Abstract
Objective
To compare the reproductive, metabolic, and skeletal profiles of young athletic women with functional hypothalamic amenorrhea (FHA) as well as clinical or biochemical hyperandrogenism (FHA-EX+HA) with body mass index matched women with FHA due to exercise (FHA-EX) or anorexia nervosa (FHA-AN) alone.
Design
Retrospective cohort study.
Setting
Tertiary care teaching hospital.
Population
Adolescents and young women, 15–30 years of age, diagnosed with FHA along with concurrent signs of hyperandrogenism (n=22) and body mass index matched control groups consisting of 22 women in each group of FHA-EX and FHA-AN.
Main outcomes
1) Reproductive hormone profile: luteinizing hormone (LH), follicle stimulating hormone (FSH), total testosterone, pelvic ultrasound features. 2) Metabolic function and skeletal health markers: fasting glucose, cholesterol, number of stress fractures and bone mineral density as assessed by spine dual-energy X-ray absorptiometry z scores.
Results
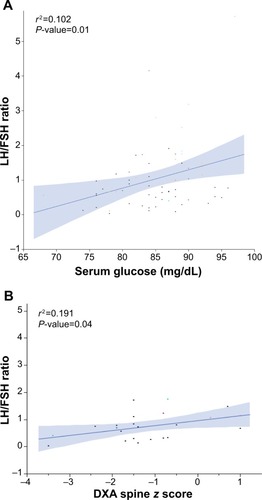
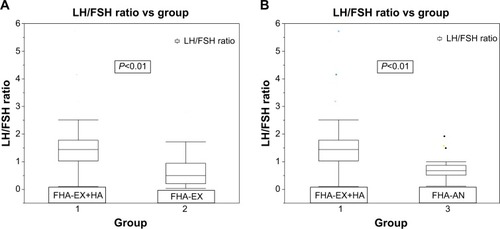
FHA-EX+HA group was older at diagnosis compared to the other groups with a median (interquartile range [IQR]) age of 22 (18.75–25.25) years versus (vs) 17.5 (15.75–19) for FHA-EX; (P<0.01) and 18 (16–22.25) years for FHA-AN (P=0.01). There were no differences among the groups based on number of hours of exercise per week, type of physical activity or duration of amenorrhea. Median (IQR) LH/FSH ratio was higher in FHA-EX+HA than both other groups, 1.44 (1.03–1.77) vs 0.50 (0.20–0.94) for FHA-EX and 0.67 (0.51–0.87) for FHA-AN (P<0.01 for both). Total testosterone concentrations were not different among the groups. Median (IQR) fasting serum glucose concentration was higher in FHA-EX+HA vs FHA-EX, 88.5 mg/dL (82.8–90 mg/dL) vs 83.5 mg/dL (78.8–86.3 mg/dL) (P=0.01) but not different from FHA-AN (P=0.31). Percentage of women with stress fractures was lower in FHA-EX+HA (4.5%) as compared to both FHA-EX (27.3%) and FHA-AN (50%); P=0.04 and 0.01 respectively. The LH/FSH ratio was weakly positively associated with serum glucose (adjusted r2=0.102; P=0.01) as well as with dual-energy X-ray absorptiometry spine score (adjusted r2=0.191; P=0.04) in the entire cohort.
Conclusion
In a small cohort of female athletes with hyperandrogenism, a distinct reproductive hormone profile consisting of higher LH to FHS ratio may be associated with adverse metabolic health markers but improved skeletal health.
