Abstract
Prevalence of pulmonary sequestration accounts for up to 6.4% of all congenital pulmonary malformations. We report on a 40-year-old woman who underwent excision of an aberrant solid retroperitoneal mass in the left subdiaphragmatic area. The mass was identified to be an extralobar pulmonary sequestration. The diagnosis could be made without surgery by percutaneous tissue biopsy and imaging. We encourage keeping in mind pulmonary sequestration anomaly presenting as an aberrant retroperitoneal mass. The aim of this case report is to increase awareness about the condition and review the criteria for its definitive diagnosis and treatment.
Case report
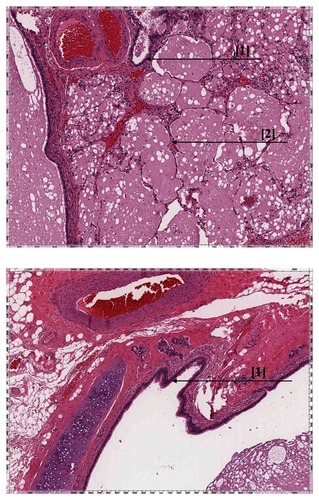
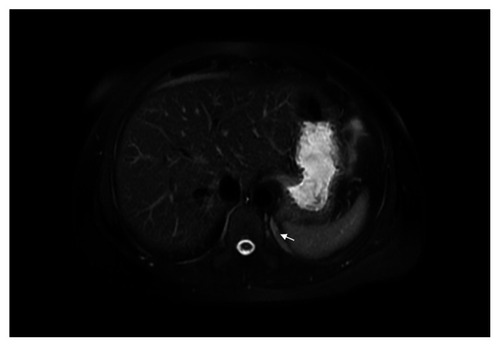
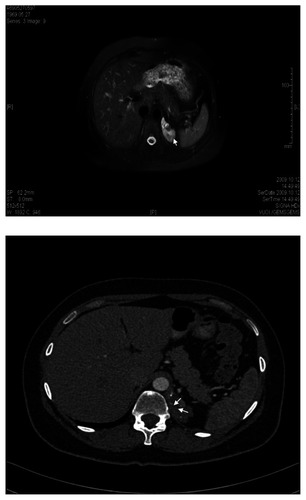
A 40-year-old woman was evaluated for abdominal pain, which had been present for two years. Abdominal ultrasound did not reveal any abnormalities. The patient had a history of endometriosis. Complete blood count, chemistry panel, urine analysis, and liver function tests were normal. Medical history, physical examination, and biochemical parameters did not suggest any dysfunction. Magnetic resonance imaging (MRI) and computed tomography (CT) of the abdomen showed a heterointensive 2.36 × 8.53 cm aberrant enhancing mass in the projection of the left diaphragmatic peduncle (). Excision of an aberrant solid retroperitoneal mass was performed. Pathology revealed an extralobar pulmonary sequestration in the left retroperitoneum, with a brown elastic 4.7 × 1.5 cm tumor. In sections, the multicameral tumor was filled with a whitish consistency substance. The tumor consisted of bronchioles lined by ciliated columnar epithelium and alveoli (). Three months later, a follow-up MRI revealed the residue of the retroperitoneal mass (). There was no negative dynamic of mass residue three months after MRI imaging.
Figure 1 A) Magnetic resonance image of the undetermined retroperitoneal left subdiaphragmatic mass (2.36 × 8.53 cm, arrow). B) Axial postcontrast arterial-phase computed tomographic image of undetermined retroperitoneal left subdiaphragmatic mass (2.36 × 8.53 cm, arrows) with small calcifications inside.
