Abstract
The aim of this study was to explore the effect of apatinib in the treatment of metastatic renal cell carcinoma (mRCC) and related adverse events. A case of mRCC was reported which recurred after surgery and roferon treatment. The patient was treated with apatinib at a dose of 500 mg orally, twice daily, 28 days/cycle. The metastatic lesions improved based on computed tomography after apatinib administration in the fourth and eighth month. The progression-free survival of the patient had increased almost to 8 months. The patient showed a good tolerance with only an adverse effect of mild-to-moderate hand-foot syndrome which was managed well. Apatinib is an option for mRCC after previous treatment. However, more and larger trials are still needed.
Introduction
The incidence of renal cell carcinoma (RCC) has continuously increased worldwide, and almost one-third of patients with RCC have been confirmed as having metastatic disease at the initial diagnosis. Generally, the prognosis of metastatic RCC (mRCC) is poor even when surgery is performed due to insensitivity to regular chemotherapy and radiotherapy agents. Pathologically, clear cell carcinoma is the most common type of RCC. Recently, in a study focusing on a Chinese population, it was found that an interleukin-6 gene polymorphism may be predictive for susceptibility and prognosis of RCC.Citation1 Some studies have shown the involvement of different pathways during the development of RCC. The RhoA–ROCK pathway can be inhibited by PARG1, a new RCC antigen, which leads to mRCC and further poor survival.Citation2 Meanwhile, the Wnt/β-catenin signaling pathway also plays an important role in the progression of RCC.Citation3 Some special factors have been identified as prognostic parameters for RCC including neutrophil to lymphocyte ratio and tumoral CCR7.Citation4,Citation5
Currently, targeted therapy is recommended to treat locally advanced RCC and mRCC before surgery due to safety, feasibility and fewer complications.Citation6 Clinically, apatinib, an oral antiangiogenic agent, is widely used during the treatment of different tumors. In particular, apatinib is the most common targeted agent aiming to improve progression-free survival (PFS) and overall survival (OS) of patients with advanced gastric cancer (AGC) and metastatic gastric cancer (mGC) after failure of chemotherapy.Citation7 Nevertheless, few publications related to the effect and safety of apatinib in treating mRCC have been found. Herein, a case of mRCC managed with apatinib is presented.
Case presentation
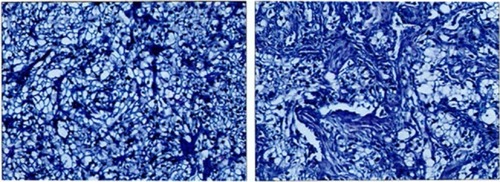
A 62-year-old male patient was admitted to our hospital on May 10, 2016, complaining of a fist-like mass on his back complicated with hemoptysis for 10 days. The patient had been diagnosed with RCC and treated by nephrectomy of the left kidney 15 years previously (May 26, 1999). The pathology test after surgery showed clear cell carcinoma of the left kidney with dimensions of 6 cm × 6 cm × 4 cm, and no metastasis was found in a total of seven tested lymph nodes or in the resection margin of the ureter. The patient had been treated with 9 MIU/week roferon for 3 consecutive months after discharge. During the follow-up, no discomfort had been reported and physical examination of the patient revealed that normal findings were maintained. However, the patient was admitted again 2 years ago (August 21, 2014) complaining of hemoptysis which had persisted for 1 week. A chest computed tomography (CT) scan showed that there was an isolated mass in the lower lobe of the right lung. Positron emission tomography (PET) showed that a peripheral mass was located in the lower lobe of the right lung without any other metastasis, and the susceptibility to cancer was not excluded in combination with a smoking history of more than 40 years. Video-assisted pulmonary wedge resection was performed on August 26, 2014. However, the pathology test indicated lung metastasis of renal clear cell carcinoma (lower lobe of right lung) with dimensions of 4 cm × 4 cm × 2 cm. One metastatic lymph node was identified among six tested subcarinal nodes, and no metastasis was found in a total of 14 mediastinal nodes. Immunolabeling revealed the tumor to be CD10+, CK8+, Vim+, EMA+, CK+, CD34−, inhibin−, and Ki-67+ (10%), TTF-1−, Napsin-A− and p63-(). Written informed consent for the publication of the patient’s clinical details and accompanying images was obtained from the patient.
Figure 1 Pathology test indicated lung metastasis of renal clear cell carcinoma.
