Abstract
Background
The reported features and effectiveness of heads-up surgery (HUS) for ophthalmic surgery include greater resolution, teaching, and significantly reduced endoillumination power.
Objective
To report how to care for severe intraoperative photophobia using the HUS system during bilateral rhegmatogenous retinal detachment (RD) surgery in a patient with severe photophobia.
Case Report
A man in his 50s, who had been followed up for photophobia and visual impairment underwent five ophthalmic surgeries for bilateral RD. In his early 40s, he had been referred to our hospital because of a complaint of bilateral visual impairment, including severe photophobia, approximately 2 years prior. His decimal best-corrected visual acuities were 0.7 and 0.6 in his right and left eyes, respectively. Optical coherence tomography showed diffuse thinning of the entire retinal layer in the macula of both eyes, which was considered to be a cause of the decrement of visual acuity and photophobia. Twelve years after his first visit, he noticed multiple floaters in his left eye. For RD excluding the macular area, we planned cataract and retinal surgery under retrobulbar anesthesia. However, as we could not continue retinal surgery after cataract surgery due to severe photophobia, we performed general anesthesia (GA) during the second surgery. Seventeen months after the surgery, he underwent the third surgery for RD in his right eye under GA. For RD recurring twice, we performed surgery with the HUS system under retrobulbar anesthesia for the fourth and fifth surgeries, which avoided photophobia due to the significantly reduced light stimulation of the HUS system.
Conclusion
Lower light intensity achieved by the HUS system enabled us to eliminate the patient’s intraoperative discomfort. Consequently, we could perform the surgery under local anesthesia in this patient with RD who complained of severe photophobia that required GA using a conventional surgical system.
Introduction
The human eyeball is a small organ with an average diameter of approximately 24 mm.Citation1 Thus, ocular surgery requires complex and complicated manipulations. Multiple surgical techniques and instruments have been developed to achieve better surgical outcomes, which have resulted in more accurate and less invasive surgery.Citation2–Citation5 In particular, intraoperative visibility, which is directly affected by the resolution and illumination of the surgical instruments, plays an important role in improving surgical outcomes. Recently, heads-up surgery (HUS) systems have been developed based on advanced technology. These systems have multiple features compared to conventional systems with a surgical microscope as they allow indirect viewing through a large three-dimensional monitor.Citation6 The main features of HUS systems are that surgeons can perform surgery in a “heads-up” position and can share the surgical view,Citation7 in addition to higher resolution and reduced illumination with newly developed image sensors.Citation8,Citation9
To safely perform operations, anesthesia and other pharmacological approaches, such as analgesics, are critical for reducing the physical and mental burdens of patients. Local anesthesia such as topical anesthesia, sub-Tenon’s anesthesia, and retrobulbar anesthesia (RBA), are common approaches in ophthalmic surgeries.Citation10 However, some patients experience intolerable intraoperative discomfort because of high sensitivity to pain and/or visual discomfort to the bright light defined as photophobia,Citation11 strong fear, psychiatric disorders including panic disorder and dementia, or other reasons, under the combination of local anesthesia and other pharmacological approaches. In such cases, general anesthesia (GA) is performed,Citation12 although it has unique risks such as postoperative nausea and vomitingCitation13 or delirium.Citation14
Herein, we describe our experiences with a male patient who reported intolerable photophobia during the first surgery under local anesthesia who underwent a total of five surgeries for bilateral rhegmatogenous retinal detachment (RD). These surgeries were performed by changing the surgical procedures and anesthesia. The purpose of this study was to report how we addressed his severe photophobia during this series of surgeries.
