Abstract
Transcatheter aortic valve replacement (TAVR) has been recently indicated for the treatment of patients with severe aortic stenosis in all risk profiles. At present, TAVR has become mature at home and abroad, but the relevant experience is deficient in the treatment of aortic valve stenosis with outflow tract stenosis. One case of a high-risk surgical patient was included in this paper who suffered from severe aortic valve stenosis with left ventricular outflow tract (LVOT) stenosis. In this case, TAVR was performed with deep implantation of a new valve and both aortic valve stenosis and LVOT stenosis were treated through a single TAVR procedure. This case highlights the vital role of such treatment in dealing with both aortic valve stenosis and LVOT stenosis through a single TAVR procedure, thus providing valuable information for similar cases.
Introduction
Aortic valve stenosis (AS) is one of the most common valvular disorders in the world. AS is the second most prevalent valvular pathology in the United StatesCitation1 where up to 10% of patients over the age of 80 years suffer from AS.Citation2–4 Transcatheter aortic valve replacement (TAVR) is a medical procedure which replaces a narrowed aortic valve by implanting a fully assembled artificial valve through a catheter. Since the first successful case in 2002,Citation5 TAVR has been a preferred treatment for elderly patients with AS.
Case Presentation
The 77-year-old female patient was admitted to Qilu Hospital of Shandong University (Qingdao) on July 25, 2022, because of paroxysmal chest tightness and shortness of breath for one year which got worse in seven days. She was hospitalized for total knee replacement over six years ago and for right tendon repair surgery three years ago without a history of diabetes, hypertension and coronary artery disease.
Physical examination: Temperature 36.2°C. Heart rate: 71/min. Cardiac rhythm was regular. Respiration 19/min. Blood pressure 125/79mmHg. The patient was conscious. No sign of cyanosis was observed on her lips. No jugular vein distension was observed on either side of her neck. No moist rales or rhonchi were heard in the lungs. A grade 4/6 systolic ejection murmur was heard between the second rib on the right edge of the sternum, accompanied by tremor. No edema of lower extremities was observed.
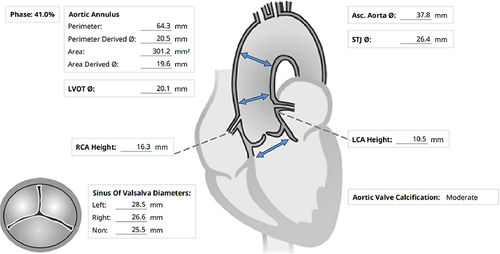
Laboratory report: haemoglobin 98g/L, triglyceride 1.87mmol/L, LDL cholesterol 4.60mmol/L, ultra-sensitive C-reactive protein 39.60mg/L. Platelet count, hypersensitive troponin I, liver and kidney function, electrolyte and blood coagulation were normal. Electrocardiogram (ECG) report: sinus rhythm (heart rate 68/min, PR interval 187 ms, no conduction block of any type), pathologic Q-waves in I, AVL. Transthoracic echocardiography (TTE) report: severe aortic valve stenosis (orifice area 0.5cm2, CW-measured peak transvalvular gradient 62mmHg and mean transvalvular gradient 41mmHg) with mild regurgitation; mild mitral valve stenosis with mild regurgitation; concentric hypertrophy of left ventricular wall (thickness of posterior wall of left ventricle 13mm); moderate left ventricular outflow tract (LVOT) stenosis (CW-measured peak cross-outflow tract gradient 58mmHg and mean cross-outflow tract gradient 21mmHg); pulmonary artery systolic pressure 36mmHg, left atrial dilatation (end diastolic diameter 47mm), left ventricular end diastolic diameter 43mm, left ventricular ejection fraction 66%. CT angiography (CTA) report (): Her aortic valve was tricuspid with thickening leaflet and mildlymoderate calcification which was unevenly distributed and mainly observed in the junction of leaflets. The perimeter of the aortic annulus was 64.3mm, the diameter of the aortic annulus was 20.5mm and the perimeter