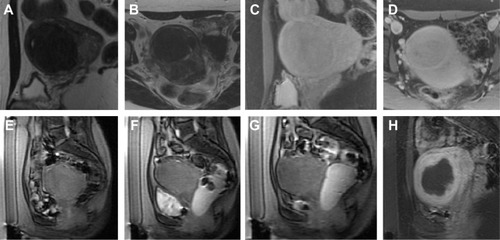
All eligible candidates undergo a contrast-enhanced MRI to determine fibroid signal intensity, size, number, and location (). The optimal scenario for MRgFUS treatment is a solitary fibroid ≤10 cm in diameter, of low signal intensity on T2-weighted images on MRI, enhances on contrast images, and is accessible by the MRgFUS system (ie, <12 cm from margin of the skin; ExAblate 2000/2100) (). Therefore, fibroids that do not demonstrate adequate enhancement or have areas of peripheral calcification () may not be good candidates. Fibroids that are too small and multiple in number () and without a dominant candidate for targeting are not suitable for MRgFUS either. As multiple sonications are needed to destroy the desired fibroid volume, treatment volume is limited by the treatment time that is realistically feasible for the patient. The total treatment time for a 7–8 cm fibroid (depending on energy absorption and location) is ~3 hours of MRgFUS ablation. Larger fibroids may require significantly longer treatment. In addition, fibroids that are of high signal intensity on T2-weighted images () may indicate cellular fibroids, which can be difficult to obtain an optimal temperature increase in.Citation75–Citation78 It is possible that pretreatment with a GnRH agonists may help “dry out” these fibroids ahead of time, allowing for higher-temperature deposition at treatment.
Figure 1 Sagittal MRI of a typical case pre/post treatment.
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; MRI, magnetic resonance image.
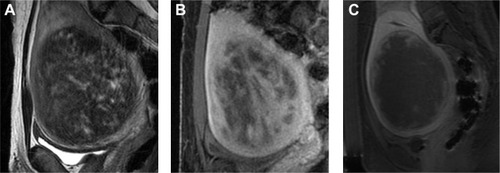
Figure 2 Examples of MRgFUS screen failures.
Abbreviation: MRgFUS, magnetic resonance-guided focused ultrasound.
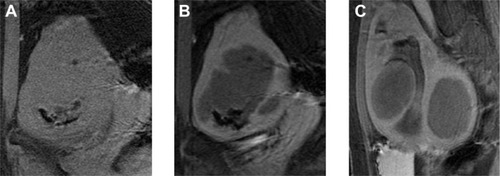
Figure 3 Example of MRgFUS screen failure due to innumerable small fibroids.
Abbreviation: MRgFUS, magnetic resonance-guided focused ultrasound.
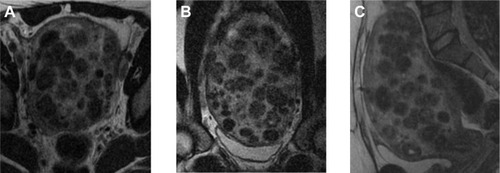
Figure 4 Illustrative example of cellular fibroid.
Table 1 MR imaging findings that require evaluation on the screening MR and on the planning MR on treatment day
For larger fibroids, pretreatment with GnRH agonists may allow for fibroid shrinkage, which can result in shortened treatment times. Overall, by reducing vascularity, energy deposition can be potentiated. Therefore, GnRH-agonist treatment prior to MRgFUS will increase efficacy.Citation4,Citation79 On the other hand, the absence of enhancement in contrast imaging may indicate nonperfused and necrotic tissue, in which case the patient may not benefit from the MRgFUS treatment. While fibroid number and size are important to consider, so also is fibroid location. If pedunculated and subserosal, there is a risk of amputation of the fibroid from the uterus.Citation80
There are physical limitations of each MRgFUS system. For example, the ExAblate system allows sonications to be delivered up to 12 cm vertical distance from the skin of the anterior abdominal wall. As the goal of treatment is to target as much of the fibroid as possible (up to 100%), fibroids where not all tissue is accessible may not have as good an outcome. Also, if the fibroid is too close to the sacrum, the heating of the sacral bone may lead to sciatic nerve injury.Citation81 Therefore, fibroids that are close to the lumbosacral plexus or to bone surfaces should only be considered suitable for MRgFUS if there is at least 4 cm distance between the fibroid and the bone surface.Citation81
Treatment procedure
Preparation for the procedure
After fasting overnight, the patient arrives at the outpatient MRI suite. The patient’s abdomen has to be clean of any type of lotion or cream, as even small amount of these products can cause skin burn during treatment. The abdomen is shaved between the umbilicus and 1 cm below the pubic bone, a urinary catheter is placed into the bladder, and an intravenous line is sited for administration of conscious sedation.