Discussion
Pulmonary sequestration was first described in 1861 by Rektorzik as an embryonic mass of lung tissue that has no identifiable bronchial communication and receives its own blood supply from an anomalous systemic artery with an origin at either the thoracic or abdominal aorta.Citation1 Prevalence of pulmonary sequestration accounts for up to 6.4% of all congenital pulmonary malformations and 1.1%–1.8% of all pulmonary resections.Citation2
The sequestration is called extralobar because the mass lies outside the normal visceral pleura, and may also lie outside of the thorax in a subdiaphragmatic position. Extralobar pulmonary sequestration accounts for 14%–25% and intralobar pulmonary sequestration accounts for 75%–86% of cases. In extralobar pulmonary sequestration, males are affected approximately four times more often than females. Sixty-one percent of patients present when they are younger than six months of age. In 10% of cases, patients are asymptomatic at the time of diagnosis.Citation2
Ideally, the diagnosis could be made without surgery by percutaneous tissue biopsy and imaging.Citation3,Citation4 However, computed tomography (CT)/magnetic resonance imaging (MRI) does not provide a conclusive diagnosis of extralobar pulmonary sequestration.Citation3,Citation4 The diagnosis of an intralobar pulmonary sequestration can be confirmed by enhanced contrast CT scanning with three-dimensional reconstruction, which is a noninvasive method.Citation3,Citation4 CT scans have 90% accuracy in the diagnosis of pulmonary sequestration.Citation3,Citation4 Arteriography (conventional or CT angiography) is the gold standard for identifying pulmonary sequestration because it confirms the anatomy and identifies the systemic arterial blood supply.Citation1 MRI and MR angiography can provide information similar to that obtained by CT scans.Citation5–Citation8 Ultrasonography is noninvasive and safe, making its use ideal in the prenatal and postnatal settings.Citation9–Citation11 Color flow and duplex Doppler ultrasound can accurately depict the ectopic blood supply and drainage.Citation12–Citation16 Radionuclide angiography is another noninvasive technique that may demonstrate the systemic arterial blood supply to the sequestration, thus establishing the diagnosis.Citation17,Citation18
Management of an asymptomatic pulmonary sequestration with no connection to the surrounding lung is controversial. At present, open surgery remains the best approach to definitive resection for intralobar or extralobar sequestration.Citation19 An alternative option to treat pulmonary sequestration is embolization of the feeding arteries.Citation19
Conclusion
The gold standard for identifying pulmonary sequestration is angiography because it confirms the anatomy, identifies the systemic supply, and shows the venous drainage. It should be done to clarify the vascular supply to the pulmonary sequestration as an essential part of the preoperative assessment of such patients, due to the risk of occult or operative hemorrhage, and to identify the exact artery to be destroyed to remove any blood supply to the mass residue. We encourage keeping in mind pulmonary sequestration anomaly presenting as an aberrant retroperitoneal mass. Prompt suspicion of nonresolving radiological lesions is needed to enable correct and early diagnosis of pulmonary sequestration. Three months later, there was no negative dynamic of mass residue on MRI in our patient. In case of mass enlargement, artery embolization or surgery is indicated.
Disclosure
The authors declare that they have no competing interests in this work.
References
- PrasadRGargRVermaKIntralobar sequestration of lungLung India200926415916120532005
- YucelOGurkokSGozubuyukADiagnosis and surgical treatment of pulmonary sequestrationThorac Cardiovasc Surg200856315415718365974
- SalmonsSPulmonary sequestrationNeonatal Netw2000197273111949021
- AmitaiMKonenERozenmanJGerniakAPreoperative evaluation of pulmonary sequestration by helical CT angiographyAm J Roentgenol19961674106910708819421
- OoiGCCheungCWLamWKTsangKWPulmonary sequestration: Diagnosis by magnetic resonance angiography and computed tomographyChin Med J (Engl)1999112766867011601267
- FuminoSIwaiNKimuraOOnoSHiguchiKPreoperative evaluation of the aberrant artery in intralobar pulmonary sequestration using multidetector computed tomography angiographyJ Pediatr Surg200742101776177917923215
- SancakTCangirAKAtasoyCOzdemirNThe role of contrast enhanced three-dimensional MR angiography in pulmonary sequestrationInteract Cardiovasc Thorac Surg20032448048217670100
- ZhangMZhuJWangQShangDContrast enhanced MR angiography in pulmonary sequestrationChin Med J (Engl)2001114121326132811793865
- MayDABarthRAYeagerSNussbaum-BlaskABulasDIPerinatal and postnatal chest sonographyRadiol Clin North Am19933134995168497587
- MacKenzieTCGuttenbergMENisenbaumHLJohnsonMPAdzickNSA fetal lung lesion consisting of bronchogenic cyst, bronchopulmonary sequestration, and congenital cystic adenomatoid malformation: The missing link?Fetal Diagn Ther200116419319511399876
- WestMSDonaldsonJSShkolnikAPulmonary sequestration. Diagnosis by ultrasoundJ Ultrasound Med1989831251292657090
- SmartLMHendryGMImaging of neonatal pulmonary sequestration including Doppler ultrasoundBr J Radiol1991647603243292025773
- NicoliniUCerriVGroliCA new approach to prenatal treatment of extralobar pulmonary sequestrationPrenat Diagn200020975876011015708
- SugaKHaraAMatsumotoTIntralobar bronchopulmonary sequestration: Evidence of air trapping shown by dynamic xenon-133 SPECTBr J Radiol20017488365766111509405
- NewmanBReal-time ultrasound and color-Doppler imaging in pulmonary sequestrationPediatrics19908646206232216630
- SauerbreiELung sequestration. Duplex Doppler diagnosis at 19 weeks’ gestationJ Ultrasound Med19911021011052020047
- GooneratneNConwayJJRadionuclide angiographic diagnosis of bronchopulmonary sequestrationJ Nucl Med1976171210351037993832
- LeeBSKimJTKimEANeonatal pulmonary sequestration: Clinical experience with transumbilical arterial embolizationPediatr Pulmonol200843440441318302235
- OsakiTKodateMTakagishiTNomiMMurakamiJYamamotoHUnique extralobar sequestration with atypical location and aberrant vesselsAnn Thorac Surg20109051711171220971304