Abstract
We report a case of a Caucasian female who developed active polypoidal choroidal vasculopathy (PCV) at the edge of a stable choroidal nevus and was successfully treated with verteporfin photodynamic therapy. No active polyp was detectable on indocyanine green angiography 2 years after treatment, and good vision was maintained. Indocyanine green angiography is a useful investigation to diagnose PCV and may be underutilized. Unlike treatment of choroidal neovascularization secondary to choroidal nevus, management of PCV secondary to nevus may not require intravitreal anti-vascular endothelial growth factor therapy. Photodynamic monotherapy may be an effective treatment of secondary PCV.
Introduction
Choroidal nevus is the most common adult intraocular tumor of the ocular fundus. It is a benign melanocytic lesion typically <2 mm in thickness, with an annual malignant transformation rate of one in 8,845.Citation1 Polypoidal choroidal vasculopathy (PCV) is a recurrent and relapsing chorioretinopathy characterized by grape-like subretinal vascular lesions associated with retinal pigment epithelium detachments (PEDs).Citation2 Indocyanine green angiography (ICGA) is the gold standard for PCV diagnosis. However, patients with PCV are often mistaken for patients with exudative age-related macular degeneration and typical choroidal neovascularization (CNV). PCV associated with choroidal nevus is a combination rarely seen in clinical practice. We previously reported the first case in the literature of a stable PCV associated with nevus, which was managed conservatively.Citation3 In this article, we performed a retrospective chart review following the written informed patient consent of a 78-year-old Caucasian female, who had active, symptomatic PCV secondary to nevus, and was successfully treated with photodynamic therapy (PDT). The patient also gave written informed consent to publish this case report.
Case report
This case involved a 78-year-old Caucasian female with a stable left-eye superotemporal extrafoveal pigmented nevus for 20 years. Her left eye had a sudden onset of paracentral scotoma for 1 week. Best-corrected visual acuity was 6/6 (0.0 logMAR) in each eye. Funduscopy showed a pigmented lesion measuring 4.8×3.2 mm in basal dimensions with overlying clumped soft drusen at the posterior pole along the 2 o’clock meridian (), corresponding to a nevus at 2.0 disc diameters from fovea. A discreet orange nodule adjacent to the pigmented lesion on the nasal aspect and associated subretinal fluid (SRF) was noted. Scattered large soft drusen was observed bilaterally.
Figure 1 PCV secondary to a stable choroidal nevus.
Abbreviations: PCV, polypoidal choroidal vasculopathy; OCT, optical coherence tomography; SRF, subretinal fluid; PED, pigment epithelium detachment; PDT, photodynamic therapy.
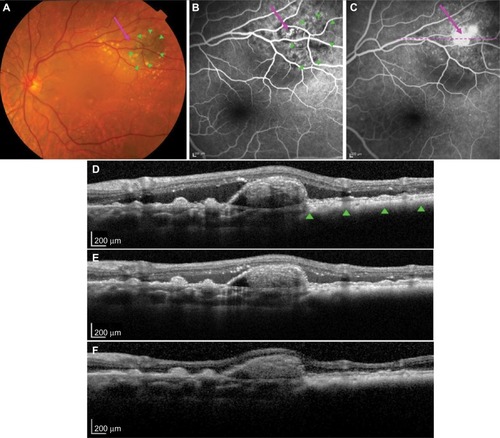
Optical coherence tomography (Heidelberg Engineering, Heidelberg, Germany) showed typical features of a flat nevus with PED associated with an underlying discreet polyp-like lesion at the nasal edge of the nevus and extensive SRF (). Fluorescein angiography () and ICGA (Heidelberg Engineering; ) demonstrated early filling of a grape-like structure suggestive of PCV with leakage in the late phase. Pulsation of the PCV lesion was noticed on ICGA. ICGA also revealed an associated small branching vascular network (BVN) in both early and late phases ().
Figure 2 Regression of polyp after PDT laser.
Abbreviations: ICGA, indocyanine green angiography; PDT, photodynamic therapy; BVN, branching vascular network.
