Abstract
Background
Mixed connective tissue disease (MCTD; also known as Sharp’s syndrome) is a rare autoimmune inflammatory disorder characterized by high titer of U1 ribonucleoprotein (U1RNP) antibody and clinical and serological overlap of systemic lupus erythematosus, systemic sclerosis, and polymyositis. The diagnosis is based on clinical and serological factors in criteria such as Alarcon-Segovia, Khan, Kusakawa, and Sharps. Cardiac disease can be a complication of connective tissue disease (CTD). There are few reports in Africa.
Aims
To present MCTD as underlying cause of heart failure with reduced ejection fraction and highlight challenges of investigations and treatment.
Objectives
To highlight the first case in our center and discuss the cardiac, respiratory, and rheumatologic management.
Patient and methods
We present a 52-year-old woman with 3 weeks history of productive cough with whitish sputum, severe dyspnea, orthopnea, paroxysmal nocturnal dyspnea, right sided abdominal pain, leg swellings, a one year history of recurrent fever, Raynaud’s phenomenon, small joint swellings and deformities with pain in both hands.
Results
On examination there was microstomia, tethered forehead and lower eyelid skin, tender swelling of the interphalangeal joints and arthritis mutilans. Laboratory findings showed estimated glomerular filtration rate <60 mL/kg/min/1.73 m2, U1RNP antibody levels were eight times upper limit of normal, elevated rheumatoid factor, speckled antinuclear antibody pattern, negative anticentromere antibody, anti Scl-70 and anticyclic citrullinated peptide. Chest X-ray/CT revealed pulmonary fibrosis. Echocardiography findings showed reduced ejection fraction of 40%, elevated pulmonary arterial pressure at rest of 60.16 mmHg. The patient showed improvement on antifailure drugs, but prednisolone was stopped for sudden reversal of previously controlled stage 2 hypertension (HTN), and the patient was discharged in a stable condition. Difficulties ensued in obtaining prompt definite results due to the unavailability of serologic tests in the hospital, and the tests were done outside the state and country.
Conclusion
Identifying MCTD is critical, especially in patients requiring steroids that may worsen systemic HTN and heart failure. There is a need to have definitive investigative facilities for such patients in hospitals.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Mixed connective tissue disease (MCTD) is a rare systemic autoimmune rheumatic disease mainly diagnosed by clinical and serologic criteria.Citation1–Citation3 There are some common underlying causes of heart failure in our environment, and MCTD is a rare cause of heart failure with unknown reports in Africa.Citation4 We hereby present a case of MCTD complicated by heart failure with the associated management challenges in our resource-limited economic environment. The way we overcame the challenges may be useful for others in similar circumstances in addition to adding information to the local and international databases about MCTD. The prevalence of MCTD in Africa is 0.11%.Citation3
Case
A 52-year-old woman presented with cough productive of whitish sputum, bilateral leg swelling, facial puffiness, right-sided abdominal pain, and severe difficulty in breathing for over 3 weeks prior to presentation. She is a computer operator and had no prior exposure to occupational lung disease hazards.
There were high-grade intermittent fevers, chills, rigors, headache, and vomiting on several occasions about a week before presentation.
Difficulty in breathing was insidious at onset, initially on exertion, and progressed to become worse at rest, with paroxysmal nocturnal dyspnea, orthopnea, palpitations, easy fatigability, and early satiety.
There is a year history of recurrent shoulder, fingers, knee, joint, and muscle pains with swellings, Raynaud’s phenomenon, deformities, and stiffness of the hands. She has been postmenopausal for 7 years and not on hormone replacement therapy. Medical history was unremarkable, and review of systems was essentially normal.