Introduction
Adolescents and young women frequently suffer from menstrual dysfunction. Functional hypothalamic amenorrhea (FHA) and hyperandrogenism syndromes such as polycystic ovary syndrome (PCOS) are the most common causes of menstrual irregularity in this age group.Citation1,Citation2 FHA is a condition characterized by the absence of menses due to the suppression of the hypothalamic–pituitary–ovarian axis, in which no anatomical or organic disease is identified.Citation3 Adolescents or young women with this condition typically present with amenorrhea of 6 months’ duration or longer.Citation4 This condition may be difficult to differentiate from immaturity of the hypothalamic–pituitary–ovarian axis during the initial post-menarchal years in adolescents but even during this time, menstrual cycles in adolescents typically are no longer than 45 days.Citation5 FHA in this population can occur secondary to disordered eating and/or excessive exercise. On the other hand, PCOS is a complex endocrinopathy with marked heterogeneity in clinical manifestations. Often associated with obesity, PCOS affects 4%–10% of women of reproductive ageCitation6–Citation10 and comprises a combination of hyperandrogenism, ovulatory dysfunction, and morphologically polycystic ovaries.Citation11–Citation14 PCOS is often associated with concomitant abnormalities of insulin resistance with or without compensatory hyperinsulinemia independent of body weight,Citation15–Citation18 dyslipidemia,Citation19 and subfertility.Citation18
There is emerging evidence of certain subsets of athletic females suffering from menstrual dysfunction with clinical features and hormonal profiles suggestive of androgen excess, in some cases meeting criteria for PCOS, challenging the contemporary concept that reproductive dysfunction in athletes is due to chronic energy deficiency alone.Citation1,Citation20 Varying athletic disciplines, based on the intensity of exercise and energy deficit incurred, may impact gonadotropin-releasing hormone pulsatility in different ways.Citation21 In fact, a systematic review assessing observational studies as well as randomized clinical trials in 2009 reported that although data are still scarce, there is a suggestion that hyperandrogenism, such as that in PCOS, may likely be associated with oligomenorrhea in exercising women, and may not always represent hypothalamic inhibition due to low energy availability. In sports emphasizing leanness, nutritional restriction may be an important causal factor in reproductive dysfunction but a distinct hormonal profile characterized by hyperandrogenism rather than hypoestrogenism has been described in sports emphasizing strength over leanness.Citation21
It has also been postulated that in subjects with FHA and concurrent features of PCOS, some athletic advantage may be afforded.Citation22
PCOS is often associated with adverse metabolic profile although it is not known if the metabolic milieu of athletes with hyperandrogenism is inferior to that of athletes with FHA alone.
The primary aim of our study was to compare reproductive hormone profile among adolescents and young women with concurrent diagnoses of exercise induced FHA as well as clinical or biochemical hyperandrogenism (FHA-EX+HA) to athletes with FHA only, either due to excessive exercise (FHA-EX) or anorexia nervosa (FHA-AN). Secondarily, we compared the metabolic and skeletal profiles of females in each group and explored the association, if any, between the reproductive and skeletal or metabolic profiles.
Methods
This was a retrospective review of medical records of female adolescents and young women seen at Mayo Clinic, Rochester, MN between 2004–2013, for female reproductive health disorders including FHA and PCOS.
Participants
After receiving the approval of the Institutional Review Board at Mayo Clinic, Rochester, MN, adolescents and young women aged 15–30 years with concurrent diagnoses of FHA and hyperandrogenism (FHA-EX+HA) or FHA-EX or FHA-AN were identified using both a free text search in clinical notes in addition to coding and billing data. Identification of the cohorts was performed using the Mayo Clinic Life Sciences System, which is a complete clinical data repository containing patient demographics; diagnoses; hospital-, laboratory-, and clinical notes; pathology; and billing data obtained from multiple clinical and hospital source systems within Mayo Clinic. Data in the Mayo Clinic Life Sciences System was accessed via the Data Discovery and Query Builder toolset, consisting of a web-based application that utilizes a unique text search engine and provides the capability to rapidly search for specific words and phrases within unstructured text documents such as clinical notes.Citation23 All adolescents and young women seen within a 10 year period (January 2004 to December 2013) with the International Classification of Diseases, Ninth Revision (ICD-9) code for PCOS (256.4) and amenorrhea (626.0) supplemented with text word search terms such as “female athlete triad”, or “exercise induced menstrual dysfunction”, or “exercise induced amenorrhea”, or “functional hypothalamic amenorrhea”, or “menstrual irregularity due to exercise” and “hyperandrogenism” or “excess androgens” were identified for Group 1 (FHA-EX+HA). Exclusion terms of “eating disorder”, “disordered eating”, “anorexia nervosa”, “bulimia nervosa”, were also applied in order to not include females with disordered eating in Group 1. The same text search terms of “female athlete triad”, “exercise induced menstrual dysfunction”, “exercise induced amenorrhea”, “functional hypothalamic amenorrhea”, “menstrual irregularity due to exercise” with negation of “PCOS”, “hyperandrogenism”, “excess androgens” and “eating disorder”, “disordered eating”, “anorexia nervosa”, “bulimia nervosa”, were used to identify Group 2 (FHA-EX). This cohort was body mass index (BMI) matched with Group 1. For identification of Group 3 (FHA-AN), search terms of “eating disorder”, “disordered eating”, “anorexia nervosa”, “bulimia nervosa”, along with “menstrual dysfunction”, “functional hypothalamic amenorrhea”, “amenorrhea”, “oligomenorrhea”, “menstrual irregularity due to eating disorder”, “menstrual irregularity due to disordered eating”, “menstrual irregularity due to anorexia nervosa” were used. This group was also BMI matched with Group 1.
