Abstract
The purpose of this study was to describe a presumed case of Epstein–Barr virus (EBV)-associated retinal vasculitis in a 42-year-old female with sudden unilateral vision loss and successful treatment with acyclovir therapy. Diagnostic vitreous biopsy of the right eye was performed to test for EBV and other known infectious causes of retinitis and evaluate vitreous cells and serological testing. Vitreous polymerase chain reaction viral DNA testing result was positive for EBV but negative for herpes simplex virus, varicella-zoster virus, and cytomegalovirus. Serologic testing was negative for toxoplasma gondii, syphilis, tuberculosis, and HIV. Histopathologic analysis of vitreous cells revealed atypical lymphocytes. Fluorescein angiography showed disk leakage, occluded retinal artery, peripheral vascular leakage, and ischemic area of the right eye. Intravenous acyclovir, 10 mg/kg/d, was prescribed for 14 days followed by oral acyclovir for 3 months. All lesions have become quiet. EBV may be a cause of retinal disease, and intravenous acyclovir is a successful treatment choice.
Case report
In 2013, a 42-year-old woman presented for the first time to Phramongkutklao Hospital, Thailand. She had suffered from floaters for 4 weeks and sudden loss of vision for a week in the right eye. She had no ocular pain or photophobia. Her left eye was not affected. The visual acuity was finger count 3’ in the right eye and was 20/25 in the left eye. Anterior segment examination revealed anterior chamber cell trace and fine keratic precipitates in the right eye and normal examination in the left eye. Intraocular pressure was 18 in both the eyes.
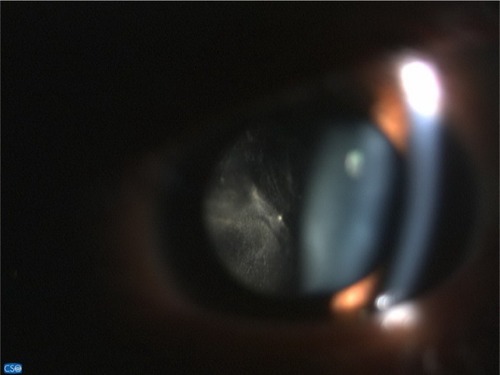
Fundus examination showed anterior vitreous cells’ grade 3+ (), vitreous haze grade 3, snow balls, and unclear optic disk in the right eye. The left eye’s fundus was normal. Three days later, the inflammation increased; therefore, diagnostic vitrectomy was performed immediately.
Intraoperatively (), we found dense vitritis, fibrous tissue on the superior disk with diffuse sclerotic vessels, and sheathing of both artery and vein. A vitreous sample was sent to the laboratory to perform polymerase chain reaction (PCR) for tuberculosis, herpesvirus family, and cytology for malignancy.
Figure 2 Intraoperative findings were dense vitritis, fibrous tissue on superior disk with diffuse periphery sclerotic vessels, and sheathing of both artery and vein.
Serum CD3 was 2,327 cells/mL (67.5%), and CD4 was 1,138 cells/mL (33%). Serum Epstein–Barr virus (EBV) immunoglobulin (Ig) M was positive (borderline 0.8), and EBV IgG was also positive (9.50; normal: 0–0.8), while serum IgM and IgG for herpes simplex virus (HSV), varicella-zoster virus, and cytomegalovirus were negative. Serum tests for HIV, syphilis, toxoplasmosis, and tuberculosis were negative. Anergy skin test and chest scan for sarcoidosis were also negative.