On examination
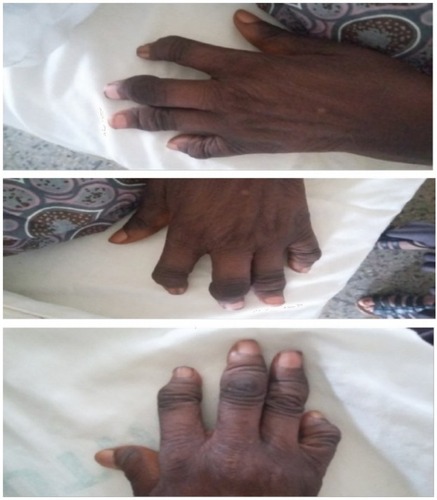
The blood pressure was 180/110 mmHg in the sitting position and 188/115 mmHg in standing position (stage 2 hypertension [HTN]), pulse rate was 120 beats/minute, respiratory rate was 28 breaths/minute, and temperature was 38°C. The patient suffered from microstomia, tethered forehead skin, lower eyelids, tender swelling of the interphalangeal (IP) joints, arthritis mutilans (la main en lorgnette; ), and bilateral pitting pedal edema up to the midlegs. There were bronchial breath sounds, coarse crepitations in left lower lung zones, bibasal fine crepitations, thickened arterial wall, locomotor brachialis, raised jugular venous pressure of 10 cm H2O, hyperdynamic precordium, apex beat in the sixth left intercostal space anterior axillary line (LICS AAL) heaving, left parasternal heave, and heart sounds (HS) – 1, 2, 3, and P2>A2. There was a tricuspid regurgitation murmur (pansystolic), enlarged liver 6 cm below the right costal margin, with a span of 14cm, tender, firm and a smooth surface.
Laboratory data revealed a hemoglobin level of 10.0 g/dL, hematocrit of 30%, and white blood cell count of 4,300/mm3 with 72% segmented neutrophils and 28% lymphocytes. Film appearance showed anisocytosis, macrocytosis, microcytosis and was positive for Plasmodium falciparum (malaria parasite). Estimated glomerular filtration rate was <60 mL/kg/min/1.73 m2 and rheumatologic tests are shown in . Chest X-ray revealed features of pulmonary fibrosis secondary to connective tissue disease (CTD), with features of pulmonary arterial hypertension (PAH) as shown in , but no pleural effusions. In , chest CT showed basal predominance, mosaic pattern, and thickening of intralobular septae. Hand X-rays (not shown) revealed global periarticular osteopenia and soft tissue swelling over the IP joints, complete resorption of the right second and eighth middle phalanges, left third and fifth phalanges, and crowding of the carpal bones. Liver function tests (LFTs) were within normal limits. Electrocardiography showed a rate of 94 beats/minute, left axis deviation, right atrial enlargement, normal ventricles and T wave inversions >2 mm in multiple leads. Echocardiography revealed a hypokinetic interventricular septum, mild aortic, mitral and tricuspid regurgitation, grade 1 diastolic dysfunction, left ventricular systolic dysfunction, an ejection fraction of 40%, no pericardial effusion and pulmonary arterial pressure of 60.1 mmHg.
Figure 2 Posteroanterior view of chest X-ray showing reticular interstitial opacities with coalesced cystic lesions giving honeycomb appearance in both lung fields, decreased left lung field, and prominent pulmonary trunk.
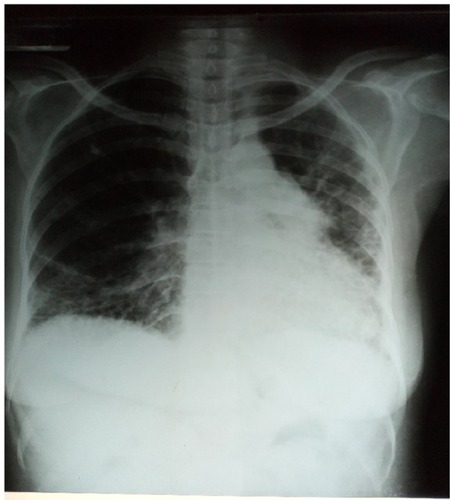
Figure 3 (A) Axial chest CT of the lung window showing reticular opacities, honeycombing, thickening of septae, and ground glass attenuation. (B) Coronal reformatted chest CT of the lung window showing honeycombing with basal predominance worse on the left and mosaic pattern, loss of left lung volume, and ground glass attenuation in the right lower lobe.