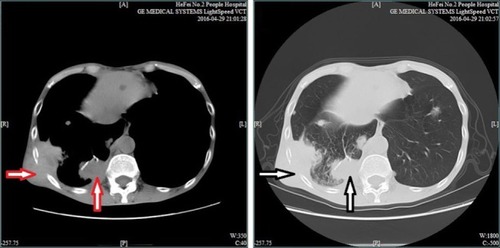
No special treatment was given after discharge. Before the third admission, the patient’s condition was stable without any complaints during the 2-year follow-up. A CT scan (April 29, 2016) showed the presence of multiple intrapulmonary metastatic lesions located mainly within the middle and lower lobes of the right lung and a larger lesion with dimensions of 3.6 cm × 6.3 cm located within the basal segment of the lower lobe extending partially outside the thoracic cavity. Osteolytic destruction was found in adjacent ribs. No swollen mediastinal lymph nodes were found (). Based on these findings together with past medical history, the patient was diagnosed with lung metastasis of renal clear cell carcinoma, TxN1M1, IV stage.
Figure 2 Chest CT scan indicates a large lesion with dimensions of 3.6 cm × 6.3 cm (red and white vertical arrows) located within the basal segment of the lower lobe and extending out of the thoracic cavity, partially complicated with osteolytic destruction (red and white horizontal arrows).
Abbreviation: CT, computed tomography.
An individual treatment strategy was prepared. Traditionally, sunitinib and sorafenib have been recommended as second- or third-line treatment for the treatment of mRCC; however, the patient refused these two agents due to poor financial support. Hence, apatinib (Hengrui Pharmaceutical Co., Ltd., Lian Yun Gang, Jiang Su, China) was used to manage the disease. After all contraindications were excluded, based on his individual condition observed in clinical practice, the patient took apatinib orally at a dose of 500 mg/day, starting on May 3, 2016 (28 days/cycle). A CT scan was performed every 4 months to evaluate the effect. Routine tests of blood and biochemical function as well as assessment of adverse events were performed every 2 weeks. Approximately 2 weeks later, the mass on the patient’s back was observably smaller and hemoptysis had cleared up; however, the patient also complained of pain in the hands and feet. During physical examination, his hands and feet were found to be red and a little swollen but without peeling or blistering. Mild hand-foot syndrome was considered and regular preventive treatment was recommended. Approximately 4 weeks later, the patient complained that the pain was not relieved as expected. Oral celecoxib 100 mg twice daily was given for the intensity of pain management. The patient reported dramatic pain relief following analgesic therapy. Regular routine test results were stable.
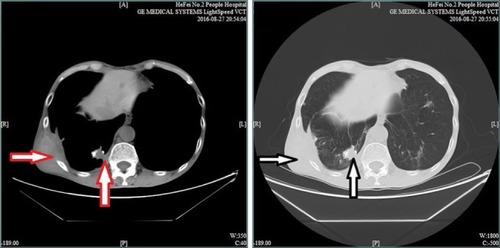
The patient was rechecked by chest CT scan 4 months later. The results (August 27, 2016) revealed multiple intra-pulmonary metastatic lesions located mainly within the middle and lower lobes of the right lung and a larger lesion with dimensions of 2.7 cm × 1.8 cm located within the basal segment of the lower lobe and partially extending out of the thoracic cavity. Worse osteolytic destruction was observed in adjacent ribs (). The effect of apatinib was evaluated as a partial response (PR). Therefore, apatinib was continued as planned and zoledronic acid was used for the inhibition of bone destruction. Results of regular routine tests including blood, urine and stool remained stable.
Figure 3 Chest CT scan indicates a larger lesion with dimensions of 2.7 cm × 1.8 cm (red and white vertical arrows) located within the basal segment of the lower lobe and extending out of the thoracic cavity, partially complicated with worse osteolytic destruction (red and white horizontal arrows).
Abbreviation: CT, computed tomography.
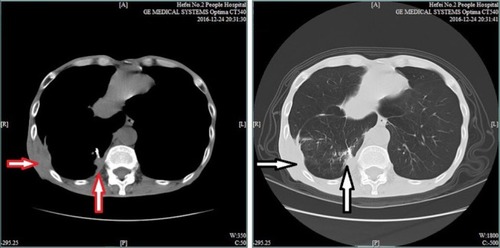
A third CT evaluation was performed on December 24, 2016. The chest CT scan showed the presence of multiple intrapulmonary metastatic lesions located mainly within the middle and lower lobes of the right lung and a larger lesion with dimensions of 2.7 cm × 1.9 cm located within the basal segment of the lower lobe and partially extending out of the thoracic cavity. Improved osteolytic destruction was observed in the adjacent ribs (). Meanwhile, abdominal and pelvic CT scans were also performed, which indicated that no metastases were found, similar to the results of previous PET. The evaluation was still PR. To date, the patient has achieved a PFS of almost 8 months. During the apatinib cycles, mild-to-moderate hand-foot syndrome has developed and has been treated with appropriate agents.
