Abstract
Purpose
The purpose of this article is to report a case of central toxic keratopathy in a patient post transepithelial photorefractive keratectomy (TransPRK), followed immediately by corneal collagen cross-linking.
Methods
This article describes the case of a 26-year-old male after bilateral aberration-free, TransPRK laser (Schwind Amaris 750S). The procedure was performed for compound myopic astigmatism in November 2015, followed immediately by accelerated corneal collagen cross-linking for early keratoconus.
Results
From day 3 post-op, tear film debris underneath both contact lenses with corneal haze and early, progressive central anterior stromal opacity formation only in the left eye were noted. At 2 weeks post-op, the left eye was noted to have a significant hyperopic shift with central corneal thinning in the anterior stroma. A central anterior stromal dense opacity had formed in the left eye with the surrounding superficial stromal haze. As of month 2, the opacity gradually started to improve in size and density. The hyperopic shift peaked at 2 months and continued to improve, largely due to epithelial compensation with a gradual recovery of stromal thickness.
Conclusion
The question remains as to what provokes the typical central corneal necrosis/thinning in central toxic keratopathy. We hypothesize that the space between the contact lens and the corneal surface post TransPRK is prone to a “pseudo-interface pathology” that could mimic diffuse lamellar keratitis-like pathology. Suboptimal lid hygiene, resulting in tear film combinations of bacteria, inflammatory cells, matrix metalloproteinases and other proteolytic enzymes, contributes to the degradation of vulnerable, exposed collagen stromal tissue post TransPRK or any surface corneal ablation. Refractive surgeons should maintain a healthy lid margin and tear film, especially in contact lens wearers, to prevent potential complications in refractive surgery procedures.
Introduction
Refractive surgeries are currently performed routinely all over the world. In the vast majority of cases, there are insignificant complications. However, there are rarely cases in which the postoperative complications can be quite severe. One of the most dreadful yet less understood postoperative complications is known as central toxic keratopathy (CTK).
CTK is a rare, self-limited, noninflammatory, post-refractive laser condition that presents with central corneal opacification and a significant hyperopic shift.Citation1–Citation4 The cause of CTK is unknown, although it has been hypothesized that photoactivation of some substance, possibly povidone-iodine, by the laser induces keratocyte apoptosis and stromal necrosis.Citation5 The inciting factor, however, is not the microkeratome itself as this condition could occur after photorefractive keratectomy (PRK) as well as after femtosecond laser-created flaps.Citation5 The hypothesis that this reaction could be an intrinsic patient response to laser ablation is also unlikely since it does not recur after enhancements.Citation5
The purpose of this article is to report a case of CTK post transepithelial photorefractive keratectomy (TransPRK) with accelerated corneal collagen cross-linking (CCL). To date, CTK has not been reported in a refractive laser-ablated eye post TransPRK with CCL. This case may also help to exclude some previously hypothesized etiologies in CTK, e.g., equipment sterilization method, iodine usage and glove powder.
Written informed consent was obtained from the patient for this case report, as well as all the images herein, to be published.
Case report
A 26-year-old male with myopic astigmatism presented for laser refractive surgery. The patient was a contact lens wearer up until 2 weeks preoperatively. The patient was noted to have blepharitis preoperatively with some debris noted within the tear film. There was no other preoperative corneal pathology noted.
The anterior segment was otherwise normal with a normal fundoscopy in both eyes.
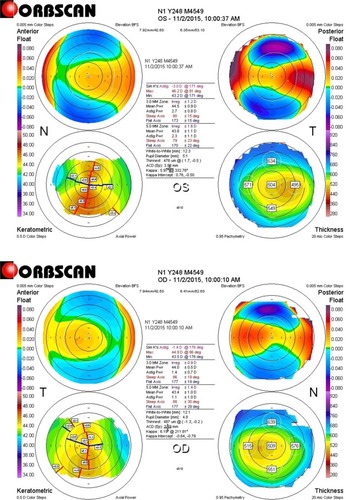
The right eye refraction was −2.50/−0.75 × 175, with the best spherical corrected visual acuity (BSCVA) 1.0 and uncorrected distance visual acuity (UDVA) 0.1 (visual acuity [VA] in a decimal format). The steepest keratometry value was 45.7 and the pachymetry value was 470 μm. The left eye (in which the CTK incident occurred) refraction was −2.00/2.75 × 170, with BSCVA 1.0 and UDVA 0.1. The steepest keratometry value was 46.2 and the pachymetry value was 469 μm ().
Figure 1 Pre-op Orbscan of the left and right eyes showing early ectasia.