Definitions
Subjects in the FHA-EX+HA group were identified if they had a diagnosis of FHA due to excessive exercise but also had either clinical or biochemical evidence of hyperandrogenism. Rotterdam criteria were used to diagnose PCOS, requiring the presence of at least two of the three criteria of: 1) clinical or biochemical evidence of hyperandrogenism, 2) oligo- or anovulation (defined as less than nine periods per year), and 3) polycystic ovarian morphology (PCOM). During chart review, we identified girls who also met the National Institutes of Health criteria for PCOS requiring the presence of both oligo-ovulation and hyperandrogenism. Determination of PCOM was based on presence of 12 or more 2–9 mm follicles in each ovary or ovarian volume >10 mL as defined by Rotterdam criteria.Citation24 FHA due to exercise was defined as absence of menstrual cycles for 6 or more months in an otherwise healthy female exercising at least 8 hours/week without any chronic severe illness.Citation3 Type of physical activity was divided into aerobic, anaerobic or both. FHA due to anorexia nervosa was defined as absence of menstrual cycles for 6 or more months in a female with previously regular menstrual cycles with a diagnosis of anorexia nervosa made by a psychiatrist or psychologist.
Subjects with Turner’s syndrome, premature ovarian failure, untreated hypothyroidism, hyperprolactinemia, congenital adrenal hyperplasia, Cushing’s syndrome, pregnancy, androgen-secreting tumors, subjects on any form of hormonal contraception or a condition as the cause of hyperandrogenism were excluded.
Procedures and measures
We employed a standardized abstraction form for data collection. For each medical record, we identified the first visit in which the diagnosis of FHA was made. We collected information regarding age at diagnosis, age at menarche, diagnosis of PCOS or hyperandrogenism (Group 1), BMI, systolic and diastolic blood pressure, number of hours of exercise per week, type of physical activity, number of fractures (stress and long bone fractures), pattern of menstrual dysfunction (primary amenorrhea, secondary amenorrhea or oligomenorrhea). Biochemical measurements collected, where available, included LH, FSH, total and free testosterone, calcium, phosphorus, alkaline phosphatase, fasting glucose, total cholesterol, triglycerides, low density lipoprotein and high density lipoprotein. Radiographic information if available was obtained on duel-energy X-ray absorptiometry (DXA) spine and hip z scores as well as pelvic ultrasonography.
All biochemical laboratory measures were obtained in the Mayo Clinic Laboratories using standard automated colorimetric enzymatic assays (Roche Diagnostics, Indianapolis, IN, USA) with the exception of testosterone, which was measured using high-throughput liquid chromatography (Hamilton Robotics Inc., Reno, NV, USA), and glucose, which was measured using a photometric rate reaction (Roche Diagnostics). Fasting glucose was obtained using a standard minimum 12 hours of fasting, which was verified on all samples.
Statistical analyses
We compared the medians and interquartile ranges (IQRs) of clinical and biochemical characteristics between FHA-EX+HA and FHA-EX and FHA-AN using Wilcoxon test. Categorical variables were compared using chi-square analysis. Linear regression models were created in a stepwise fashion using JMP 9.0.1 (SAS Institute Inc., Cary, NC, USA) to identify associations of luteinizing hormone (LH)/follicle stimulating hormone (FSH) ratio with skeletal and metabolic parameters.