Treatment planning
The MRgFUS is planned and performed with the patient in a prone position (). The patient lies on a custom-built patient-scanner table, which houses all of the electronics and the phased array transducer in a sealed water bath. This table docks to the magnet. To ensure good acoustic coupling with the patient’s abdomen, a gel pad is placed between the transducer and the patient’s skin. A mixture of degassed water and US gel is applied to both sides of the gel pad to avoid air bubbles. Multiplanar T2-weighted images are taken of the pelvis, and are transferred to the ExAblate/Sonalleve planning program. The region of treatment is manually drawn and defined within the capsule of the fibroid. The target volume is analyzed with superimposed US-beam paths in all three planes. By angling the beam path, the target can be optimally accessed and unwanted heating can be avoided. When bowel loops are found beneath the abdominal wall, they can usually be moved out of the beam path by distending the bladder with saline or using large gel pads on the abdomen wall to apply external pressure.Citation75,Citation81
Figure 5 Photograph depicting a patient lying in the prone position on the magnetic resonance (MR) table.

Procedure
Just prior to the procedure being started, titrated doses of fentanyl and midazolam are administered intravenously to alleviate anxiety and relieve pain. The patient, however, should remain conscious, responsive, and able to give feedback throughout the procedure. The MRgFUS procedure starts with low-power sonications (50–100 W). Real-time thermometry is performed through the proton-resonance frequency-shift method.Citation66,Citation69 Sonication location is determined, and the targeting accuracy can be confirmed. If the targeting is accurate, additional sonications are delivered at therapeutic power level to confirm the appropriate thermometry. The goal of the treatment is to increase the local temperature in the selected tissue volume over 60°C, which ensures coagulative necrosis in the tissue.Citation81,Citation82 Between each sonication, adequate cooling time is ensured to avoid thermal buildup and damage to normal tissues: the ExAblate 2100 and the Sonalleve systems do this calculation automatically, and they apply subsequent sonications in a relatively distant place to allow adequate cooling time for the tissue. The procedure continues with delivery of all planned sonications. During the treatment, to ensure no complications occur, continuous communication with the patient is very important.
At the end of the MRgFUS treatment, we assess the treatment effect by measuring the nonperfused volume (NPV) of fibroid tissue on gadolinium contrast-enhanced T1-weighted images. Higher NPV ratios are associated with lower probabilities of recurrence, due to fibroid regrowth and a reduced need for additional procedures.Citation76–Citation78,Citation83
Obstacles in the FUS-beam path
US energy is absorbed to a greater extent by high-density tissues as abdominal wall scar tissue, and is reflected by low-density tissues (eg, containing air), such as the intestines or the air-containing catheter in the bladder.Citation84,Citation85 In some cases, the angulation (tilting the beam path away) of the US-beam path can help to avoid these tissues. Alternatively, the bladder can be filled with saline to enable the beam to pass through the bladder. The presence of bowel loops behind the anterior abdominal wall and in front of the uterus fibroids can sometimes be manipulated by filling the bladder with saline and filling the rectum with US gel, and displacing the bowel loops to create a sonication window ().
Figure 6 Illustrative example of manipulation of beam path obstruction.
Clinical trial results
The initial Phase I/II trial of the ExAblate 2000 device was conducted to evaluate the safety and feasibility of MRgFUS.Citation86 In this trial, premenopausal women with symptomatic fibroids, defined by their SSS from the UFS-QOL questionnaire, were recruited. Treatment was limited to 100 mL of fibroid tissue, a 15 mm safety margin from both the serosal surface and the endometrium of the uterus, and 2 hours of sonication. In this trial, all patients went to a planned hysterectomy within 1 month, allowing for direct pathological correlations. It was demonstrated that MRgFUS could successfully cause thermal coagulation and necrosis in uterine fibroids.
Subsequently, a multicenter Phase III clinical trial was carried out to evaluate the effectiveness and durability of the treatment.Citation82,Citation87,Citation88 In this trial, patients did not receive any additional forms of therapy after MRgFUS. Enrollment criteria included symptomatic fibroids in patients who had no desire for further pregnancy. The patients were followed, and the outcome was documented using the UFS-QOL questionnaire scores at 6, 12, and now 24 months after treatment, and by fibroid volume on MRI. In 2004, after receiving FDA clearance, the treatment guidelines were changed to allow a treatment volume of 150 mL per fibroid, with no restriction on the distance from the endometrium. Fennessy et alCitation82 first analyzed the effect of the different protocols on treatment results, and found that the relaxed treatment protocol resulted in a greater NPV, with 25.79% achieved versus the 16.65% of the restricted protocol (P<0.001).Citation82 For those treated with the restricted protocol, clinical follow-up revealed that 74% of patients at 6 months and 73% at 12 months had a greater than 10-point improvement in symptom score. In those treated according to the more relaxed protocol, 88% reported a 10-point or greater symptom improvement at 6 months, and 91% had significant symptom improvement at 12 months. This study was the first to show that treating a greater amount of fibroid tissue resulted in a larger treatment volume and better symptom improvement and durability.