Case Report
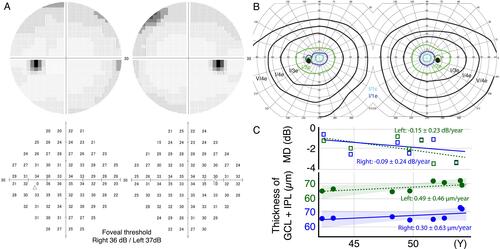
A man in his early 40s presented to our hospital with a complaint of bilateral visual impairment starting approximately 2 years prior. He had noticed this visual impairment because any glasses failed to correct his decimal visual acuity up to 1.0 at an eyeglass shop. The patient had no ocular or family histories. At the first examination in our clinic, his decimal best-corrected visual acuity (BCVA) was 0.7 (equivalent to 0.155 on logMAR visual acuity) with a spherical refractive error of −0.25 diopter (D) and cylinder –1.00 D Axis 170° and 0.6 (0.22 on logMAR) with a cylinder refractive error of–1.25 D Axis 5° in the right and left eyes, respectively. The ocular tension was within the normal range in both eyes. Slit-lamp examinations showed mildly senile cataracts bilaterally and no other abnormalities in the anterior and medial segments. Dilated fundus examinations showed no apparent abnormalities in either eye (). Optical coherence tomography (OCT) images showed sustained macular structure but diffuse thinning of the entire retinal layers in the macular area in both eyes (, Cirrus HD-OCT model 4000, Carl Zeiss, Germany). Fluorescein angiography (FA) revealed no apparent abnormalities (). A static visual field test using a Humphrey field analyzer (HFA II 750, Carl Zeiss, Germany) showed slight relative visual field defects in both eyes (). A kinetic visual field test using the Goldmann perimeter did not reveal any obvious abnormalities (). During several ophthalmic examinations, especially the indirect ophthalmic scope and FA, the patient experienced photophobia such that he was unable to keep his eyes open and complained of intolerable discomfort due to the bright light. Thus, we avoided examinations with bright light or reduced the luminance of the light source as much as possible. Reducing the luminance enabled us to perform these ophthalmic examinations. Some retinal diseases, including inherited retinal dystrophy, were suspected to be the cause of visual impairment and photophobia; however, a clear diagnosis was not possible as he was reluctant to undergo further examinations. There was no abnormality in the fundus or visual field; however, OCT demonstrated diffuse retinal thinning, which could be considered as the major cause for his visual impairment and photophobia. Follow-up ophthalmic examinations were performed every 6 months. His visual acuity and visual field were stable for over 10 years ().
Figure 1 Clinical fundus images of the patient in his 40s. (A) Fundus photographs showing no apparent abnormalities. (B) Horizontal optical coherence tomography (OCT) images showing sustained macular structure whereas the macular maps show diffuse thinning of the entire retinal layers in the macular area. (C) Fluorescence angiography images in the left eye. Both images obtained 50 sec (upper) and 20 min (lower) after injection show no apparent abnormalities.
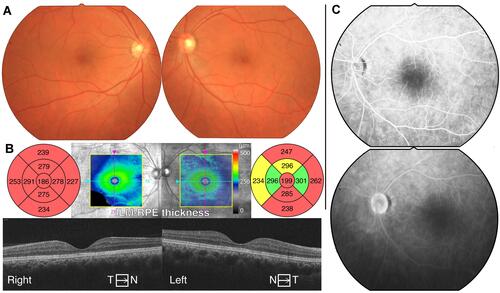
Figure 2 Visual field testing and retinal thickness. (A) Humphrey visual field testing showing slight relative visual field defects in both eyes. The foveal thresholds are within the normal range. (B) Kinetic visual field testing showing no obvious abnormalities in all isopters. (C) The change of median deviation (MD) in Humphrey field analyzer and averaged thickness of ganglion cell layer (GCL) and inner plexiform layer (IPL) in optical coherence tomography images showing no progressive change as glaucoma during the time course of 12 years.
Twelve years later, the patient reported multiple floaters in his left eye. A slit-lamp examination revealed multiple pigments in the vitreous of this eye. Dilated fundus examination showed partial rhegmatogenous retinal detachment that did not include the macula. The RD occurred in the superior retina with five atrophic holes and one retinal tear and in the inferior retina with lattice breaks. We first planned cataract surgery with phacoemulsification and intraocular lens implantation and vitreous surgery under local anesthesia with RBA. In the preparation for surgery using a surgical microscope (OPMI Lumera 700, Carl Zeiss, Germany), the patient reported severe photophobia with body motion. After RBA, the photophobia was reduced and cataract surgery was completed without any complications. However, after inserting the fiber optic light pipe into a cannula for vitrectomy with Constellation instruments (Alcon Laboratories, Fort Worth, TX, USA), with a light level set to 100% (approximately 3.2×105 cd/m2, at 40 cm by PR-655 Spectrascan®, Photo Research, Inc., CA, USA), the patient experienced intolerable photophobia with body motion (, Supplemental video). Although we reduced the light dose to 60% (1.9 × 105 cd/m2) and administered pentazocine and hydroxyzine hydrochloride as analgesics, the intolerable photophobia persisted. After consultation with the patient, we interrupted the surgery and performed the second surgery under GA 7 days later. Retinal reattachment was achieved after vitrectomy, laser photocoagulation (LPC), and 20% SF6 gas tamponade.