Figure 4 Chest CT scan indicates a larger lesion with dimensions of 2.7 cm × 1.8 cm (red and white vertical arrows) located within the basal segment of the lower lobe and partially extending out of the thoracic cavity, complicated with improved osteolytic destruction (red and white horizontal arrows).
Abbreviation: CT, computed tomography.
Discussion
Generally, the approved targeted agents for mRCC are sunitinib and sorafenib. Sunitinib is considered as a small molecule inhibitor of receptor TKI including platelet-derived growth factor receptor (PDGFR) and vascular endothelial growth factor receptor (VEGFR). Therefore, besides the inhibition of PDGFR and VEGFR, the mechanism of sorafenib also activates the inhibition of RAF/MEK/ERK signaling. In a study in a Latin-American population, sunitinib has been confirmed to be safe and effective even with worse prognosis. The median PFS (mPFS) and OS were 12.1 and 16.9 months, respectively, while neutropenia, thrombocytopenia and anemia were common adverse hematologic events.Citation7 Rovithi et alCitation8 indicated that the optimal treatment with sunitinib was based on a pulsatile, high-dose strategy, which led to an antitumor effect. Chang et alCitation9 suggested that among patients with RCC, the genotype of cyclooxygenase 2 (COX2) could be used as a novel marker for the diagnosis of RCC. In 14 patients with inoperable mRCC, neoadjuvant sunitinib therapy showed a satisfactory response rate although a small number are operable after treatment.Citation10 It has been indicated that among Chinese patients with mRCC, both sorafenib and sunitinib are effective agents. The median OS of patients treated with the two agents is similar (24.0 vs 24.0 months; P = 0.298), while the mPFS with sorafenib is higher compared with sunitinib (11.1 vs 10.0 months; P = 0.028). In addition, sorafenib is recommended to elderly patients aged over 65 years.Citation11 A study in Tsc 2± mice by Yang et alCitation12 indicated that everolimus used as monotherapy or combined with sorafenib is effective in controlling tumor size, based on the inhibitory mechanism of mTORC1 and the mitogen-activated protein kinase (MAPK) pathway. Nevertheless, there is also a difference between sorafenib and sunitinib. Ma et alCitation13 indicated that there is a correlation between these two agents and the expression levels of hypoxia-inducible factor 2, alpha subunit (HIF-2α), CD31, carbonic anhydrase IX (CAIX), vascular endothelial growth factor receptor 1 (VEGFR-1) and platelet-derived growth factor beta polypeptide (PDGFRB), and interestingly the opposite trend is also found for sorafenib and sunitinib. In a single-center retrospective study, a total of 169 patients receiving sorafenib (400 mg, twice daily, 4 weeks/cycle) and 165 patients receiving sunitinib (50 mg/day, 6 weeks/cycle) were enrolled. The PFS and OS of sorafenib and sunitinib were similar (9.0 vs 11.0 months, respectively; 28.0 vs 28.0 months, respectively). For vascular endothelial growth factor-TKI (VEGF-TKI)-naive patients with mRCC, the effect of sorafenib and sunitinib was also comparable (9.0 vs 11.0 months, respectively). However, sorafenib was found to be superior in subsets such as non-clear cell carcinoma, with Karnofsky Performance Status (KPS) < 90.Citation14 Bourlon et alCitation15 found that compared with sorafenib, the incidence of macrocytosis was significantly higher during sunitinib treatment. Moreover, organ atrophy has been demonstrated during treatment with either sorafenib or sunitinib. The atrophy of the spleen, thyroid gland and pancreas was significant as assessed by CT scan within 1 year of treatment.Citation16
Other targeted therapies for mRCC are potentially significant. For clear-cell mRCC, bevacizumab and pazopanib, as first-line treatment, can significantly improve PFS. On the other hand, temsirolimus is better for improving poor prognosis of clear cell and non-clear cell mRCC.Citation17
For mRCC, the pathways of VEGFR and mammalian target of rapamycin (mTOR) are considered to be the main mechanisms of carcinogenesis. It has been proposed that dysfunction of von Hippel–Lindau (VHL) is the key factor in dysregulation of VEGF signaling which leads to overexpression of VEGF protein and increased tumor angiogenesis.Citation18 Recently, it has been proposed that combination therapy with TKIs of VEGFR, such as sorafenib and sunitinib and the mTOR inhibitors, vatalanib and everolimus, is effective and tolerable for patients suffering from mRCC.Citation19 Terakawa et alCitation20 suggested that VEGFR-2 is the most significant factor in PFS of mRCC in a study involving a total of 40 patients with mRCC after radical nephrectomy. It has been shown in a registry-based analysis that anti-VEGFR agents are effective in improving the prognosis of mRCC.Citation21 Apatinib, as a small-molecule multi-targeted TKI of VEGFR-2, potentially interacts with CYP1A2, CYPAD4 and CYP3A1 of the cytochrome P450 family in vivo.Citation22 Apatinib, which inhibits VEGFR-2, may further reduce endothelial cell migration and proliferation as well as decrease tumor microvascular density.Citation23 Peng et alCitation24 have found that among patients with intrahepatic cholangiocarcinoma (ICC), apatinib plays an important role in inhibiting VEGF–VEGFR-2–PI3K–AKT signaling and inducing apoptosis of ICC cells.