This need for increased NPVs and better outcomes has been replicated in multiple papers since then.Citation77,Citation83,Citation89–Citation91 In all, these papers underscore that the learning process and the worldwide accumulation of data on MRgFUS of uterine fibroids has led to treatment optimization to safely achieve large-enough NPVs that will ensure sustained clinical benefit.
MRgFUS as a treatment option for African–American women
Uterine fibroids have a disproportionate impact on African–American (AA) women. They have an earlier age of onset with more symptomatic fibroids than non-AA women,Citation92–Citation94 with different effects on employment and therapy-seeking behavior.Citation95 A recent study by our group found that AA women tended to have differences in the pattern of fibroid disease, with a higher total number of fibroids that were of smaller volume, compared to non-AA women ().Citation96 Specific to MRgFUS, AA patients were more likely to fail screening than non-AA patients, ie, they were more likely to be unsuitable candidates for MRgFUS. This was more likely to be due to MRI findings that indicated that safe delivery of treatment was not possible, such as significant anterior abdominal wall scarring or multiple small fibroids. However, once AA patients pass screening and were treated with MRgFUS, the two groups do not differ in terms of their need for surgical intervention for failed MRgFUS treatment and in terms of their overall symptom improvement and fibroid shrinkage.
MRgFUS and fertility and pregnancy
Early clinical trials and initial treatment protocols of MRgFUS included only women who were family-complete.Citation87,Citation88 In recent years, there have been several case reports of successful pregnancies following MRgFUS treatment,Citation97–Citation104 which are summarized in a recent review article by Clark et al.Citation105 As MRgFUS does not target the uterus, it remains intact, and anecdotal pregnancies are to be expected. Rabinovici et al published the largest case study results of 54 pregnancies in 51 patients who received MRgFUS on average 8 months before conception.Citation98 The term-delivery rate was reported to be 93%, and the C-section rate was remarkably lower than that seen after UAE.Citation106–Citation108 In addition, it is notable that in this series, there were neither low-birth-weight infants nor stillbirths, which have been reported (albeit at a low frequency) in women who become pregnant post-UAE. While women who conceive after MRgFUS should be informed that normal pregnancy outcomes and normal vaginal deliveries are possible, since our knowledge is currently limited, these pregnancies need to be followed up carefully.
Technical improvements
In recent years, different approaches have been developed to increase the treatment volume and decrease the treatment time of MRgFUS. In the early treatments, the sonications were delivered at one or a small number of treatment depths in a grid pattern.Citation86 The tissue was allowed to cool back to baseline before a subsequent sonication was performed, in order to avoid accumulation of heating in the near field due to overlap of the US beam. This need to take time for cooling limited the time available for treatment, and thus reduced overall efficacy. Optimization of placement of focal spots to avoid accumulated heating in the near field has greatly improved the treatment time. The systems have also evolved so that larger areas can be ablated during each sonication by electronically steering the US beam over a larger area. The ExAblate system steers the focal beam laterally to increase the width of the focal area, and when in “elongated” mode it steers the focal beam from a deeper to a more shallow location over the course of the sonication. For large fibroids, this steering in the depth direction can cover several centimeters in one sonication. Improved robotics and the ability to disable portions of the array that place unwanted tissue in the US-beam path have also been developed with the aim of increasing the patient population that are candidates for MRgFUS.