Figure 3 Intraoperative images. (A) The first operation for the left eye with conventional bright light under retrobulbar anesthesia (RBA). The patient moved his head as he felt intolerable discomfort from the bright light of the light pipe. Although we reduced the luminance level to 60%, he refused vitrectomy. (B) The second operation for the right eye, the fourth surgery in total, using the heads-up surgery system with minimal illumination under RBA. Although the luminance of the light from the light pipe was as low as a quarter of that used in the previous operation (15%), the intraoperative visibility was sufficient to perform vitrectomy. The patient tolerated this light level and did not complain of photophobia. (C) The third operation for the right eye, the fifth operation in total, with inadvertent bright illumination under RBA. After encircling and vitrectomy (approximately 85 min), we inadvertently set the brightness level of the twin light chandelier to 100%. The luminance was the same as the 60% from the light pipe, which was high enough to evoke his photophobia. However, he did not feel photophobia, presumably because he had had enough time for light adaptation.
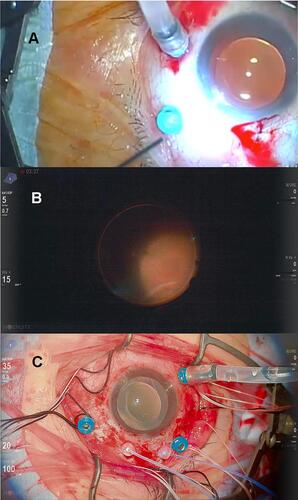
Seventeen months after vitrectomy for his left eye, the patient reported multiple floaters in his right eye. His right BCVA was 0.8. In the right eye, slit lamp examination revealed multiple pigments in the vitreous. Dilated fundus examination showed partial RD in the superior retina with a giant retinal tear, which did not include the macula. Similar to his left eye, we performed cataract surgery, vitrectomy, LPC, and gas tamponade with 20% SF6 under GA, which resulted in retinal reattachment. However, the RD recurred in his right eye 16 days after the operation. For the next operation (the fourth operation in total), we proposed to him and obtained his agreement to use an HUS system with minimal illumination under RBA as the patient had felt nausea and complained of throat pain after GA. In case that the patient experienced the bright light from the light pipe to be intolerable, we requested anesthesiologists to be on standby during the operation. We performed vitrectomy, LPC, and gas tamponade with 12% C3F8 under RBA with the HUS system, which was consisted of an NGENUITY 3D® visualization system (Alcon Laboratories) attached to a VISU 210 microscope (Carl Zeiss Meditec, Jena, Germany). We set the brightness of the light pipe to 15% (4.8 x 104 cd/m2) and inserted the light pipe into a cannula, which provided excellent intraoperative visibility with minimal illumination (, Supplemental video). The patient did not complain of photophobia during the operation, and retinal reattachment was achieved. Twenty-six days after the re-operation, the RD recurred. For the second RD recurrence, and the fifth operation in total, we performed vitrectomy, encircling, LPC, and gas tamponade with 12% C3F8 under RBA with the HUS system under the same conditions as the fourth operation. After approximately 60 min of encircling and 25 min of vitrectomy, we inadvertently set the brightness of the twin light chandelier to 100% (1.9 × 105 cd/m2) as the default brightness setting. Upon noticing the wrong setting, we immediately reduced the brightness to 30% (5.9 × 104 cd/m2) and asked the patient if he felt photophobia (, Supplemental video). Interestingly, he did not experience photophobia. Retinal reattachment was achieved and was sustained until 18 months after surgery. His decimal BCVAs were 0.6 and 0.9 in the right and left eyes, respectively (0.22 and 0.046 on logMAR).