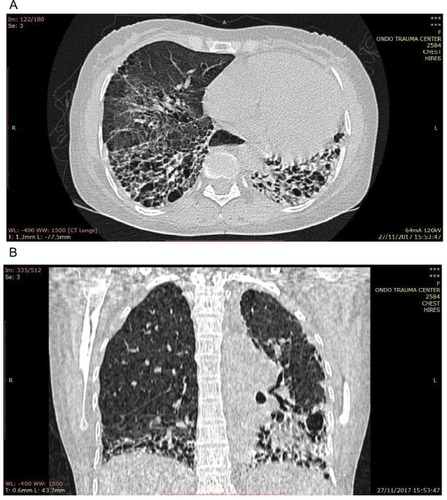
Table 1 Laboratory results and reference values
Treatment
On admission the patient was treated immediately for malaria with an adult dose for patients 35 kg or greater which is artemether+lumefantrine 80/480 mg one tab orally two times daily for 3 days. She was commenced on both oral and intravenous antifailure drugs and antibiotics namely, aldactone 25 mg, lisinopril 5 mg, lasix 60 mg, ramipril 10 mg, digoxin 0.125 mg, warfarin 2.5 mg (goal international normalized ratio, 2–3), clexane 40 IU, augmentin 600 mg intravenous q12h, clarithromycin 500 mg IV q12h, with daily weighing and monitoring of fluid input and output.
Treatment with 20 mg prednisolone orally once daily was started on the 18th day of admission, and joint pains, stiffness, and myalgia gradually improved. Two days after commencement of prednisolone, previously controlled blood pressure from 180/110 mmHg to 130/80 mmHg, increased back to 186/100 mmHg with clinical deteriorations evidenced by worsening dyspnea at rest, orthopnea, and palpitations. Prednisolone was discontinued on 22nd day of admission with resultant reduction in blood pressure to 130/70 mmHg and improvement in clinical status. A provisional diagnosis of acute heart failure precipitated by CTD was made but could not be confirmed due to initial lack of serological results. She was discharged home in a stable condition 25 days after admission and was asked to report after 2 weeks to both Cardiology and Rheumatology clinics for follow-up. Serological tests were eventually done outside the state and country because facilities for the tests were not available within the hospital.
Discussion
Even though MCTD has generally a good prognosis, significant cardiac involvement is rare.Citation5 Rheumatoid arthritis, systemic lupus erythematosus (SLE), inflammatory myopathies, systemic sclerosis, and sarcoidosis are among CTDs with severe cardiovascular association; this is due to numerous causative features such as micro/macrovascular disease, myopericarditis, myocardial fibrosis, coronary artery disease, pulmonary hypertension (PH), and finally cardiac failure.Citation6,Citation15 Concentric intimal proliferation and plexogenic pulmonary arteriopathy were described at autopsy as evidence for PH in young females who were diagnosed with MCTD.Citation7 Briasoulis et alCitation8 reported a young woman with new-onset heart failure with features suggesting cardiac tamponade and a related diagnosis of progressive MCTD associated with severe hypothyroidism, and despite prompt recognition and rapid management, she worsened and died within 4 weeks. Although MCTD has been known to be widely associated with PH, the pathogenesis of other cardiovascular manifestations such as left heart failure that could lead to right heart failure is not well understood. Kawano-Dourado et alCitation17 in a study of 39 MCTD patients found that functional and radiologic alterations suggestive of interstitial lung disease in long-term MCTD patients were prevalent. Caminati et alCitation18 mentioned that PH is a well-known outcome of ILDs associated with CTDs.