The Sonalleve system uses volumetric heating with real-time feedback. This sonication approach is based on the work of Salomir et al and Mougenot et alCitation109,Citation110 and utilizes heat diffusion by electrically steering the focal point in a predetermined circular path in the focal plane. Over time, the center of the “treatment cell” is filled in via thermal conduction and perfusion. To control the temperature increase in each sonication location, the Sonalleve system uses the feature “real-time thermometry feedback”, by which it can automatically modify the sonication parameters to generate a spatially controlled temperature profile at the target location. The combination of the volumetric ablation and the real-time multislice temperature monitoring provides well-controlled maximum lesion temperature, predictable lesion volumes, and faster ablation of larger fibroids.Citation111
Cost-effectiveness
As discussed, studies have concluded that MRgFUS is safe and effective in shrinking fibroids and producing symptom relief. It is a short outpatient procedure, and there are few adverse events or minor complications associated with it. However, according to the Blue Cross and Blue Shield technology assessment of MRgFUS for treatment of uterine fibroids,Citation112 MRgFUS is still considered investigational, without sufficient proof that it “improves the net health outcome for any clinical application”, and currently not all insurance companies cover MRgFUS for uterine fibroid treatment. To address this assessment, there have been a number of cost-effectiveness analyses performed that have compared MRgFUS with conventional treatments for fibroids.Citation113–Citation115
O’Sullivan et al found MRgFUS to be in the range of currently accepted criteria for cost-effectiveness, along with hysterectomy and UAE, while Zowall et al found that a treatment strategy for symptomatic uterine fibroids starting with MRgFUS is likely to be cost-effective.Citation113,Citation114 Our group found that MRgFUS, as the first-line treatment for symptomatic fibroids, is preferred over both UAE and hysterectomy from a cost-effectiveness standpoint.Citation115 Fennessy et al applied specific short-term quality-of-life decreases (“disutilities”) related to MRgFUS, UAE, and hysterectomy, for the cost-effectiveness analyses.Citation116 This approach strengthened our results, as we took into account the difference in the quality of life and duration of recovery following each specific treatment procedure for uterine fibroids.
Conclusion
To determine the value and effectiveness of MRgFUS in the widely available therapeutic options for uterine fibroids, one can consider the feasibility studies that have proven the safety and efficacy of MRgFUS (which eventually led the FDA approval of this technology), together with multicenter clinical trial results that showed the treatment’s efficacy in symptom reduction and resolution with the low occurrence rate of side or adverse effects. As the technology advances and improves, and the operators become more aware of how to circumvent anatomical obstacles, such as bowel and scar tissue, MRgFUS treatment time is decreasing and more patients become treatment-eligible. Recent studies have indicated that MRgFUS is a safe and effective treatment option in AA women, in whom this disease is more common and more symptomatic. Preliminary evidence would suggest that MRgFUS is not a definitive roadblock to future pregnancies, although this is an area that will require more exploration for confirmation. Furthermore, cost-effectiveness studies have concluded that MRgFUS is cost-effective as a uterine-sparing, noninvasive therapy for eligible patients. As MRgFUS continues to mature and undergo technical improvements, we believe that it will be a first-choice therapy for many women and an important tool in the personalized therapy arsenal for uterine fibroids.
Acknowledgments
We acknowledge the pioneering image-guided therapy work of Dr Ferenc A Jolesz, MD, who passed away on December 31, 2014. He was instrumental in the concept and introduction of MRgFUS, and is missed by his friends, colleagues, and mentees. This study was supported by grant R25 CA089017-06A2.
Disclosure
FMF, CMT, and NJM have received support at different times over the past 13 years from InSightec Inc for the performance of uterine fibroid clinical trials at Brigham and Women’s Hospital, as part of multicenter clinical trials. The other authors report no conflicts of interest in this work.
References
- FalconeTParkerWHSurgical management of leiomyomas for fertility or uterine preservationObstet Gynecol2013121485686823635687