Discussion
Case Summary
We described how we addressed severe photophobia during five retinal surgeries in a patient. At the first surgery, the patient experienced intolerable light stimulation from conventional illumination systems under RBA and administration of analgesics. During the second and third surgeries, the patient did not experience photophobia as he was unconscious under GA. Lastly, he completely tolerated light stimulation, which was markedly reduced, with the introduction of the HUS system during the fourth and fifth surgeries for RBA.
Previous studies have demonstrated the effectiveness of the HUS system.Citation6,Citation9 However, to our knowledge, this is the first case report directly describing how the HUS system allowed us to operate on a patient for whom intraoperative bright light was intolerable without GA. To reduce intolerable intraoperative discomfort experienced by patients, such as pain and photophobia, clinicians generally attempt at least two methods. One is to increase the discomfort threshold of the patient’s tolerance to the sensory inputs. The other is to reduce the total amount of sensory inputs during the operation. In cases of ophthalmic operations with photophobia, 1) increasing the threshold of photophobia and 2) reducing the level of luminance during operation are considered. Previous reports for intraoperative photophobia were intracameral illumination for cataract surgeryCitation15 and the HUS system only with room lighting for strabismus surgery,Citation16 which were following the method of reducing the level of luminance. We discuss the specific methods derived from this rare case.
Anesthesia for Ophthalmic Surgery
Light stimulation of the retina and associated phenomena is a unique problem in ophthalmic surgeries. Topical anesthesia, sub-Tenon’s anesthesia, and RBA are commonly used for local anesthesia in ophthalmic surgery; among these, RBA can increase the photophobic threshold owing to reduced photosensitivity,Citation17 using neither tropical anesthesia,Citation18 anti-inflammatory eye dropsCitation19 nor intracameral anesthesia.Citation20 Analgesics such as sedatives and analgesics are used in combination to induce drowsiness,Citation21,Citation22 which can suppress photophobia through both neural inputs. In our case, however, the combination of RBA and analgesics was insufficient to control the photophobia to a tolerable level in conventional illumination systems.
For the second and third surgeries, we selected GA, which had the advantage of release from intolerable discomfort but it also had risks of intra- and postoperative complications related to GA. Nausea, vomiting, and delirium are the major postoperative complications associated with GA.Citation23 Additionally, GA can (rarely) cause lethal complications such as malignant hyperthermia or cardiac, respiratory, and renal complications.Citation23 Surgery under local anesthesia has several merits compared to GA, including fewer postoperative complications, faster postoperative recovery, shorter or no administration period, less surgical preparation time, and lower financial cost.Citation24,Citation25 Thus, surgery under local anesthesia had benefits for our patient if the problem of photophobia could be solved.
Light Adaptation
Another method to increase the threshold of intolerant discomfort to bright light is light adaptation. Eye surgeons often notice patient reactions to bright light, such as blinking, head motion, and complaints of photophobia at the beginning of surgery. However, patients generally adapt to the bright light, which immediately becomes tolerable. A previous study suggested that photophobic patients could have an abnormality in the dynamic range of lightness perception and photophobia could be evoked by luminance over the highest distinguishable luminance.Citation26 Our patient tolerated the inadvertent bright light that occurred during the fifth surgery, presumably because his visual system had slowly adapted to the bright light over 85 min. If neither the HUS nor GA are available and surgery is required for photophobic patients, waiting for a much longer time and gradually increasing the brightness of the light from tolerable to operable luminance may provide an alternative solution.