The patient underwent single-step TransPRK treatment in both eyes using the Amaris 750S laser platform (SCHWIND eye-tech-solutions GmbH, Kleinostheim, Germany) aiming for plano refraction. The optical zone was 6.50 mm in both eyes with a transition zone of 1.14 mm and 0.82 mm in the right and left eyes, respectively, and the residual stromal thickness was 366 μm and 344 μm in the right and left eyes, respectively. Accelerated CCL protocol followed the laser procedure in both eyes. The accelerated CCL has been shown to penetrate less corneal stroma than the standard Dresden protocol. The authors, however, recommend that proven cornea thickness safety profiles are adhered to when performing CCL. The accelerated CCL protocol included saturation of the post-ablative exposed stroma with riboflavin (VibeX Rapid; Avedro Inc., Waltham, MA, USA) every minute for 4 minutes. The CCL was performed using the KXLI UV-A source (Avedro Inc.). On the right eye, the settings were 30 mW × 80 seconds = 2.4 J/cm2 and on the left eye, 30 mW × 120 seconds = 3.6 J/cm2. A higher CCL energy was used on the left eye due to the slightly higher K reading pre-op and because more corneal tissue ablation was planned in comparison to the right eye due to the higher astigmatism; hence, a thinner residual stromal bed was expected.
Iodine was not instilled preoperatively as the patient gave a history of iodine allergy. No mitomycin C was used intraoperatively. A bandage contact lens (Acuvue Oasys; Jacksonville, FL, USA) of base curve 8.8 mm and diameter 14 mm was inserted in both eyes at completion of CCL.
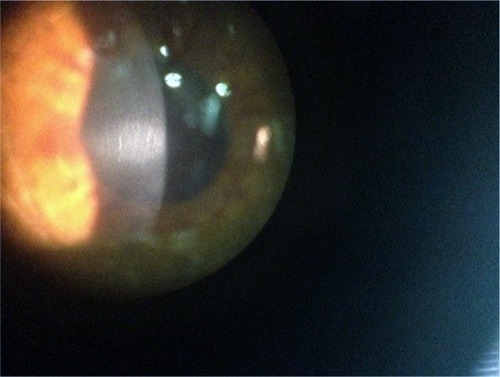
Postoperative treatment protocol was commenced on day 0, which included topical antibiotic (ofloxacin, qid), topical steroid (Maxidex, qid) and topical tear lubricant hourly (Thealoz Duo, Thea, France). On day 1, there was no corneal opacity or haze and there was a healing epithelial defect in both eyes. On day 3, debris was noted beneath the left contact lens within the tear film. Fine corneal haze with anterior stromal opacity had begun to appear in the left eye with a healing epithelial defect. A mild anterior chamber inflammatory reaction of 1+ cells was noted. On day 5, the contact lenses were removed as epithelialization had occurred in the right eye. The left eye still had a very small healing epithelial defect of <1 mm, the steroid was stopped in the left eye and ReGeneraTing Agent (RGTA) (glycosaminoglycan mimetic/matrix therapy agent to promote epithelial healing, Cacicol; Thea, Clermont-Ferrand, France) was added 4 times daily only in the left eye. At 1 week post-op, the right eye was healing well; however, some stromal haze (Grade 1 – Fantes scale) was present. The left eye epithelium had healed, but the eye had developed a diffuse anterior stromal haze with a dense central anterior stromal opacity.
At 2 weeks post-op, the left eye was noted to have a significant hyperopic shift (+5, 25 D) with central corneal thinning of the superficial stroma, and a corneal optical coherence tomography (OCT) was performed to confirm this. A central corneal opacity had increased in density at the central anterior stroma with surrounding diffuse superficial stromal haze (). Fine vertical striae were noted at the center of the opacity. Autologous serum q6h and Zovirax q6h were added to the current topical post-op treatment in the left eye.
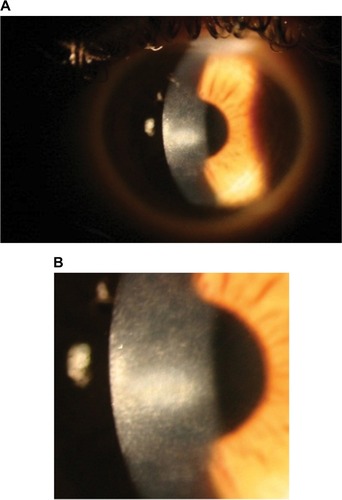
At 1 month post-op, the left central white opacity remained consistent in size and density. A large hyperopic shift had occurred (+9.25/−3.25 × 63). Vertical stromal striae at the center of the dense opacity were appreciated. Corneal thickness on Orbscan of the right eye was measured at 334 mm. The left eye was noticeably thinner at 257 mm. Pred Forte tds, autologous serum q6h and minocycline 50 mg bd by mouth (PO) were commenced. Ganciclovir gel bd was commenced in the left eye as there was uncertainty around the underlying pathology at this stage and a reactivated necrotic viral infection could not be excluded. At 9 weeks post-op, the refraction was +8.75/−3.00 × 116. Central opacity, infiltrate and haze showed mild improvement. Lubricants and minocycline 100 mg bd PO were continued. The left corneal opacity with vertical striae and fine iron line remained visible at 4 months post-op (). The opacity gradually continued to improve in size and density at 6 months post-op.