- StewartEAUterine fibroids and evidence-based medicine – not an oxymoronN Engl J Med2012366547147322296082
- SmartOCHindleyJTReganLGedroycWMMagnetic resonance guided focused ultrasound surgery of uterine fibroids – the tissue effects of GnRH agonist pre-treatmentEur J Radiol200659216316716740371
- SmartOCHindleyJTReganLGedroycWGGonadotrophin-releasing hormone and magnetic-resonance-guided ultrasound surgery for uterine leiomyomataObstet Gynecol20061081495416816055
- KhanATShehmarMGuptaJKUterine fibroids: current perspectivesInt J Womens Health201469511424511243
- American College of Obstetricians and GynecologistsACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomasObstet Gynecol20081122 Pt 138740018669742
- FunakiKSawadaKMaedaFNagaiSSubjective effect of magnetic resonance-guided focused ultrasound surgery for uterine fibroidsJ Obstet Gynaecol Res200733683483918001451
- MauskopfJFlynnMThiedaPSpaldingJDuchaneJThe economic impact of uterine fibroids in the United States: a summary of published estimatesJ Womens Health (Larchmt)200514869270316232101
- CardozoERClarkADBanksNKHenneMBStegmannBJSegarsJHThe estimated annual cost of uterine leiomyomata in the United StatesAm J Obstet Gynecol20122063211.e1e922244472
- BairdDDDunsonDBHillMCCousinsDSchectmanJMHigh cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidenceAm J Obstet Gynecol2003188110010712548202
- MarshallLMSpiegelmanDBarbieriRLVariation in the incidence of uterine leiomyoma among premenopausal women by age and raceObstet Gynecol19979069679739397113
- ButtramVCJrReiterRCUterine leiomyomata: etiology, symptomatology, and managementFertil Steril19813644334457026295
- StewartEAUterine fibroidsLancet2001357925229329811214143
- SolnikMJMunroMGIndications and alternatives to hysterectomyClin Obstet Gynecol2014571144224488051
- SinghSSBellandLContemporary management of uterine fibroids: focus on emerging medical treatmentsCurr Med Res Opin201531111225365466
- MarretHFritelXOuldamerLTherapeutic management of uterine fibroid tumors: updated French guidelinesEur J Obstet Gynecol Reprod Biol2012165215616422939241
- HirstADuttonSWuOA multi-centre retrospective cohort study comparing the efficacy, safety and cost-effectiveness of hysterectomy and uterine artery embolisation for the treatment of symptomatic uterine fibroids. The HOPEFUL studyHealth Technol Assess2008125124818331704
- MareshMJMetcalfeMAMcPhersonKThe VALUE national hysterectomy study: description of the patients and their surgeryBJOG2002109330231211950186
- HillDJComplications of hysterectomyBaillieres Clin Obstet Gynaecol19971111811979155943
- BrownJSSawayaGThomDHGradyDHysterectomy and urinary incontinence: a systematic reviewLancet2000356922953553910950229
- MoormanPGMyersERSchildkrautJMIversenESWangFWarrenNEffect of hysterectomy with ovarian preservation on ovarian functionObstet Gynecol201111861271127922067716
- CabnessJThe psychosocial dimensions of hysterectomy: private places and the inner spaces of women at midlifeSoc Work Health Care201049321122620229394
- SawinSWPilevskyNDBerlinJABarnhartKTComparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomasAm J Obstet Gynecol200018361448145511120509
- AdvinculaAPXuXGoudeauS4thRansomSBRobot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and immediate costsJ Minim Invasive Gynecol200714669870517980329
- SmorgickNDaltonVKPatzkowskyKEHoffmanMRAdvinculaAPAs-SanieSComparison of 2 minimally invasive routes for hysterectomy of large uteriInt J Gynaecol Obstet2013122212813123664102
- SizziORossettiAMalzoniMItalian multicenter study on complications of laparoscopic myomectomyJ Minim Invasive Gynecol200714445346217630163
- JinCHuYChenXCLaparoscopic versus open myomectomy – a meta-analysis of randomized controlled trialsEur J Obstet Gynecol Reprod Biol20091451142119398260
- AlessandriFLijoiDMistrangeloEFerreroSRagniNRandomized study of laparoscopic versus minilaparotomic myomectomy for uterine myomasJ Minim Invasive Gynecol2006132929716527709
- CicinelliETinelliRColafiglioGSalianiNLaparoscopy vs minilaparotomy in women with symptomatic uterine myomas: a prospective randomized studyJ Minim Invasive Gynecol200916442242619573818
- PalombaSZupiEFalboAA multicenter randomized, controlled study comparing laparoscopic versus minilaparotomic myomectomy: reproductive outcomesFertil Steril200788493394117434505
- BrowerVFDA likely to further restrict or ban morcellationLancet Oncol2014159e36925225696
- BrowerVFDA considers restricting or banning laparoscopic morcellationJ Natl Cancer Inst201410610