Results
Cohort derivation
Group 1: FHA due to exercise with hyperandrogenism (FHA-EX+HA)
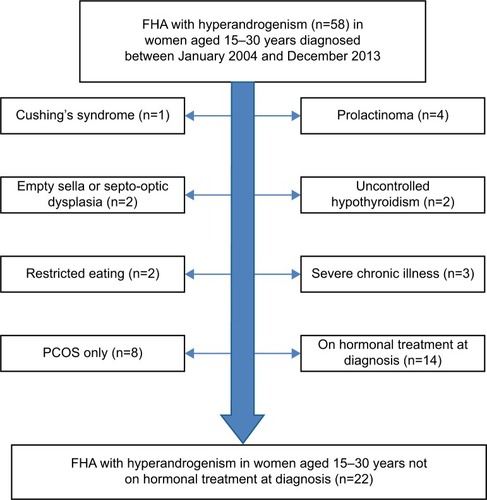
Between January 2004 and December 2013, a total of 58 women were identified, who were aged between 15–30 years with a concurrent electronic documentation of both exercise related FHA and clinical or biochemical hyperandrogenism. We determined by individual chart if the subject met criteria for PCOS (). After exclusion of subjects with other chronic illnesses and women on hormonal medication use at the time of diagnosis, a total of 22 subjects with FHA due to excessive exercise and evidence of hyperandrogenism at the time of presentation were included in the analysis.
Group 2: FHA due to excessive exercise without hyperandrogenism (FHA-EX)
For every case of FHA-EX+HA, women aged 15–30 years with FHA due to excessive exercise but no evidence of disordered eating, not on any hormonal treatment at diagnosis and with no features of hyperandrogenism were BMI matched (within 1 kg/m2) with the corresponding case of FHA-EX+HA. Exclusion criteria were: 1) presence of any restricted or disordered eating, 2) anorexia nervosa or bulimia nervosa, 3) clinical and/or biochemical hyperandrogenism (ie, presence of hirsutism or Ferriman Gallwey score >6 or serum total testosterone >60 ng/dL), 4) hyperprolactinemia or 5) untreated hypothyroidism.
Group 3: FHA due to anorexia nervosa without hyperandrogenism (FHA-AN)
For every case of FHA-EX+HA, women aged 15–30 years with FHA due to anorexia nervosa (FHA-AN), not on any hormonal treatment at diagnosis and no features of hyperandrogenism (as described in the Methods section), were BMI matched (within 1 kg/m2) with the corresponding case of FHA-EX+HA.
Clinical and anthropometric characteristics
FHA-EX+HA subjects were older at diagnosis compared to the other groups with a median (IQR) age of 22 (18.8–25.3) years versus (vs) 17.5 (15.8–19) for FHA-EX (P<0.01) and 18 (16–22.3) for FHA-AN; P=0.01. There was no difference between the two groups based on ethnicity, BMI or age at menarche (). Median (IQR) reported weight loss (kg) at the time of diagnosis was not different between the groups (FHA-EX+HA 2.25 (1.58–3.6) vs FHA-EX 2.7 (2.25–3.49) vs FHA-AN 2.25 (1.69–2.8); P=0.17 and P=0.42 respectively. There was no difference among the groups based on type of activity (aerobic versus anaerobic) or number of hours of exercise per week ().
Table 1 Baseline characteristics of all three groups
Reproductive phenotype
There was no difference among the groups based on duration of amenorrhea (). Clinical signs of hyperandrogenism such as hirsutism were present in 54.5% of FHA-EX+HA subjects while none of the subjects with FHA-EX and FHA-AN had hirsutism (P<0.01 for both). PCOM was present in 95% of FHA-EX+HA group as compared to 17.7% of FHA-EX and 0% in the FHA-AN group (P<0.01 for both). Based on Rotterdam criteria, all subjects in the FHA-EX+HA group met criteria for PCOS.
Table 2 Comparison of reproductive, metabolic, and skeletal phenotypes between FHA-EX+HA, FHA-EX, and FHA-AN
Mean LH/FSH ratio was higher in FHA-EX+HA than both other groups (P<0.001) (). Estradiol concentrations were not available in most subjects, precluding analysis. Progesterone withdrawal was performed in 16/22 of FHA-EX+HA and 22/22 of FHA-EX as well as 17/22 of FHA-AN with percentage of girls failing to menstruate after this being 37.5% for FHA-EX+HA, 45.5% for FHA-EX, and 82.4% for FHA-AN. Estradiol concentrations did not differ between the subsets who failed to menstruate in the FHA-EX+HA and FHA-EX groups. Total testosterone concentrations were available in 20 subjects with FHA-EX+HA, 12 subjects with FHA-EX, and eleven subjects with FHA-AN. Total testosterone concentrations were higher in the FHA-EX+HA group as compared to FHA-EX (P=0.04) but did not differ from FHA-AN (P=0.06) (). Dehydroepiandrostenedione sulfate (DHEA-S), free testosterone, and androstenedione concentrations were not available in most subjects, precluding analysis. Pelvic ultrasound evaluation was completed at the time of diagnosis in 20 subjects in the FHA-EX+HA group, 17 subjects in FHA-EX group and 19 FHA-AN subjects.