HUS System and Luminance Levels
Microscopic surgery usually requires a bright light source because the high magnification reduces the number of photons. The image quality is relevant to the number of photons.Citation27 Thus, microscopes and intraocular lighting devices for ophthalmic surgery have extremely high-power light bulbs, such as halogen or xenon.Citation12 In our case, the patient tolerated light from the microscope but felt intolerable discomfort from the light pipe into the cannula for vitrectomy. We measured the luminance from the fiber optic light pipe and confirmed the linear relationship between luminance and the output setting of the intraocular lightning device used for his operation. For him, a luminance of 60% (1.9 × 105 cd/m2) was intolerable to proceed with the operation. When we set the output to 15% (4.8 × 104 cd/m2), he was able to tolerate the light from the light pipe. In a previous case report, using a 27G system and the same type of intraocular lightning device, an eye surgeon succeeded in vitrectomy at the minimum output level of 1% (0.1 lm).Citation28 Reducing the luminance level during the operation can effectively prevent photophobia, even in patients with severe photophobia, as in our case. Additionally, not only the luminance level but also the spatial pattern of light inputs are important for brightness perception.Citation29 Using only chandelier illumination systems without the light pipe might help reduce photophobia as the chandelier illumination systems supply more diffuse light than the light pipe.Citation30 During the fifth surgery in our case, the patient did not feel photophobia to the light from the twin light chandelier at 100%. We speculate that the light adaption of his visual system dominantly prevents photophobia; however, diffuse light from the chandelier may not facilitate photophobia.
Conclusions
In this report, the HUS system reduced the luminance of the light source and helped eliminate intolerable discomfort from intraoperative bright light. Our data showed that reducing the luminance level using the HUS system enabled retinal surgery under local anesthesia in a patient with severe photophobia who required general anesthesia with conventional operating systems.
Ethical Approval
Ethical approval was deemed unnecessary by The Jikei University School of Medicine Institutional Review Board, as in cases where a procedure is part of a patient’s standard care, following the principals of the Declaration of Helsinki is recommended.
Patient Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Acknowledgment
Our study was supported by the grant of Japan Society for the Promotion of Science (JSPS) KAKENHI (JP18K16939 and 21K09729 to H.H.), Charitable Trust Fund for Ophthalmic Research in Commemoration of Santen Pharmaceutical’s Founder (H.H.).
Disclosure
The authors declare that there is no conflict of interest regarding this paper.
References
- Bekerman I, Gottlieb P, Vaiman M. Variations in eyeball diameters of the healthy adults. J Ophthalmol. 2014;2014:503645. doi:10.1155/2014/503645
- Ashwin PT, Shah S, Wolffsohn JS. Advances in cataract surgery. Clin Exp Optom. 2009;92(4):333–342. doi:10.1111/j.1444-0938.2009.00393.x
- Fujii GY, De Juan E, Humayun MS, et al. A new 25-gauge instrument system for transconjunctival sutureless vitrectomy surgery. Ophthalmology. 2002;109(10):1807–1812. doi:10.1016/S0161-6420(02)01179-X
- Oshima Y, Wakabayashi T, Sato T, Ohji M, Tano Y. A 27-gauge instrument system for transconjunctival sutureless microincision vitrectomy surgery. Ophthalmology. 2010;117(1):93–102. doi:10.1016/j.ophtha.2009.06.043
- Lavia C, Dallorto L, Maule M, Ceccarelli M, Fea AM. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: a systematic review and meta-analysis. PLoS One. 2017;12(8):e0183142. doi:10.1371/journal.pone.0183142
- Eckardt C, Paulo EB. Heads-Up Surgery For Vitreoretinal Procedures: an Experimental And Clinical Study. Retina. 2016;36(1):137–147. doi:10.1097/IAE.0000000000000689
- Coppola M, La Spina C, Rabiolo A, Querques G, Bandello F. Heads-up 3D vision system for retinal detachment surgery. Int J Retina Vitreous. 2017;3:46. doi:10.1186/s40942-017-0099-2
- Matsumoto CS, Shibuya M, Makita J, et al. Heads-Up 3D surgery under low light intensity conditions: new high-sensitivity hd camera for ophthalmological Microscopes. J Ophthalmol. 2019;2019:5013463. doi:10.1155/2019/5013463
- Del Turco C, D’Amico Ricci G, Dal Vecchio M, et al. Heads-up 3D eye surgery: safety outcomes and technological review after 2 years of day-to-day use. Eur J Ophthalmol. 2021;3:11206721211012856.
- Nouvellon E, Cuvillon P, Ripart J. Regional anesthesia and eye surgery. Anesthesiology. 2010;113(5):1236–1242. doi:10.1097/ALN.0b013e3181f7a78e
- Digre KB, Brennan KC. Shedding light on photophobia. J Neuroophthalmol. 2012;32(1):68–81. doi:10.1097/WNO.0b013e3182474548
- Rizzo S, Patelli F, Chow D. Vitreo-Retinal Surgery. Berlin Heidelberg. Springer-Verlag; 2009.