- FalconeTBedaiwyMAMinimally invasive management of uterine fibroidsCurr Opin Obstet Gynecol200214440140712151830
- RavinaJHHerbreteauDCiraru-VigneronNArterial embolisation to treat uterine myomataLancet199534689766716727544859
- GoodwinSCSpiesJBUterine fibroid embolizationN Engl J Med2009361769069719675331
- SiskinGPQuality improvement guidelines for uterine artery embolization: an evolution in patient selectionJ Vasc Interv Radiol201425111748174925442137
- DariushniaSRNikolicBStokesLSSpiesJBQuality improvement guidelines for uterine artery embolization for symptomatic leiomyomataJ Vasc Interv Radiol201425111737174725442136
- EdwardsRDMossJGLumsdenMAUterine-artery embolization versus surgery for symptomatic uterine fibroidsN Engl J Med2007356436037017251532
- ToorSSJaberiAMacdonaldDBMcInnesMDSchweitzerMERasuliPComplication rates and effectiveness of uterine artery embolization in the treatment of symptomatic leiomyomas: a systematic review and meta-analysisAJR Am J Roentgenol201219951153116323096193
- PronGMocarskiECohenMHysterectomy for complications after uterine artery embolization for leiomyoma: results of a Canadian multicenter clinical trialJ Am Assoc Gynecol Laparosc20031019910612555002
- PelageJPLe DrefOSoyerPFibroid-related menorrhagia: treatment with superselective embolization of the uterine arteries and midterm follow-upRadiology2000215242843110796920
- WalkerWJPelageJPUterine artery embolisation for symptomatic fibroids: clinical results in 400 women with imaging follow upBJOG2002109111262127212452465
- PintoIChimenoPRomoAUterine fibroids: uterine artery embolization versus abdominal hysterectomy for treatment – a prospective, randomized, and controlled clinical trialRadiology2003226242543112563136
- GoodwinSCSpiesJBWorthington-KirschRUterine artery embolization for treatment of leiomyomata: long-term outcomes from the FIBROID RegistryObstet Gynecol20081111223318165389
- SpiesJBCurrent evidence on uterine embolization for fibroidsSemin Intervent Radiol201330434034624436560
- SpiesJBBrunoJCzeyda-PommersheimFMageeSTAscherSAJhaRCLong-term outcome of uterine artery embolization of leiomyomataObstet Gynecol20051065 Pt 193393916260509
- LawPGedroycWMReganLMagnetic-resonance-guided percutaneous laser ablation of uterine fibroidsLancet199935491952049205010636374
- LawPReganLInterstitial thermo-ablation under MRI guidance for the treatment of fibroidsCurr Opin Obstet Gynecol200012427728210954146
- DonnezJSquiffletJPoletRNisolleMLaparoscopic myolysisHum Reprod Update20006660961311129695
- SilvermanSGTuncaliKAdamsDFMR imaging-guided percutaneous cryotherapy of liver tumors: initial experienceRadiology2000217365766411110925
- SakuharaYShimizuTKodamaYMagnetic resonance-guided percutaneous cryoablation of uterine fibroids: clinical experiencesCardiovasc Intervent Radiol200629455255816532267
- CowanBDMyomectomy and MRI-directed cryotherapySemin Reprod Med200422214314815164309
- BergaminiVGhezziFCromiALaparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomasAm J Obstet Gynecol2005192376877315746670
- MilicAAschMRHawrylyshynPALaparoscopic ultrasound-guided radiofrequency ablation of uterine fibroidsCardiovasc Intervent Radiol200629469469816502165
- KimHSTsaiJJacobsMAKamelIRPercutaneous image-guided radiofrequency thermal ablation for large symptomatic uterine leiomyomata after uterine artery embolization: a feasibility and safety studyJ Vasc Interv Radiol2007181 Pt 1414817296703
- RecaldiniCCarrafielloGLaganàDPercutaneous sonographically guided radiofrequency ablation of medium-sized fibroids: feasibility studyAJR Am J Roentgenol200718961303130618029862
- JonesSO’DonovanPToubDRadiofrequency ablation for treatment of symptomatic uterine fibroidsObstet Gynecol Int2012201219483921961009
- WoodRWLoomisLThe physical and biological effects of high frequency sound waves of great intensityPhilos Mag1927422417436
- LynnJGZwernerRLChickAJThe biological application of focused ultrasound wavesScience194296248311912017809987
- FryWJBarnardJWFryFJBrennanJFUltrasonically produced localized selective lesions in the central nervous systemAm J Phys Med195534341342314376518
- FryWJFryFJFundamental neurological research and human neurosurgery using intense ultrasoundIRE Trans Med Electron1960ME-716618113702332
- LizziFLDrillerJOstromogilskyMThermal model for ultrasonic treatment of glaucomaUltrasound Med Biol19841032892986464216
- HynynenKDamianouCDarkazanliAUngerESchenckJFThe feasibility of using MRI to monitor and guide noninvasive ultrasound surgeryUltrasound Med Biol199319191928456533
- ClineHEHynynenKWatkinsRDFocused US system for MR imaging-guided tumor ablationRadiology199519437317377862971