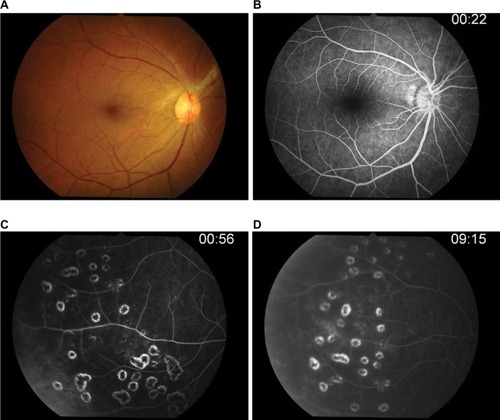
Three days after vitrectomy, the right fundus showed mild vitreous haze, fibrous tissue, and optic disk edema with normal retinal vasculature at the posterior pole in the right eye. Fluorescein angiography () confirmed leakage at the optic disk and occluded retinal artery and retinal vein leakage at the periphery in all quadrants of the right eye. The ischemic area was also observed at the periphery. Vitreous PCR viral DNA testing result was positive for EBV but negative for HSV-1 and -2, varicella-zoster virus, and also cytomegalovirus.
Figure 3 Fluorescein angiography.
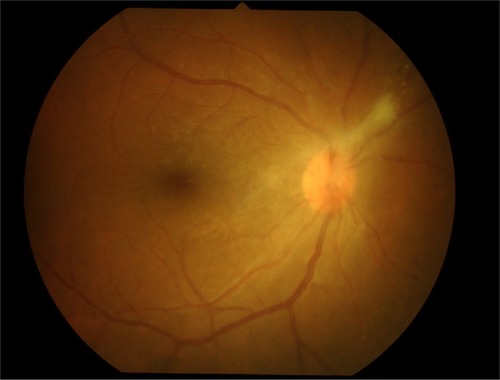
After vitrectomy, her right visual acuity improved to 20/25, intraocular pressure was 30, anterior chamber cells’ grade was 2+, and anterior vitreous cells’ grade was 2+. She was treated with intravenous acyclovir 10 mg/kg for 2 weeks; antiglaucoma drugs, topical prednisolone acetate, and antibiotics were prescribed. Panretinal photocoagulation of the right eye at the ischemic area was performed ().
Figure 4 Panretinal photocoagulation of the right eye at ischemic area was performed.
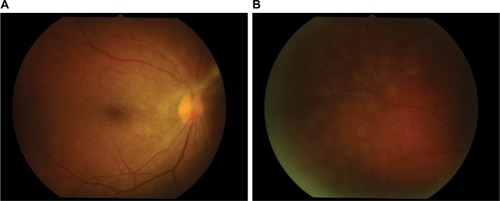
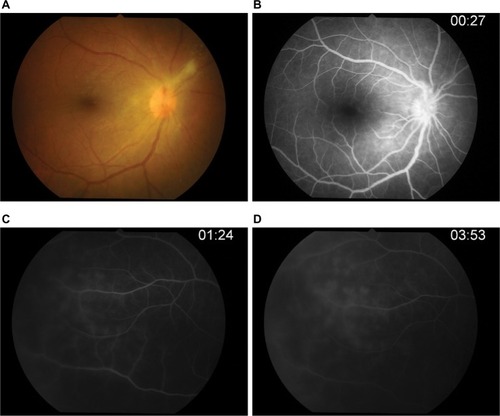
After 14-day therapy () with intravenous acyclovir, her anterior chamber activity in the right eye was much improved, but intraocular pressure still rose to 32. The patient was continued on oral acyclovir (800 mg) five times daily and antiglaucoma drugs.
Figure 5 Post-IV acyclovir after 14 days of therapy.
Abbreviation: IV, intravenous; FA, fluorescein angiography.
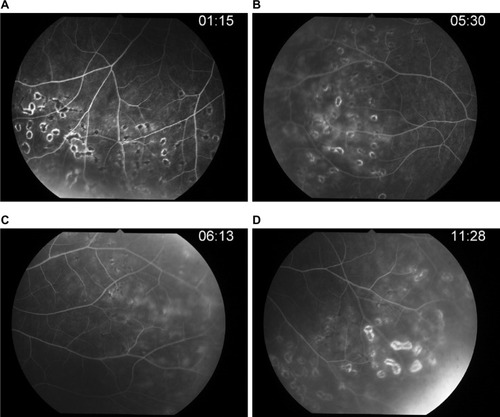
After 4 weeks of therapy (), the fluorescein angiography showed that her right optic disk edema had decreased and retinal phlebitis had improved.