Undiagnosed HTN overtime, in the setting of an unrecognized MCTD, may lead to left heart failure and, with the concomitant PH, culminated in biventricular failure. Ungprasert et alCitation19 reported that cardiac involvement was common among MCTD patients and was one of the leading causes of mortality. Similarly, Schwagten et alCitation9 described combined systolic and diastolic heart failures as the first presentation of MCTD in a middle-aged female, and Gunnarsson et alCitation16 in a nationwide multicenter cohort of 147 adult MCTD patients found that the prevalence of PH is much lower than expected from previous studies but confirm the seriousness of the disease complication. On the other hand, pulmonary manifestations have been drawing attention as they are found in more than 50% of the patients with MCTD.Citation10 However, PAH and Raynaud’s phenomenon of the small vessels of the heart, similar to that observed in the fingers, are some of the most important cardiac lesions in MCTDs.Citation11 Similarly, Hajas et alCitation12 in a prospective study of 280 patients with MCTD found that overall, PAH remained the leading cause of death in patients with MCTD and that the prevalence of cardiovascular morbidity and mortality, malignancy, and thrombotic events increased during the disease course of MCTD.
Whitlow et alCitation13 described a young black woman with clinical and serologic features of MCTD who developed myocarditis, congestive heart failure, and ventricular ectopic activity with high titer of antibody to U1 ribonucleoprotein (U1RNP). The combination of features is the core of criteria by Sharp,Citation20 Alarcón-Segovia and Villareal,Citation21 and Khan and AppeboomCitation22 in the presence of synovitis. MCTD is diagnosed by these criteria with a focus on a high U1RNP titer: synovitis, PAH, and Raynaud’s phenomenon in patients.Citation2 Among the rheumatic diseases, the diagnosis of well-established rheumatoid arthritis, progressive systemic sclerosis (PSS), dermatomyositis–polymyositis, and SLE is usually obvious, even though some cases display the characteristic of more than one rheumatic disease and are referred to as “overlap” syndromes.
Most of these have the features of two to three rheumatic diseases, such as SLE, PSS, and PM or DM. SharpCitation20 designated an overlap syndrome of SLE, generalized scleroderma, and PM, which they considered to be a novel class of a “rheumatic disease syndrome dissimilar from the overlap syndromes” and termed it “mixed connective tissue disease”. The discrete feature of this model was the high titer of antibodies to a saline extractable nuclear antigen, which were isolated into ribonucleoprotein found in a lesser number of patients with classical PSS and SLE.Citation7 Patients with MCTD may show a favorable response to corticosteroid therapy and a benign course, but the presence of cardiorespiratory complications may spell a rapid devastating clinical course in patients.
In our case, a major treatment challenge among others was that the onset of MCTD could not be accurately ascertained, so the temporal relationship between MCTD and cardiac problems was unclear, but as she presented with acute decompensated heart failure, there was prompt management with antifailure drugs with clinical improvement, but with persistence of the rheumatologic manifestations, such as fever, polyarthritis, joint swelling, stiffness, and pain, the patient responded positively, but briefly, to the administration of prednisolone with resulting sudden deterioration in clinical status and impending death, which led to its immediate withdrawal.
Another treatment challenge was the delay in obtaining prompt serology to confirm the suspected CTD at early stage due to the unavailability of the serologic lab tests in our hospital.
Furthermore, a declining renal status prompted consideration for renal replacement therapy. Immune complex glomerulonephritis with MCTD has often been reported; in addition, MCTD associated with myeloperoxidase-anti- neutrophil cytoplasmic antibody positive polyangiitis occurred in a patient with advanced renal failure, anemia, and pulmonary alveolar hemorrhage and died on the 16th day of admission.Citation14
On echocardiography, our patient had several myocardial involvements, and Lash et alCitation15 reported a relatively poor prognosis in MCTD patients with primary myocardial involvement as in SLE.
We believe that our patient benefited from the prompt management of acute heart failure complicating MCTD, but we encountered difficulties in establishing an early link between both entities.
Conclusion
Identifying MCTD is critical, chiefly in patients requiring glucocorticoids or steroids that may worsen systemic HTN and heart failure. There is a need to have definitive investigative facilities for such patients in hospitals.
Ethics approval
The ethics and research committee approval was obtained before the commencement of the report.