Figure 2 Comparison of LH/FSH ratio between FHA-EX+HA and FHA-EX and FHA-AN.
Abbreviations: FHA, functional hypothalamic amenorrhea; LH/FSH, luteinizing hormone/follicle stimulating hormone; vs, versus.
Metabolic phenotype
FHA-EX+HA group had higher diastolic blood pressure (DBP) compared to FHA-EX group and higher systolic blood pressure and DBP than FHA-AN group (all P-values <0.05) (). Mean fasting serum glucose was higher in FHA-EX+HA as compared to FHA-EX (P=0.01) but did not differ from FHA-AN group (P=0.31). Total cholesterol concentrations were available for 14 subjects in the FHA-EX+HA group, nine in the FHA-EX group, and eight in the FHA-AN group. There was no difference between the groups based on mean total cholesterol concentrations ().
Skeletal phenotype
Percentage of subjects with stress fractures was lower in FHA-EX+HA (4.5%) as compared to both FHA-EX (27.3%) and FHA-AN (50%) groups (P=0.04 and 0.01 respectively). DXA scores at the spine were available for four FHA-EX+HA, 14 FHA-EX, and six FHA-AN subjects. Mean z scores were higher in FHA-EX+HA as compared to FHA-EX (P=0.02) but did not differ from FHA-AN group (). Total alkaline phosphatase, calcium, phosphorus, and 25 hydroxy vitamin D concentrations were not obtained in most subjects, precluding analysis.
Relationship between LH/FSH ratio and metabolic phenotype
There was a positive association between LH/FSH ratio and serum glucose in the entire cohort (P<0.01) (). This relationship persisted even after adjusting for BMI (P=0.01). When assessed separately in the groups, the relationship was significant only in the FHA-EX+HA cohort (P=0.04), even after adjustment for BMI (P=0.04). There was a positive association between the LH/FSH ratio and DBP (P=0.01), persisting after adjustment for BMI (P=0.02). No association was present between the LH/FSH ratio and systolic blood pressure or total cholesterol (all P-values >0.05).
Relationship between LH/FSH ratio and skeletal phenotype
There was no association between the LH/FSH ratio and the number of stress fractures.
However, there was a positive association between LH/FSH ratio and DXA spine score in the entire cohort (P=0.04), attenuated after adjusting for BMI (P=0.09) ().
Discussion
Although reproductive dysfunction in female athletes is typically ascribed to energy deficit and inhibition of gonadotropin-releasing hormone pulsatility,Citation25 our study highlights the unique reproductive phenotype, with higher LH/FSH ratios of athletes with FHA due to excessive exercise who also have evidence of clinical or biochemical hyperandrogenism as compared to BMI matched females with FHA due to exercise or anorexia nervosa alone. We also report a possible adverse metabolic profile with higher fasting glucose in these athletes with hyperandrogenism as well as an improved bone mineral density resulting in fewer stress fractures in this cohort.
Rickenlund et al describe the existence of hyperandrogenism in a subset of endurance athletes with menstrual dysfunction with elevated LH/FSH ratios and androgen concentrations.Citation22 In a previous study of 90 Swedish Olympic athletes, the most common cause of menstrual dysfunction was PCOS and not hypothalamic inhibitionCitation26 but although previous studies have reported existence of a subset of athletes with menstrual dysfunction attributable, at least in part, to hyperandrogenism,Citation22,Citation26 our study is the first to our knowledge to compare the reproductive, metabolic, and skeletal health parameters among females with both menstrual dysfunction and hyperandrogenism (FHA-EX+HA) to BMI matched groups of females with FHA due to exercise or anorexia nervosa alone. In addition to a higher LH/FH ratio in the subset of athletic women with hyperandrogenism, we also report higher blood pressure and fasting glucose in this group as compared to BMI matched subjects with FHA due to excessive exercise or anorexia nervosa alone. The association of LH/FSH ratio with fasting glucose has not previously been reported in young female athletes with menstrual dysfunction and hyperandrogenism. In subjects with PCOS, and nationally representative women without PCOS, a distinct subset with excess LH and hyperinsulinemia has been reported. These women typically have higher adrenal androgenic activity and markers of chronic inflammation and dyslipidemia which have been shown to be significantly associated with LH/FSH ratio.Citation27,Citation28 To further confirm this association in our cohort, this association of LH/FSH ratio with fasting glucose persisted only in the subgroup of athletic women with hyperandrogenism (FHA-EX+HA).