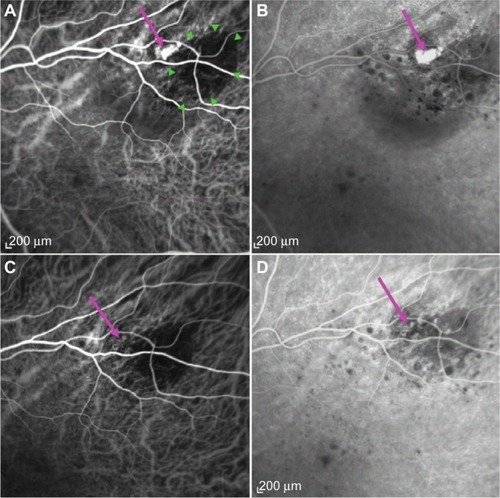
A diagnosis of PCV adjacent to nevus was made. Due to the peripheral location of PCV and the presence of SRF threatening the fovea, PDT was performed after written informed consent was obtained. Intravenous verteporfin (6 mg/m2) was followed by standard-fluence PDT (50 J/cm2) at the center of the PCV with a spot size of 1,600 µm over 83 seconds from a 689 nm laser. Following treatment, the patient noted an improvement in her paracentral scotoma. SRF was reduced at 2 weeks (). At 2 months, SRF was absent; however, a persisting small PED was observed (). ICGA at 7 months showed an absence of leakage, with complete regression of the polypoidal lesion (). No active polyp could be detected on ICGA at 2 years, with a stable best-corrected visual acuity of 6/6 (0.0 logMAR).
Discussion
Studies have found that patients with choroidal nevus may develop typical CNV.Citation4–Citation7 However, PCV secondary to nevus has rarely been reported. We previously reported a case of a quiescent PCV arising from a stable choroidal nevus where the polyp was located above the nevus, however, between the retinal pigment epithelium (RPE) and Bruch’s membrane. This location of the PCV corresponded to a study by Uyama et al on ICGA findings of Japanese patients with PCV, in which they proposed that PCV may be a peculiar form of CNV beneath the RPE and above the Bruch’s membrane.Citation8 Similar to this, histopathological examinations revealed that vessels found at the margins of type-1 choroidal neovascular membranes tend to be matured and dilated, and therefore, PCV seemed to originate from longstanding type-1 (occult) CNV above Bruch’s membrane and is not a primary choroidal vascular disorder.Citation9 We postulated that a choroidal nevus may cause chronic inflammatory or degenerative changes overlying RPE, which may result in the growth of type-1 CNV and eventually lead to PCV lesions developing adjacent to the nevus.Citation3
In our case, optical coherence tomography and angiography revealed a discrete orange grape-like lesion below the PED with early filling and leakage in the late phase associated with a BVN and an SRF, which were more typical of active PCV. Some may argue the current case to represent malignant transformation of choroidal nevus, since SRF was observed with an orange structure, which could be interpreted as pigment associated with choroidal melanoma. However, the pigmented lesion was flat, and the orange structure was adjacent to, not overlying, the lesion. Moreover, our case responded well to PDT being applied at the center of the PCV, not over the pigmented lesion, and has remained stable for 2 years post-PDT. These features support our case to be PCV secondary to choroidal nevus rather than malignant transformation.
Various treatments including thermal laser therapy have been used for PCV.Citation10–Citation15 ICGA-guided PDT was found to be effective in treating CNV associated with choroidal nevus; however, variable outcomes have been reported.Citation11 PDT is also one of the most effective treatments for PCV, resulting in acuity improvement, leakage reduction, and complete PCV regression.Citation13,Citation14 However, a high recurrence rate and minimal BVN regression have been documented, with possible complications such as subretinal hemorrhage and RPE tears.Citation16 The favorable outcome of PDT in our case was most likely due to the extrafoveal location of PCV and the patient’s good initial acuity.