Disclosure
The authors report no conflicts of interest in this work.
References
- Martínez-BarrioJValorLLópez-LongoFJFacts and controversies in mixed connective tissue diseaseMed Clin (Barc)20181501263228864092
- Alarcón-SegoviaDCardielMHComparison between 3 diagnostic criteria for mixed connective tissue disease. Study of 593 patientsJ Rheumatol19891633283342724251
- MissoungaLBaJINseng Nseng OndoIRMixed connective tissue disease: prevalence and clinical characteristics in African black, study of 7 cases in Gabon and review of the literaturePan Afr Med J20172716228904690
- AdebayoRAAkinwusiPOBalogunMOTwo-dimensional and Doppler echocardiographic evaluation of patients presenting at Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife, Nigeria: a prospective study of 2501 subjectsInt J Gen Med2013654154423861593
- LundbergIECardiac involvement in autoimmune myositis and mixed connective tissue diseaseLupus200514970871216218472
- MavrogeniSMarkousis-MavrogenisGKoutsogeorgopoulouLKolovouGCardiovascular magnetic resonance imaging: clinical implications in the evaluation of connective tissue diseasesJ Inflamm Res201710556128546762
- UedaNMimuraKMaedaHMixed connective tissue disease with fatal pulmonary hypertension and a review of literatureVirchows Arch A19844044335340
- BriasoulisAHalpernDBhattiMDodellGHerzogEAcute biventricular failure as a sequela of multiple autoimmune disordersCan J Cardiol2012285611.e7e9
- SchwagtenBVerheyeSVan den HeuvelPCombined systolic and diastolic heart failure as the first presentation of mixed connective tissue diseaseActa Cardiol200762442142317824306
- DerderianSSTellisCJAbbrechtPHWeltonRCRajagopalKRPulmonary involvement in mixed connective tissue diseaseChest198588145484006555
- TaniCCarliLVagnaniSThe diagnosis and classification of mixed connective tissue diseaseJ Autoimmun201448–494649
- HajasASzodorayPNakkenBClinical course, prognosis, and causes of death in mixed connective tissue diseaseJ Rheumatol20134071134114223637328
- WhitlowPLGilliamJNChubickAZiffMMyocarditis in mixed connective tissue disease. Association of myocarditis with antibody to nuclear ribonucleoproteinArthritis Rheum19802378088157406932
- KitauraKMiyagawaTAsanoKMixed connective tissue disease associated with MPO-ANCA-positive polyangiitisIntern Med200645201177118217106166
- LashADWittmanALQuismorioFPJrMyocarditis in mixed connective tissue disease: clinical and pathologic study of three cases and review of the literatureSemin Arthritis Rheum19861542882962940685
- GunnarssonRAndreassenAKMolbergØPrevalence of pulmonary hypertension in an unselected, mixed connective tissue disease cohort: results of a nationwide, Norwegian cross-sectional multicentre study and review of current literatureRheumatology (Oxford)20135271208121323407386
- Kawano-DouradoLBaldiBGKayFUPulmonary involvement in long-term mixed connective tissue disease: functional trends and image findings after 10 yearsClin Exp Rheumatol201533223424025896472
- CaminatiACassandroRHarariSPulmonary hypertension in chronic interstitial lung diseasesEur Respir Rev20132212929230123997057
- UngprasertPWannarongTPanichsillapakitTCardiac involvement in mixed connective tissue disease: a systematic reviewInt J Cardiol2014171332633024433611
- SharpGCDiagnostic criteria for classification of MCTD Mixed connective tissue disease and antinuclear antibodiesAmsterdamElsevier19872332
- Alarcon-SegoviaDVillarealMClassification and diagnostic criteria for mixed connective tissue diseaseKasukawaRSharpGCMixed connective tissue disease and antinuclear antibodiesAmsterdamElsevier19873340
- KahnMFAppeboomTSyndrome de SharpKahnMFPeltierAPMeyerOPietteJCLes Maladies Systemiques3rd edParisFlammarion1991545556