It has been confirmed that VEGF plays a key role in the development of carcinoma and that VEGFRs function as regulators of this process.Citation25 Apatinib, a small-molecule antiangiogenic agent, shows an inhibitory effect on VEGFR-2 and a moderate inhibitory effect on c-Kit and c-Src TKIs.Citation26 Apatinib exerts its inhibitory effect by binding to VEGFR-2 and inhibiting phosphorylation of extracellular signal-regulated kinase (ERK).Citation27 Currently, apatinib is used as monotherapy or combination therapy with other chemotherapy agents against various solid tumors and shows better tolerance. The most common adverse events of apatinib are hypertension, proteinuria and hand-foot syndrome.
Apatinib has been assessed in a randomized Phase II trial focusing on AGC or mGC with high expression of VEGFR.Citation28 Placebo, apatinib 850 mg/day and apatinib 425 mg twice daily were the three study groups. The PFS and OS of the two apatinib groups were significant better compared with the placebo group; however, there was no significant difference between the two groups, which meant that apatinib improved PFS and OS for patients with AGC or mGC after failure of chemotherapy. Recently, Peng et alCitation24 demonstrated that the development of ICC relied on the interaction between VEGF and VEGFR-2. Consequently, targeting of the VEGF signaling pathway by apatinib may be recommended as treatment for ICC. Treatment of metastatic non-triple-negative breast cancer (TNBC) is challenging due to drug resistance. In a Phase IIb study, a total of 59 patients with TNBC received apatinib at a dose of 500 mg/day. The mean PFS and mean OS were 3.3 and 10.6 months, respectively, and the most common adverse events were thrombocytopenia, leukopenia, neutropenia and anemia.Citation29 Song et alCitation30 reported that mPFS and OS of patients with non-small cell lung cancer (NSCLC) treated with apatinib was 4.2 and 6 months, respectively, in a trial involving 42 patients with NSCLC who received apatinib at a dose of 500 mg/day.
In the case presented in this article, the patient refused sorafenib and sunitinib because of poor financial support. However, the patient also had a strong desire to continue treatment after failure of previous treatments including interferon-γ (IFN-γ). Based on individual condition and clinical effects as well as the benefits of apatinib, this drug was selected for the patient. We report that it is a reasonable and feasible therapy when given at a level of 500 mg orally twice per day. Mild-to-moderate hand-foot syndrome was observed but was well managed.
Conclusion
In this case, apatinib was used to treat mRCC and, so far, the effect is good. However, the effect of apatinib on different tumors is variable due to the different mechanisms involved. Hence, more and larger trials are needed in future to further explore the effects. Moreover, the indications concerning monotherapy and combination therapy are also identified during clinical practice.
Disclosure
The authors report no conflicts of interest in this work.