Although a previous small series has reported an association between improved bone mineral density and hyperandrogenism in female athletesCitation22 and in lean women with PCOS,Citation29 the findings of our study are novel in the sense of reporting a positive association between LH/FSH ratio and stress fractures as well as bone mineral density (BMD) DXA z scores at the spine in athletes with and without evidence of hyperandrogenism as well as another comparator group of females with anorexia nervosa. Although causality is difficult to establish in a retrospective study, the LH/FSH ratio may well serve as a marker of metabolic and skeletal health in young athletic females. Therefore, it may serve not just as a diagnostic tool but also indicate when further screening for metabolic risk factors may be warranted. An important clinical implication of our study findings pertains to management of these females. While the existing treatment focuses on energy repletion above all else, one may have to exercise some caution prior to recommending significant increase in caloric intake or weight in this subset of hyperandrogenic young women as their metabolic profiles may be adversely impacted. In the FHA-EX+HA group, a significant number of women had withdrawal bleeding after progesterone challenge, serving as further evidence that menstrual dysfunction in this subset may arise from excessive androgen concentration or activity rather than hypoestrogenism.
Strengths
The strengths of our study include the comparison of athletic young women with menstrual dysfunction along with clinical or biochemical hyperandrogenism with two comparator groups ie, women with FHA due to excessive exercise (FHA-EX) or anorexia nervosa (FHA-AN). Also, exclusion of subjects with potential disordered eating from FHA-EX+HA and FHA-EX was performed after extensive review of clinical charts. Further, the matching of BMI between groups allowed us to negate the impact of weight associated changes on metabolic and skeletal health parameters. Lastly, unlike prior investigations on the subject, we excluded women who were on any hormonal medications at the time of diagnosis in order to not impact the gonadotropin or androgen concentrations.
Limitations
There is a potential risk of selection bias conducting a retrospective observational study that selects groups from different specialties. Also, despite careful clinical review, it is possible that some subjects in the FHA-EX+HA and FHA-EX groups had some disordered eating while others meeting criteria for anorexia nervosa also had significant exercise habits but not documented in charts. Similarly, the distinction between amenorrhea secondary to FHA or hyperandrogenism may be quite difficult as we relied on medical record diagnosis of FHA along with evidence of hyperandrogenism to define the group FHA-EX+HA. We hope, however, that comparison to two groups with documented diagnoses of FHA due to exercise or anorexia nervosa alone with no clinical or biochemical evidence of hyperandrogenism would further support the conclusions reported. Additionally, missing or limited data for some parameters is also a challenge of retrospective data collection. Similarly, we relied solely on medical records to define the group FHA-EX+HA as athletes with FHA along with evidence of androgen excess, making the distinction between amenorrhea secondary to FHA or to FHA with hyperandrogenism difficult. We also acknowledge that in 2006, there was a change in the LH assay but this did not affect reference ranges and a validation study showed good correlation between the two immunoassays. Future prospective studies systematically studying the hormonal profile of young athletic women and body composition as well as markers of metabolic function are needed to further elucidate any underlying relationships.
Conclusion
Young athletic women may suffer from menstrual dysfunction due to reasons other than energy depletion and FHA. In the subset of young active women with clinical or biochemical hyperandrogenism, higher LH/FSH ratios than similar aged women with comparable weight who suffer from FHA due to excessive exercise or anorexia nervosa can be seen. This can serve as an important diagnostic indicator and have clinical implications on management of this subset of young women since indiscriminate caloric repletion and weight gain may adversely impact their metabolic profile. Large, prospective studies are needed to delineate the relationship between these phenotypes in athletic women.
Disclosure
The authors report no conflicts of interest in this work.