- Apfel CC, Heidrich FM, Jukar-Rao S, et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth. 2012;109(5):742–753. doi:10.1093/bja/aes276
- Ehsani R, Djalali Motlagh S, Zaman B, Sehat Kashani S, Ghodraty MR. Effect of General Versus Spinal Anesthesia on Postoperative Delirium and Early Cognitive Dysfunction in Elderly Patients. Anesth Pain Med. 2020;10(4):e101815. doi:10.5812/aapm.101815
- Seo H, Nam DH, Lee JY, et al. Macular photostress and visual experience between microscope and intracameral illumination during cataract surgery. J Cataract Refract Surg. 2018;44(2):190–197. doi:10.1016/j.jcrs.2017.11.016
- Hamasaki I, Shibata K, Shimizu T, Kono R, Morizane Y, Shiraga F. Lights-out surgery for strabismus using a heads-up 3D vision system. Acta Med Okayama. 2019;73(3):229–233.
- Verma L, Arora R, Kumar A. Temporary conduction block of optic nerve after retrobulbar anesthesia. Ophthalmic Surg. 1990;21(2):109–112.
- Gills JP, Cherchio M, Raanan MG. Unpreserved lidocaine to control discomfort during cataract surgery using topical anesthesia. J Cataract Refract Surg. 1997;23(4):545–550. doi:10.1016/S0886-3350(97)80211-8
- McDonald MB, Wyse TB, Borodkin MJ, Ocmand A, Shoelson B, Thompson H. Comparison of the effectiveness of 4 anti-inflammatory drops in relieving photophobia after pupil dilation. J Cataract Refract Surg. 1999;25(3):405–410. doi:10.1016/S0886-3350(99)80090-X
- Pang MP, Fujimoto DK, Wilkens LR. Pain, photophobia, and retinal and optic nerve function after phacoemulsification with intracameral lidocaine. Ophthalmology. 2001;108(11):2018–2025. doi:10.1016/S0161-6420(01)00798-9
- Kumar CM. Orbital regional anesthesia: complications and their prevention. Indian J Ophthalmol. 2006;54(2):77–84. doi:10.4103/0301-4738.25826
- Greenhalgh DL, Kumar CM. Sedation during ophthalmic surgery. Eur J Anaesthesiol. 2008;25(9):701–707. doi:10.1017/S0265021508004389
- Harris M, Chung F. Complications of general anesthesia. Clin Plast Surg. 2013;40(4):503–513. doi:10.1016/j.cps.2013.07.001
- Costen MT, Newsom RS, Wainwright AC, Luff AJ, Canning CR. Expanding role of local anaesthesia in vitreoretinal surgery. Eye. 2005;19(7):755–761. doi:10.1038/sj.eye.6701640
- Simanjuntak GW, Djatikusumo A, Adisasmita A, Nadjib M, Mailangkay H, Hussain N. Cost analysis of vitrectomy under local versus general anesthesia in a developing country. Clin Ophthalmol. 2018;12:1987–1991. doi:10.2147/OPTH.S179369
- Horiguchi H, Suzuki E, Kubo H, et al. Efficient measurements for the dynamic range of human lightness perception. Jpn J Ophthalmol. 2021;65(3):432–438. doi:10.1007/s10384-020-00808-2
- Morris PA, Aspden RS, Bell JE, Boyd RW, Padgett MJ. Imaging with a small number of photons. Nat Commun. 2015;6:5913. doi:10.1038/ncomms6913
- Kunikata H, Abe T, Nakazawa T. Heads-Up Macular Surgery with a 27-Gauge Microincision Vitrectomy System and Minimal Illumination. Case Rep Ophthalmol. 2016;7(3):265–269. doi:10.1159/000452993
- Anderson BL, Winawer J. Image segmentation and lightness perception. Nature. 2005;434(7029):79–83. doi:10.1038/nature03271
- Oshima Y, Awh CC, Tano Y. Self-retaining 27-gauge transconjunctival chandelier endoillumination for panoramic viewing during vitreous surgery. Am J Ophthalmol. 2007;143(1):166–167. doi:10.1016/j.ajo.2006.07.051