References
- LiuZWangZXiaoYLuYLuYAssociation between the interleukin-6 gene polymorphisms and renal cancer riskImmunol Lett2015164212512825766682
- MiyazakiJItoKFujitaTProgression of human renal cell carcinoma via inhibition of RhoA-ROCK axis by PARG1Transl Oncol201710214215228131798
- LiuZLiuXWLiuSALvJJFuQClinical significance of changes of expression of the Wnt/β-catenin signaling pathway in renal clear cell carcinomaEur Rev Med Pharmacol Sci201620234840484527981554
- BazziWMTinALSjobergDDBernsteinMRussoPThe prognostic utility of preoperative neutrophil-to-lymphocyte ratio in localized clear cell renal cell carcinomaCan J Urol20162318151815426892055
- XiaYLiuLXiongYPrognostic value of CC-chemokine receptor seven expression in patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitorBMC Cancer2017171708028114889
- BorregalesLDAdibiMThomasAZWoodCGKaramJAThe role of neoadjuvant therapy in the management of locally advanced renal cell carcinomaTher Adv Urol20168213014127034725
- BarriosCHHerchenhornDChacónMCabrera-GaleanaPSajbenPZhangKSafety and efficacy of sunitinib in patients from Latin America: subanalysis of an expanded access trial in metastatic renal cell carcinomaOnco Targets Ther20169235839584527713637
- RovithiMde HaasRRHoneywellRJAlternative scheduling of pulsatile, high dose sunitinib efficiently suppresses tumor growthJ Exp Clin Cancer Res201635113814927604186
- ChangWSLiaoCHMiaoCEThe role of functional polymorphisms of cyclooxygenase 2 in renal cell carcinomaAnticancer Res201434105481548625275044
- KimSHSeoSILeeHMA prospective multicenter trial of the efficacy and tolerability of neoadjuvant sunitinib for inoperable metastatic renal cell carcinomaJ Korean Med Sci201631121983198827822939
- ZhangHLShengXNLiXSSorafenib versus sunitinib as first-line treatment agents in Chinese patients with metastatic renal cell carcinoma: the largest multicenter retrospective analysis of survival and prognostic factorsBMC Cancer2017171162628056874
- YangJSamselPANarovKCombination of everolimus with sorafenib for solid renal tumors in Tsc2 ± mice is superior to everolimus aloneNeoplasia201719211212028092822
- MaXWangLLiHZPredictive immunohistochemical markers related to drug selection for patients treated with sunitinib or sorafenib for metastatic renal cell cancerSci Rep201663088627488093
- ShengXNChiZHCuiCLEfficacy and safety of sorafenib versus sunitinib as first-line treatment in patients with metastatic renal cell carcinoma: largest single-center retrospective analysisOncotarget20161972704427054
- BourlonMTGaoDXTrigeroSClinical significance of sunitinib-associated macrocytosis in metastatic renal cell carcinomaCancer Med20165123386339327758076
- TakahashiHNasuKMinamiMOrgan atrophy induced by sorafenib and sunitinib-quantitative computed tomography (CT) evaluation of the pancreas, thyroid gland and spleenPol J Radiol2016812255756527956943
- AfriansyahAHamidARMochtarCAUmbasRTargeted therapy for metastatic renal cell carcinomaActa Med Indones201648433534728143997
- RiniBIEscudierBTomczakPComparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trialLancet201137898071931193922056247
- RhondaLBPatrickHPatriciaACA phase Ib study of combined VEGFR and mTOR inhibition with vatalanib and everolimus in patients with advanced renal cell carcinomaClin Genitourin Cancer2014412241250
- TerakawaTMiyakeHKusudaYFujisawaMExpression level of vascular endothelial growth factor receptor-2 in radical nephrectomy specimens as a prognostic predictor in patients with metastatic renal cell carcinoma treated with sunitinibUrol Oncol201331449349821478036
- TomasBZbynekBAlexandrPOutcomes for patients with metastatic renal cell carcinoma achieving a complete response on targeted therapy: a registry-based analysisEur Urol201670346947526746623
- ZhouYFWangSHDingTEvaluation of the effect of apatinib (YN968D1) on cytochrome P450 enzymes with cocktail probe drugs in rats by UPLC-MS/MSJ Chromatogr B Analyt Technol Biomed Life Sci2014159736875
- LiJZhaoXMChenLSafety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignanciesBMC Cancer20101052920923544
- PengHZhangQLiJApatinib inhibits VEGF signaling and promotes apoptosis in intrahepatic cholangiocarcinomaOncotarget2016713172201722926967384
- ZhangHJApatinib for molecular targeted therapy in tumorDrug Des Devel Ther201591360756081
- TianSQuanHXieCYN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivoCancer Sci201110271374138021443688
- ScottAJMessersmithWAJimenoAApatinib: a promising oral antiangiogenic agent in the treatment of multiple solid tumorsDrugs Today (Barc)201551422323926020064
- LiJQinSXuJApatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trialJ Clin Oncol201331263219322523918952
- HuXZhangJXuBMulticenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancerInt J Cancer201413581961196924604288
- SongZBYuXMLouGYSalvage treatment with apatinib for advanced non-small-cell lung cancerOnco Targets Ther2017231018211825