- ClineHESchenckJFWatkinsRDHynynenKJoleszFAMagnetic resonance-guided thermal surgeryMagn Reson Med1993301981068371680
- ChungAHJoleszFAHynynenKThermal dosimetry of a focused ultrasound beam in vivo by magnetic resonance imagingMed Phys19992692017202610505893
- BotnarRMSteinerPDubnoBErhartPvon SchulthessGKDebatinJFTemperature quantification using the proton frequency shift technique: in vitro and in vivo validation in an open 0.5 Tesla interventional MR scanner during RF ablationJ Magn Reson Imaging200113343744411241819
- JoleszFAHynynenKMcDannoldNTempanyCMR imaging-controlled focused ultrasound ablation: a noninvasive image-guided surgeryMagn Reson Imaging Clin N Am200513354556016084419
- McDannoldNQuantitative MRI-based temperature mapping based on the proton resonant frequency shift: review of validation studiesInt J Hyperthermia200521653354616147438
- McDannoldNTempanyCJoleszFHynynenKEvaluation of referenceless thermometry in MRI-guided focused ultrasound surgery of uterine fibroidsJ Magn Reson Imaging20082841026103218821603
- KennedyJEHigh-intensity focused ultrasound in the treatment of solid tumoursNat Rev Cancer20055432132715776004
- GedroycWMNew clinical applications of magnetic resonance-guided focused ultrasoundTop Magn Reson Imaging200617318919417414076
- FischerKGedroycWJoleszFAFocused ultrasound as a local therapy for liver cancerCancer J201016211812420404608
- SpiesJCoyneKGuaou GuaouNBoyleDSkyrnarz-MurphyKGonzalvesSThe UFS-QOL, a new disease specific symptom and health-related quality of life questionnaire for leiomyomataObstet Gynecol200299229030011814511
- ShenSHFennessyFMcDannoldNJoleszFTempanyCImage-guided thermal therapy of uterine fibroidsSemin Ultrasound CT MR20093029110419358440
- LénárdZMMcDannoldNJFennessyFMUterine leiomyomas: MR imaging-guided focused ultrasound surgery – imaging predictors of successRadiology2008249118719418695211
- FunakiKFukunishiHSawadaKClinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-upUltrasound Obstet Gynecol200934558458919852041
- ZhaoWPChenJYChenWZEffect of biological characteristics of different types of uterine fibroids, as assessed with T2-weighted magnetic resonance imaging, on ultrasound-guided high-intensity focused ultrasound ablationUltrasound Med Biol201541242343125542494
- LethabyAVollenhovenBSowterMEfficacy of pre-operative gonadotrophin hormone releasing analogues for women with uterine fibroids undergoing hysterectomy or myomectomy: a systematic reviewBJOG2002109101097110812387461
- ParkHYoonSWKimKAKimDJJungSGMagnetic resonance imaging-guided focused ultrasound treatment of pedunculated subserosal uterine fibroids: a preliminary reportJ Vasc Interv Radiol201223121589159323099002
- InSightec [homepage on the Internet] Available from: http://www.insightec.comAccessed June 17, 2005
- FennessyFMTempanyCMMcDannoldNJUterine leiomyomas: MR imaging-guided focused ultrasound surgery – results of different treatment protocolsRadiology2007243388589317446521
- OkadaAMoritaYFukunishiHTakeichiKMurakamiTNoninvasive magnetic resonance-guided focused ultrasound treatment of uterine fibroids in a large Japanese population: impact of the learning curve on patient outcomeUltrasound Obstet Gynecol200934557958319852042
- ZaherSGedroycWLyonsDReganLA novel method to aid in the visualisation and treatment of uterine fibroids with MRgFUS in patients with abdominal scarsEur J Radiol201076226927319665856
- MachtingerRTempanyCMKanan RoddyAFennessyFMSuccessful MRI-guided focused ultrasound uterine fibroid treatment despite an ostomy and significant abdominal wall scarringISRN Obstet Gynecol2011201196262121647219
- TempanyCMStewartEAMcDannoldNQuadeBJJoleszFAHynynenKMR imaging-guided focused ultrasound surgery of uterine leiomyomas: a feasibility studyRadiology2003226389790512616023
- HindleyJGedroycWMReganLMRI guidance of focused ultrasound therapy of uterine fibroids: early resultsAJR Am J Roentgenol200418361713171915547216
- StewartEARabinoviciJTempanyCMClinical outcomes of focused ultrasound surgery for the treatment of uterine fibroidsFertil Steril2006851222916412721
- KimHSBaikJHPhamLDJacobsMAMR-guided high-intensity focused ultrasound treatment for symptomatic uterine leiomyomata: long-term outcomesAcad Radiol201118897097621718955
- KimYSKeserciBPartanenAVolumetric MR-HIFU ablation of uterine fibroids: role of treatment cell size in the improvement of energy efficiencyEur J Radiol201281113652365921959213
- TrummCGStahlRClevertDAMagnetic resonance imaging-guided focused ultrasound treatment of symptomatic uterine fibroids: impact of technology advancement on ablation volumes in 115 patientsInvest Radiol201348635936523385396