of LVOT was 65.2mm with a mean diameter of 20.7mm. The perimeter measured at 4mm above aortic annulus was 58.5 mm with a mean diameter of 18.6mm. The perimeter measured at 6mm above aortic annulus was 56.9mm with a mean diameter of 18.1mm. The perimeter measured at 8mm above aortic annulus was 59.7mm with a mean diameter of 19.0mm. The perimeter measured at 2mm below aortic annulus was 61.3mm with a mean diameter of 19.5mm. The perimeter measured at 6mm below aortic annulus was 59.7mm with a mean diameter of 19.0mm. The perimeter measured at 8mm below aortic annulus was 57.3mm with a mean diameter of 18.2mm. The distance from the virtual annulus to the membrane was 2.41mm, and the mean diameter of sinotubular junction and the diameter of ascending aorta were 26.4mm and 37.8mm respectively. The diameters of left coronary artery ostium and right coronary artery ostium were 10.5mm and 16.3mm respectively. Coronary angiography report: No stenosis was observed in the left main artery (LM), left anterior descending branch (LAD), left circumflex branch (LCX) and right coronary artery (RCA). TIMI flow marked grade 3.
Preliminary diagnosis: 1. valvular disease; aortic valve stenosis (severe) with aortic valve insufficiency (mild), mitral stenosis (mild) with mitral valve insufficiency (moderate). 2. total knee replacement (post-operation). 3. right tendon repair surgery (post-operation).
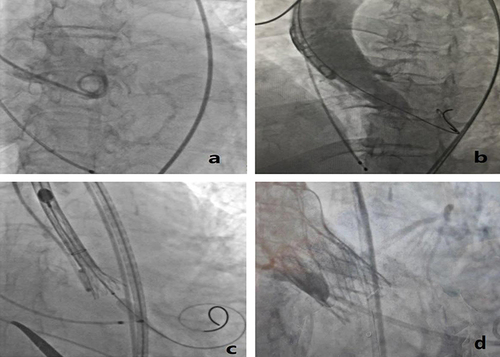
After a comprehensive evaluation of the patient, the cardiology team concluded that the surgical risk score of the patient given by the American Association of Thoracic Surgeons was in the high risk group, and the mortality rate was 11.2%, so the surgical risk of valve replacement and LVOT dredging was extremely high, and intervention treatment seemed to be the most practicable. Transcatheter aortic valve replacement (TAVR) may lead to worsening LVOT stenosis which poses a risk of circulatory collapse on the patient. One thousand five hundred milliliters of preoperative fluid was provided to avoid the risk. Meanwhile, a high deployment technique was adopted for valve implantation so as to support the LVOT and reduce the risk of conduction block. The operation was performed in a hybrid operation room where emergency surgery was fully prepared. After a thorough preoperative assessment of the vascular pathway, aortic root and LVOT anatomy by CTA, a 16mm×18 mm balloon was used to previously stretch the narrowed place through the right femoral artery as the pathway and then a 24 minimal invasive valve (produced by Shanghai MicroPort Medical Co., Ltd) was implanted. The valve was placed in a high position and gradually approached the target area (). Intraoperatively, the patient’s hemodynamic indexes remained stable and her left ventricular apex pressure (preoperative pressure 165/20mmHg, postoperative pressure 155/22mmHg), aortic valve pressure (preoperative pressure 154/19mmHg, postoperative pressure 145/8mmHg) and aortic root pressure (preoperative 112/60 mmHg, postoperative 138/69mmHg) were measured. The postoperative TTE report showed that the artificial valve functioned well to support the LVOT and no perivalvular leakage was found. The temporary pacemaker was removed after 24 hours of the operation as there was no new bundle branch block. The patient’s chest tightness and shortness of breath significantly improved, and she was eventually discharged from the hospital. Three months later, the clinical follow-up showed that the patient had no obvious sign of heart failure. The ECG report did not reveal any new bundle branch block as well. Besides, the TTE report indicated that the artificial valve worked well, the mean LVOT gradient was 24mmHg and the peak cross-outflow tract gradient reduced from 65mmHg to 41mmHg.