Figure 6 Post-IV acyclovir after 4 weeks of therapy.
Abbreviation: IV, intravenous; FA, fluorescein angiography.
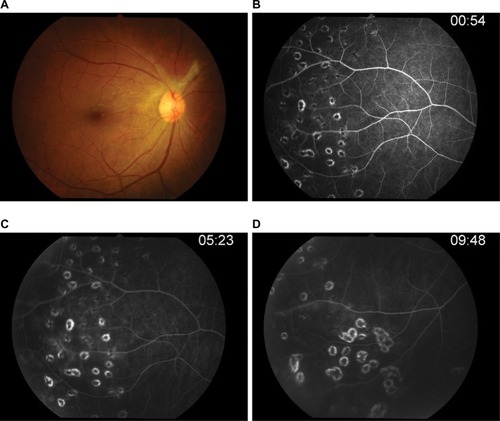
After 3 months of therapy (), her right visual acuity increased up to 20/20, intraocular pressure was 17, and no anterior chamber cells were observed. Repeat fluorescein angiography showed neither retinal vascular nor optic disk leakage. She discontinued oral acyclovir; however, she remained on brimonidine/timolol ophthalmic solution and brinzolamide.
Figure 7 Post-acyclovir after 3 months of therapy.
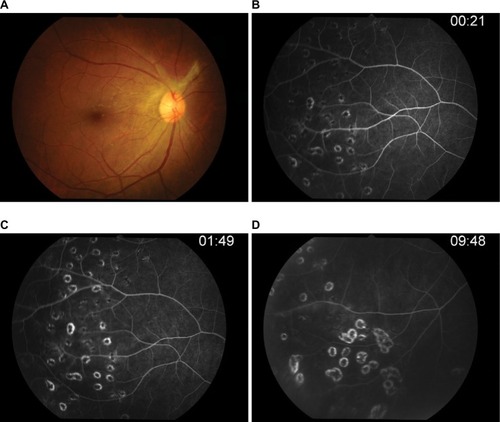
After 6 months of therapy (), the visual acuity was 20/20 in both eyes, and intraocular pressure was controlled. Anterior chamber and vitreal cells were also quiet, and inactive retinal vasculitis was confirmed by fluorescein angiography. This case report was approved by The Institutional Review Board, Royal Thai Army Medical Department who have deemed that informed consent is not required when the patient cannot be identified, directly or through identifiers linked to the patient.
Discussion
EBV, also known as human herpesvirus 4, is a member of the herpesvirus family and is associated with infectious mononucleosis. The Center for Disease Control estimates that 90% of adults between the ages of 35 years and 40 years have been infected; however, only a few patients have presented with ocular disease. Mitchell et alCitation1 found that the presence of detectable HSV-1, EBV, and human herpesvirus 6 in vitreous biopsy was not associated with clinical disease, so EBV may be detected in the retina without the virus being the cause of the retinal infection.
Previously described cases of “ocular EBV infection”, such as uveitis, vitritis, and optic disk vasculitis,Citation2–Citation5 are not typical for viral retinitis. One study reported EBV-related typical acute retinal necrosis (ARN) and EBV detected by vitreous PCR and molecular pathology within retinal cells that were confirmed as the sole causative agent.Citation6 In our case, EBV-associated retinal vitritis and vasculitis was proved by PCR from vitreous biopsy and also serological testing. The clinical signs were vitritis and retinal ischemia in the area of occlusive vasculitis, which was shown by fluorescein angiography. Despite the usual unilateral onset such as ARN, left eye was followed up regularly.Citation7
Several antiviral drugs have been used to inhibit EBV replication; however, none of them are licensed for the treatment of EBV in the clinic.Citation8 Andersson et al found a significant reduction in spontaneous outgrowth of in vivo EBV-infected B-lymphocytes after acyclovir therapy. Meanwhile, some studies suggest that acyclovir, which effectively inhibits in vitro EBV production,Citation9–Citation11,Citation15 was the first-line regimen. Rafailidis et alCitation12 reviewed the usage of antivirals for severe EBV infection studies from PubMed and Scopus from 1982 to 2009 and found that acyclovir monotherapy was the most commonly prescribed antiviral regimen.