References
- Pinhas-HamielOPilpelNCarelCSingerSClinical and laboratory characteristics of adolescents with both polycystic ovary disease and anorexia nervosaFertil Steril20068561849185116650415
- GoldenNHCarlsonJLThe pathophysiology of amenorrhea in the adolescentAnn N Y Acad Sci2008113516317818574222
- GordonCMClinical practice. Functional hypothalamic amenorrheaN Engl J Med2010363436537120660404
- LiuJHBillAHStress-associated or functional hypothalamic amenorrhea in the adolescentAnn N Y Acad Sci2008113517918418574223
- LegroRSLinHMDemersLMLloydTRapid maturation of the reproductive axis during perimenarche independent of body compositionJ Clin Endocrinol Metab20008531021102510720033
- BaumannEERosenfieldRLPolycystic ovary syndrome in adolescenceThe Endocrinologist200212333348
- KnochenhauerESKeyTJKahsar-MillerMWaggonerWBootsLRAzzizRPrevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective studyJ Clin Endocrinol Metab1998839307830829745406
- DunaifAThomasACurrent concepts in the polycystic ovary syndromeAnnu Rev Med20005240141911160786
- AzzizRWoodsKSReynaRKeyTJKnochenhauerESYildizBOThe prevalence and features of the polycystic ovary syndrome in an unselected populationJ Clin Endocrinol Metab20048962745274915181052
- GabrielliLAquinoEMPolycystic ovary syndrome in Salvador, Brazil: a prevalence study in primary healthcareReprod Biol Endocrinol2012109623173761
- AzzizRCarminaEDewaillyDPosition statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guidelineJ Clin Endocrinol Metab200691114237424516940456
- ZawadzkiJKDunaifADiagnostic criteria for polycystic ovary syndrome: towards a rational approachDunaifAGivensJRHaseltineFMerriamGPolycystic Ovary SyndromeCambridge, MABlackwell Scientific1992377384
- AzzizRCarminaEDewaillyDThe Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force reportFertil Steril200991245648818950759
- Centers for Disease Control and Prevention [homepage on the Internet]CDC Growth Charts [updated September 9, 2010]. Available from: http://www.cdc.gov/growthcharts/cdc_charts.htmAccessed November 10, 2014
- DeUgarteCMBartolucciAAAzzizRPrevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessmentFertil Steril20058351454146015866584
- MathurRAlexanderCJYanoJTrivaxBAzzizRUse of metformin in polycystic ovary syndromeAm J Obstet Gynecol2008199659660919084097
- PalmertMRGordonCMKartashovAILegroRSEmansSJDunaifAScreening for abnormal glucose tolerance in adolescents with polycystic ovary syndromeJ Clin Endocrinol Metab20028731017102311889155
- SamSDunaifAPolycystic ovary syndrome: syndrome XX?Trends Endocrinol Metab200314836537014516934
- CovielloADLegroRSDunaifAAdolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistanceJ Clin Endocrinol Metab200691249249716249280
- ConstantiniNWWarrenMPMenstrual dysfunction in swimmers: a distinct entityJ Clin Endocrinol Metab1995809274027447673417
- WarrenMPPerlrothNEThe effects of intense exercise on the female reproductive systemJ Endocrinol2001170131111431132
- RickenlundACarlstromKEkblomBBrismarTBvon SchoultzBHirschbergALHyperandrogenicity is an alternative mechanism underlying oligomenorrhea or amenorrhea in female athletes and may improve physical performanceFertil Steril200379494795512749436
- AlsaraAWarnerDOLiGHerasevichVGajicOKorDJDerivation and validation of automated electronic search strategies to identify pertinent risk factors for postoperative acute lung injuryMayo Clin Proc201186538238821531881
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop GroupRevised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndromeFertil Steril20048111925
- JavedATebbenPJFischerPRLteifANFemale athlete triad and its components: toward improved screening and managementMayo Clin Proc2013889996100924001492
- HagmarMBerglundBBrismarKHirschbergALHyperandrogenism may explain reproductive dysfunction in olympic athletesMed Sci Sports Exerc20094161241124819461542
- BanaszewskaBSpaczynskiRZPeleszMPawelczykLIncidence of elevated LH/FSH ratio in polycystic ovary syndrome women with normo- and hyperinsulinemiaRocz Akad Med Bialymst20034813113414737959
- BeydounHABeydounMAWigginsNStadtmauerLRelationship of obesity-related disturbances with LH/FSH ratio among post-menopausal women in the United StatesMaturitas2012711556122088801
- ZborowskiJVCauleyJATalbottEOGuzickDSWintersSJClinical Review 116: Bone mineral density, androgens, and the polycystic ovary: the complex and controversial issue of androgenic influence in female boneJ Clin Endocrinol Metab200085103496350611061489