Discussion
Aortic valve stenosis with cardiac hypertrophy is common in clinical medicine. In terms of aortic valve stenosis with LVOT stenosis, different planes should be selected for measurement to avoid interference as it is difficult to accurately measure the transvalvular gradient and LVOT gradient with TTE. In addition, the doctor should have a clear understanding of the patient’s medical history, auxiliary examination and treatment process before making a clinical diagnosis of aortic valve stenosis and LVOT stenosis. Accurate measurement of intraoperative catheter pressure can contribute to determining the dominant stenosis, aortic valve stenosis or LVOT stenosis. The degree of LVOT stenosis can always be underestimated or neglected because of an increase in LVOT afterload caused by aortic valve stenosis. TAVR may cause or exacerbate LVOT obstruction because (1) a sudden drop in left ventricular afterload and an increase in blood flow velocity at LVOT give rise to Venturi effect that promotes forward movement of the anterior mitral leaflet and chordae tendineae, and (2) a decrease in left ventricular filling pressure (especially in patients with small left ventricular diameter) aggravate LVOT obstruction. Numerous cases of LVOT obstruction after TAVR have been reported internationally.Citation6–9 Zgheib AZCitation10 presented a case of an 82-year-old frail female patient with severe AS that was treated with TAVR, but the procedure was made complicated by hemodynamic compromise due to LVOT obstruction, which was treated with alcohol septal ablation. In addition, Krishnaswamy ACitation7 reported a case where alcohol septal ablation either before, or concomitant with, the TAVR procedure may be necessary.
However, for this case, transferring some controllable risks in advance was taken into consideration to avoid the rescue operation due to circulatory collapse, thus increasing the chances of a successful treatment. Meanwhile, it is of great significance that this case provides a treatment that can deal with both aortic valve stenosis and LVOT stenosis through a single TAVR procedure. Exactly, the deeply implanted artificial valve can be used to treat valve stenosis and the valve stent can treat LVOT stenosis by assisting LVOT. However, the anatomy of aortic root and the diameters of each plane should be carefully evaluated so as to fully assess the possible risks associated with deep implantation of the valve, such as paravalvular leak, mitral valve stenosis, mitral valve complex damage and conduction block.Citation11 A large valve should not be selected because an oversized valve may severely press the LVOT and increase the risk of conduction block. It can also increase the risk of downward displacement of the valve, which may negatively affect the function of mitral valve. The intraoperative CTA and TTE monitoring can provide critical information for implanting the valve at an appropriate depth. Besides, for most patients suffering from aortic valve stenosis with LVOT stenosis, the treatment of aortic valve stenosis will not cause LVOT obstruction because the reduced LVOT afterload can lead to a decrease in myocardial contractility, greatly relieving LVOT stenosis. Accordingly, the operator should treat aortic valve stenosis first before evaluating the outflow tract. In this case, the patient’s family made a strong request for interventional therapy due to the high risk of surgery. According to the evaluation results, the risk of the operation was controllable, and a contingency plan had been made before the TAVR. For example, the patient was provided with an amount of preoperative fluid because her small left ventricle may result in a high risk of circulatory collapse. In addition, the depth of implanting the artificial valve was adjusted based on the anchoring of the valve so as to avoid conduction block. In response to conduction block, a heart pacemaker was placed in the right jugular vein and adequate preparations was made for emergency surgery. In this case, by deeply implanting the artificial valve and covering part of the LVOT, aortic valve stenosis was repaired and no LVOT obstruction was observed, confirming the efficacy of the treatment. Moreover, the deep implantation of the valve did not lead to complications such as conduction block. The key lies in the detailed preoperative assessment of the anatomy, the selection of a proper valve and the precise intraoperative positioning of the implanted valve. It should be noted that surgery should be recommended first if the patient is at low surgical risk. Considering the high risk of intervention, a strong cardiology team is critical to the performance of the operation. At present, aortic valve stenosis with severe LVOT obstruction but moderate LVOT obstruction is regarded as a contraindication for TAVR. However, for patients who are at high surgical risk, intervention treatment seems to be the most practicable. If the patient is evaluated to be at high risk for LVOT obstruction and the surgery can be risky for him/her, the cardiology team should provide a detailed assessment of risks and benefits before determining the treatment method, surgery or intervention. In a word, the success of this case provides a useful reference for the treatment of patients suffering from aortic valve stenosis with LVOT stenosis and opens up a new way for TAVR indications.