- KjerulffKHLangenbergPSeidmanJDStolleyPDGuzinskiGMUterine leiomyomas. Racial differences in severity, symptoms and age at diagnosisJ Reprod Med19964174834908829060
- CatherinoWHEltoukhiHMAl-HendyARacial and ethnic differences in the pathogenesis and clinical manifestations of uterine leiomyomaSemin Reprod Med201331537037923934698
- HuyckKLPanhuysenCICuencoKTThe impact of race as a risk factor for symptom severity and age at diagnosis of uterine leiomyomata among affected sistersAm J Obstet Gynecol20081982168.e1e918226615
- StewartEANicholsonWKBradleyLBorahBJThe burden of uterine fibroids for African–American women: results of a national surveyJ Womens Health (Larchmt)2013221080781624033092
- MachtingerRFennessyFMStewartEAMissmerSACorreiaKFTempanyCMMR-guided focused ultrasound (MRgFUS) is effective for the distinct pattern of uterine fibroids seen in African–American women: data from phase III/IV, non-randomized, multicenter clinical trialsJ Ther Ultrasound201312325232480
- HanstedeMMTempanyCMStewartEAFocused ultrasound surgery of intramural leiomyomas may facilitate fertility: a case reportFertil Steril2007882497.e5e717292361
- RabinoviciJDavidMFukunishiHMoritaYGostoutBSStewartEAPregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroidsFertil Steril201093119920919013566
- Gavrilova-JordanLPRoseCHTraynorKDBrostBCGostoutBSSuccessful term pregnancy following MR-guided focused ultrasound treatment of uterine leiomyomaJ Perinatol2007271596117180132
- MoritaYItoNOhashiHPregnancy following MR-guided focused ultrasound surgery for a uterine fibroidInt J Gynaecol Obstet2007991565717599842
- ZaherSLyonsDReganLUncomplicated term vaginal delivery following magnetic resonance-guided focused ultrasound surgery for uterine fibroidsBiomed Imaging Interv J201062e2821611041
- ZaherSLyonsDReganLSuccessful in vitro fertilization pregnancy following magnetic resonance-guided focused ultrasound surgery for uterine fibroidsJ Obstet Gynaecol Res201137437037321392163
- YoonSWKimKAKimSHPregnancy and natural delivery following magnetic resonance imaging-guided focused ultrasound surgery of uterine myomasYonsei Med J201051345145320376901
- QinJChenJYZhaoWPHuLChenWZWangZBOutcome of unintended pregnancy after ultrasound-guided high-intensity focused ultrasound ablation of uterine fibroidsInt J Gynaecol Obstet2012117327327722465558
- ClarkNAMumfordSLSegarsJHReproductive impact of MRI-guided focused ultrasound surgery for fibroids: a systematic review of the evidenceCurr Opin Obstet Gynecol201426315116124751998
- RavinaJHVigneronNCAymardALe DrefOMerlandJJPregnancy after embolization of uterine myoma: report of 12 casesFertil Steril20007361241124310856491
- PronGBennettJCommonAWallJAschMSnidermanKThe Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroidsFertil Steril200379112012712524074
- WalkerWJMcDowellSJPregnancy after uterine artery embolization for leiomyomata: a series of 56 completed pregnanciesAm J Obstet Gynecol200619551266127116796984
- SalomirRPalussièreJVimeuxFCLocal hyperthermia with MR-guided focused ultrasound: spiral trajectory of the focal point optimized for temperature uniformity in the target regionJ Magn Reson Imaging200012457158311042639
- MougenotCQuessonBde SennevilleBDThree-dimensional spatial and temporal temperature control with MR thermometry-guided focused ultrasound (MRgHIFU)Magn Reson Med200961360361419097249
- PhilipsExpanding therapy options for women’s health and oncology: Sonalleve MR-HIFU2014 Available from: http://www.healthcare.philips.com/main/products/mri/systems/sonalleve/index.wpdAccessed June 17, 2015
- Blue Cross and Blue Shield of North CarolinaCorporate Medical Policy: MRI-Guided Focused Ultrasound (MRgFUS)Durham, NCBCBSNC2014 Available from: https://www.bcbsnc.com/assets/services/public/pdfs/medicalpolicy/mri_guided_focused_ultrasound_%28MRgFUS%29.pdfAccessed June 17, 2015
- O’SullivanAKThompsonDChuPLeeDWStewartEAWeinsteinMCCost-effectiveness of magnetic resonance guided focused ultrasound for the treatment of uterine fibroidsInt J Technol Assess Health Care2009251142519126247
- ZowallHCairnsJABrewerCLampingDLGedroycWMReganLCost-effectiveness of magnetic resonance-guided focused ultrasound surgery for treatment of uterine fibroidsBJOG2008115565366218333948
- KongCYMengLOmerZBMRI-guided focused ultrasound surgery for uterine fibroid treatment: a cost-effectiveness analysisAJR Am J Roentgenol2014203236137125055272
- FennessyFMKongCYTempanyCMSwanJSQuality-of-life assessment of fibroid treatment options and outcomesRadiology2011259378579221364084