Figure 3 (A and B) Left eye 4 months post-op showing central corneal opacity with dense central vertical striae.
The vision and hyperopic refraction continued to slowly improve over 6 months post-op, and the central opacity still gradually continued to improve in size and density (). At 2 weeks post-op, the right eye had healed well with an uncorrected visual acuity (UCVA) of 1.0; however, fine anterior stromal reticular haze had formed centrally, which remained visible 6 months post-op (). A corneal OCT comparison of 2 weeks and 6 months post-op of the left eye showed how the epithelium compensated largely for the stromal thinning and hyperopic shift (). There was no anterior stromal thinning or epithelium thickening in the right eye on corneal OCT at 6 months post-op ().
Figure 4 Right eye 6 months post-op showing diffuse anterior stromal haze without the central dense opacity.
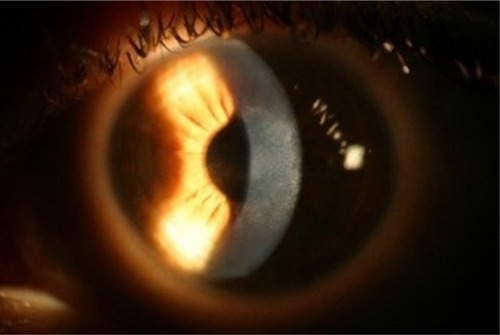
Figure 5 Early post-op (3 weeks) vs late post-op (6 months).
Abbreviations: OCT, optical coherence tomography; SSI, scan score index; OS, left eye.
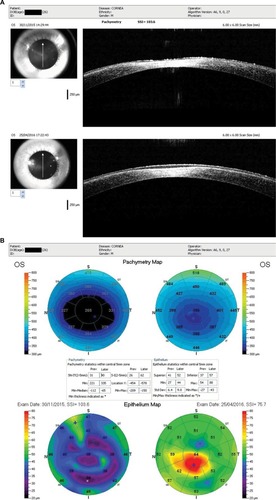
Figure 6 Post-op right eye (4 months) showing normalized postoperative stromal and epithelial thicknesses.
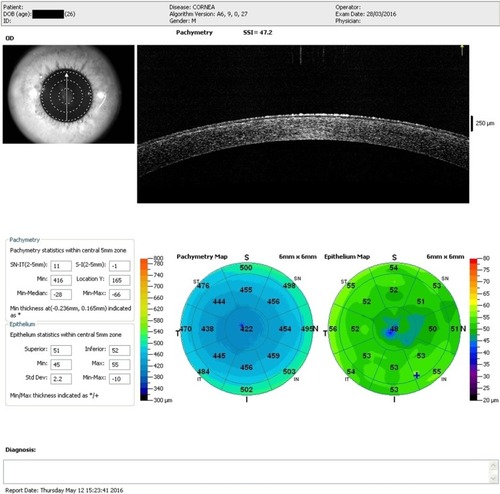
Table 1 Changes in refractive values and visual acuity in the left eye over 6 months post-op
Discussion
This may be the first documented report of a patient who developed CTK in a cornea post-TransPRK with accelerated CCL. Although the possibility of CCL as a cause for the CTK could not be entirely excluded, the authors deemed the CCL treatment an unlikely etiology based on the time of onset and depth of the pathology. CTK usually starts within 3–9 days after laser refractive surgery.Citation2,Citation3,Citation5–Citation7 The inciting procedure may be PRK, laser-assisted in situ keratomileusis (LASIK) or intra-LASIK. The opacity clears gradually, usually within 12–18 months.Citation5 It is rare for the opacity to persist beyond 18 months.Citation5 Recurrence of CTK after enhancement surgeries has not been observed.Citation5
The etiology of CTK is still under contention; however, some of the proposed etiologies may perhaps be excluded from this report. No iodine was administered in this case preoperatively as the patient had given a history of iodine allergy. Powder-free gloves were used, and other than the speculum, no sterilized instrumentation touched the eye intraoperatively. Cases of deep stromal scarring post-CCL have been reported, although these cases tend to present much later postoperatively (~3 months) and the scarring is deep stromal, unlike in this case.Citation8 The authors could not find any literature or case reports of CCL resulting in corneal pathology of this similar nature.