In PCV patients, vascular endothelial growth factor (VEGF) was found to be elevated in RPE and vascular endothelial cells.Citation17 Intravitreal anti-VEGF injections for PCV have been shown to be effective in reducing SRF and improving vision; however, vascular abnormalities have often persisted.Citation12,Citation15 A previous study reported that combination treatment with PDT and anti-VEGF resulted in better acuity outcomes and a lower risk of developing PDT complications than photodynamic monotherapy.Citation15 Lowering VEGF levels after PDT may be the key to preventing PCV recurrence and CNV development. However, Koh et al reported that in follow-up visits of up to 6 months, although PDT combined with ranibizumab was superior to ranibizumab monotherapy in achieving complete regression of PCV, no difference was found between the combination treatment and the photodynamic monotherapy.Citation12
In this report, we present the case of a Caucasian female who developed symptomatic, active PCV at the edge of a stable choroidal nevus, which was successfully treated with one session of PDT, resulting in improved symptoms and fluid resolution, and no further treatment was required. ICGA is invaluable for diagnosing PCV and differentiating PCV from typical CNV, as PCV is a highly variable disorder. PCV secondary to choroidal nevus may be underdiagnosed. Further studies are required to understand the mechanisms of pathogenesis and evaluate optimal treatment options.
Disclosure
The authors report no conflicts of interest in this work.
References
- SinghADKalyaniPTophamAEstimating the risk of malignant transformation of a choroidal nevusOphthalmology2005112101784178916154197
- YannuzziLASorensonJSpaideRFLipsonBIdiopathic polypoidal choroidal vasculopathy (IPCV)Retina1990101181693009
- WongJGLaiXJSarafianRYWongHSSmithJBPolypoidal choroidal vasculopathy secondary to a stable choroidal nevusRetin Cases Brief Rep201610322122426509999
- ShieldsCLMashayekhiAMaterinMAOptical coherence tomography of choroidal nevus in 120 patientsRetina200525324325215805899
- CallananDGLewisMLByrneSFGassJDChoroidal neovascularization associated with choroidal neviArch Ophthalmol199311167897947685589
- ZografosLMantelISchalenbourgASubretinal choroidal neovascularization associated with choroidal naevusEur J Ophthalmol200414212313115134109
- PapastefanouVPNogueiraVHayGChoroidal naevi complicated by choroidal neovascular membrane and outer retinal tabulationBr J Ophthalmol20139781014101923686326
- UyamaMMatsubaraTFukushimaIIdiopathic polypoidal choroidal vasculopathy in Japanese patientsArch Ophthalmol199911781035104210448746
- FreundKBDifferent types of neovascularization that occur in AMD. The diagnosis and management of polypoidal choroidal vasculopathy (PCV)Presented at: The 8th Annual Retinal Physician SymposiumMarch 28–31, 2012Miami Beach, FL
- YuzawaMMoriRHaruyamaMA study of laser photocoagulation for polypoidal choroidal vasculopathyJpn J Ophthalmol200347437938412842207
- ParodiMBBosciaFPiermarocchiSFerrariTMFurinoCSborgiaCVariable outcome of photodynamic therapy for choroidal neovascularization associated with choroidal nevusRetina200525443844215933589
- KohALeeWKChenLJEVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathyRetina20123281453146422426346
- OtaniASasaharaMYodoiYIndocyanine green angiography: guided photodynamic therapy for polypoidal choroidal vasculopathyAm J Ophthalmol2007144171417467649
- GomiFOhjiMSayanagiKOne-year outcomes of photodynamic therapy in age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patientsOphthalmology2008115114114617582498
- LaiTYChanWMLiuDTLukFOLamDSIntravitreal bevacizumab (Avastin) with or without photodynamic therapy for the treatment of polypoidal choroidal vasculopathyBr J Ophthalmol200892566166618356265
- KangHMKimYMKohHJFive-year follow-up results of photodynamic therapy for polypoidal choroidal vasculopathyAm J Ophthalmol2013155343844723218705
- MatsuokaMOgataNOtsujiTNishimuraTTakahashiKMatsumuraMExpression of pigment epithelium derived factor and vascular endothelial growth factor in choroidal neovascular membranes and polypoidal choroidal vasculopathyBr J Ophthalmol200488680981515148217