Concerning intraocular treatment, one report has shown the intravitreal acyclovir concentration of 17.9 mM after given intravenously at 13 mg/kg three times daily,Citation13,Citation14 while acyclovir has an ED50 (mean effective dose) of only 0.3 mM against EBV replication in vitro.Citation15 Accordingly, some studies showed good results of treating EBV-associated ocular involvement,Citation16 and ARN,Citation17 with systemic acyclovir therapy.
Intraocular EBV infection can be found in immunocompetent patients, where there are no systemic diseases. We suggest that early vitrectomy and fluorescein angiography should be performed when occluded vascular is observed, and antiviral drugs should be considered. Corticosteroids are controversial for severe inflammation. This is the first case report of EBV vasculitis in an immunocompetent patient, which was identified by fluorescein angiography and proved by vitreous PCR; then the patient was successfully treated with early vitrectomy, focal retinal photocoagulation, and acyclovir therapy.
Disclosure
The author reports no conflicts of interest in this work.
References
- MitchellSMFoxJDTedderRSGazzardBGLightmanSVitreous fluid sampling and viral genome detection for the diagnosis of viral retinitis in patients with AIDSJ Med Virol19944343363407964643
- KimSJBarañanoDEGrossniklausHEMartinDFEpstein–Barr infection of the retina: case report and review of the literatureRetin Cases Brief Rep2011511525389670
- SugitaSShimizuNWatanabeKUse of multiplex PCR and real-time PCR to detect human herpes virus genome in ocular fluids of patients with uveitisBr J Ophthalmol200892792893218408082
- YamamotoMOhgaSOhnishiYInomataHOptic disk vasculitis associated with chronic active Epstein–Barr virus infectionOphthalmologica2002216322122512065861
- MatobaAYOcular disease associated with Epstein–Barr virus infectionSurv Ophthalmol19903521451502173161
- SchaalSKaganAWangYChanCCKaplanHJAcute retinal necrosis associated with Epstein–Barr virus: immunohistopathologic confirmationJAMA Ophthalmol2014132788188224743882
- HollandGNExecutive Committee of the American Uveitis SocietyStandard diagnostic criteria for the acute retinal necrosis syndromeAm J Ophthalmol199411756636668172275
- GershburgEJosephSPagano: Epstein Barr virus infections: prospect for treatmentJ Antimicrob Chemother200556227728116006448
- AnderssonJSköldenbergBHenleWAcyclovir treatment in infectious mononucleosis: a clinical and virological studyInfection198715suppl 1S14S203036715
- AnderssonJSköldenbergBErnbergIBrittonSHenleWAnderssonUAcyclovir treatment in primary Epstein–Barr virus infection. A double-blind placebo-controlled studyScand J Infect Dis Suppl1985471071153006226
- YaroAEpstein–Barr infection: current treatment optionsAnn Trop Med Public Health2013611013
- RafailidisPIMavrosMNKapaskelisAFalagasMEAntiviral treatment for severe EBV infections in apparently immunocompetent patientsJ Clin Virol201049315115720739216
- SchulmanJAPeymanGAFiscellaRGPulidoJSugarJParentally administered acyclovir for viral retinitis associated with AIDSArch Ophthalmol19841021217506508612
- PatrickMKClaireYHSusanLAntiviral selection in the management of acute retinal necrosisClin Ophthalmol20104112020169044
- PaganoJSSixbeyJWLinJCAcyclovir and Epstein–Barr virus infectionJ Antimicrob Chemother198312suppl B1131216313591
- WongKWD’AmicoDJHedgesTR3rdSoongHKSchooleyRTKenyonKROcular involvement associated with chronic Epstein–Barr virus diseaseArch Ophthalmol198710567887923034222
- RobertoGMiquelHJoseJGInmaculadaSEnriqueEMariaDPEpstein–Barr virus and acute retinal necrosis in a 5-year-old immunocompetent childClin Ophthalmol20082245145519668736