Tu and AslanidesCitation9 reported on 3 patients with bilateral CTK post LASIK where 2 of the 3 patients received early surgical intervention in the form of flap lift and irrigation. In 1 eye, an arrested CTK resulted from the surgical intervention. The eyes with initial hyperopic/astigmatic errors had gradual improvement in these errors. Imaging confirmed early stromal loss posterior to the flap with the stroma regaining some thickness over the following months. The authors concluded that early surgical intervention in the form of flap lift and irrigation could at least arrest part of the process of progressive corneal thinning/necrosis.Citation2,Citation3,Citation9,Citation10
This successful intervention of early flap lift and irrigation may contribute to the hypothesis that an overload of bacteria, proteolytic enzymes and/or inflammatory cells within the tear film, with trapping of the cellular components underneath the postoperative contact lens, may contribute to the formation of CTK and possibly even diffuse lamellar keratitis (DLK)-like pathology. There has been some controversy as to whether CTK may be considered an advanced form of DLK. DLK is generally accepted as an interface pathology occurring post LASIK flap creation.Citation7 We would like to hypothesize that perhaps the space between the contact lens and corneal surface post TransPRK could be prone to a pseudo-interface pathology that could mimic DLK-like pathology.
Until the pathology of CTK is better understood, the optimal intervention options for this dreaded condition may remain unclear. Perhaps microbiological/cytology studies of early CTK corneas and/or tear film may help to provide some clarity on the subject. Interestingly, in a study by Larkin and Leeming,Citation11 contact lens wearers were found to have a significantly higher number of commensal bacterial species at the lid margin and within the tear film than controls. Significant quantitative changes were identified on the lid margin and tear film, with particularly high bacterial counts in some lens wearers.Citation12–Citation14 Quantitative/osmolarity changes in the tear film are thought to be secondary to changes at the lid margin, for which no explanation is apparent.Citation11 The authors conclude that suboptimal lid hygiene with high concentrations or osmolarity of tear film bacteria, inflammatory cells, matrix metalloproteinases and/or other proteolytic enzymes contributes to the degradation of the vulnerable, exposed collagen stromal tissue, potentially resulting in stromal degradation and opacity formation.
Particular attention needs to be drawn to a healthy lid margin and tear film pre-ablation treatment, especially in contact lens wearers. Perhaps this may contribute to a decline of this dreaded postoperative dilemma for the refractive surgeon.
Acknowledgments
No funding was received for this work.
Disclosure
The authors report no conflicts of interest in this work.
References
- SonmezBMaloneyRKCentral toxic keratopathy: description of a syndrome in laser refractive surgeryAm J Ophthalmol2007143342042717222794
- HazinRDaoudYKhalifaYWhat is central toxic keratopathy syndrome if it is not diffuse lamellar keratitis grade IVMiddle East Afr J Ophthalmol2010171606220543938
- MoshirfarMMadsenMWolseyDCentral toxic keratopathy: description of a syndrome in laser refractive surgeryAm J Ophthalmol20071442332
- LyleWJinGCentral lamellar keratitisJ Cataract Refract Surg200127448749011392315
- MoshirfarMHazinRKhalifaYMCentral toxic keratopathyCurr Opin Ophthalmol201021427427920531192
- Marí CotinoJFSurianoMMDe La Cruz AguilóRIVila-ArteagaJCentral toxic keratopathy: a clinical case seriesBr J Ophthalmol201397670123536420
- ParoliniBMarconGPanozzoGACentral necrotic lamellar inflammation after laser in situ keratomileusisJ Refract Surg200117211011211310759
- GüellJVerdaguerPEliesDGrisOManeroFLate onset of a persistent, deep stromal scarring after PRK and corneal cross-linking in a patient with forme fruste keratoconusJ Refract Surg201430428628824702582
- TuKLAslanidesIMSurgical intervention in central toxic keratopathyEur J Ophthalmol201323133
- FraenkelGECohenPRSuttonGLLawlessMARogersCMCentral focal interface opacity after laser in situ keratomileusisJ Refract Surg19981455715769791825
- LarkinDLeemingJQuantitative alterations of the commensal eye bacteria in contact lens wearEye19915pt 170742060675
- McBrideMEvaluation of the microbial flora of the eye during wear of soft contact lensesAppl Environ Microbiol1979372233236434806
- SmolinGOkumotoMNozikRThe microbial flora in extended-wear soft contact lens wearersAm J Ophthalmol1979883 pt 2543547484683
- AllansmithMOstlerBButterworthMConcomitance of bacteria in various areas of the eyeArch Ophthalmol196982137424894514