In the current era of transcatheter therapies for patients with severe aortic stenosis and inoperable or high surgical risk, it is important to recognize that a treatment that can deal with both aortic valve stenosis and LVOT stenosis through a single TAVR procedure may be necessary. It is also important for operators to understand the physiological changes arising from relieving valvular obstruction and vigilantly monitor the hemodynamic tracings and adjunctive imaging to respond emergently and appropriately.
Ethics and Consent
Institutional approval is not required for publication, so informed written consent for using patient information and case report publication was obtained from the patient as long as identifying data are anonymous.
Disclosure
All authors report no conflicts of interest in this work.
Additional information
Funding
References
- Osnabrugge RL, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62(11):1002–1012. doi:10.1016/j.jacc.2013.05.015
- Carabello BA, Paulus WJ. Aortic stenosis. Lancet. 2009;373(9667):956–966. doi:10.1016/S0140-6736(09)60211-7
- Eveborn GW, Schirmer H, Heggelund G, et al. The evolving epidemiology of valvular aortic stenosis. The Tromsø study. Heart. 2013;99(6):396–400. doi:10.1136/heartjnl-2012-302265
- Horstkotte D, Loogen F. The natural history of aortic valve stenosis. Eur Heart J. 1988;9(Suppl E):57–64. doi:10.1093/eurheartj/9.suppl_e.57
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106(24):3006–3008. doi:10.1161/01.cir.0000047200.36165.b8
- Gerckens U, Pizzulli L, Raisakis K. Alcohol septal ablation as a bail-out procedure for suicide left ventricle after transcatheter aortic valve implantation. J Invasive Cardiol. 2013;25(5):E114–E117.
- Krishnaswamy A, Tuzcu EM, Svensson LG, et al. Combined transcatheter aortic valve replacement and emergent alcohol septal ablation. Circulation. 2013;128(18):e366–e368. doi:10.1161/CIRCULATIONAHA.112.000470
- Sorajja P, Booker JD, Rihal CS. Alcohol septal ablation after transaortic valve implantation: the dynamic nature of left outfl ow tract obstruction. Catheter Cardiovasc Interv. 2013;81(2):387–391. doi:10.1002/ccd.23454
- Olsen KR, LaGrew JE, Awoniyi CA, et al. Undiagnosed hypertrophic obstructive cardiomyopathy during transcatheter aortic valve replacement: a case report. J Med Case Rep. 2018;12(1):372. doi:10.1186/s13256-018-1904-8
- Zgheib AZ, Iskandarani DZ, Jdaidani J, et al. Post trans-catheter aortic valve replacement shock: back to the basics. J Cardiol Cases. 2022;26(1):1–4. doi:10.1016/j.jccase.2022.01.007
- Sammour Y, Krishnaswamy A, Kumar A, et al. Incidence, predictors, and implications of permanent pacemaker requirement after transcatheter aortic valve replacement. JACC: Cardiovasc Interv. 2021;14(2):115–134. doi:10.1016/j.jcin.2020.09.063