Abstract
Severe sickle retinopathy is commonly known in adults but not in children, hence any related treatment for sickle retinopathy in children was not well described. We reported 2 paediatric sickle patients (aged 12 and 13) presented with severe sickle retinopathy and described details of their disease progression and treatments over 2–3 years, along with the challenges faced when managing this particular group of young age sickle cell patients. Our case reports also demonstrated the benefits of laser photocoagulation treatment to early sickle proliferative disease, and how complications from advanced severe retinopathy hindered effective treatments.
Introduction
Sickle cell disease (SCD) is an inherited blood disorder primarily affecting African descendants.Citation1 The clumping of abnormal sickle-shaped hemoglobin causes vascular occlusion, reduces oxygenation and blood supply to specific body parts, leading to complications such as localized organ necrosis and commonly severe pain experienced by patients during crisis. In the case of the eyes, it may cause asymptomatic painless retinal ischemic change which could lead to retinal neovascularization (known as seafans lesion of proliferative sickle retinopathy) and vitreous hemorrhage and blindness.Citation1,Citation2
The prevalence of proliferative sickle retinopathy is higher in sickle patients with the HbSC genotype than the HbSS genotype.Citation1 Published literature showed children as young as age 10 have evidence of sickle retinopathy, but severe proliferative retinopathy was reported as low as 5.6%, incidence of vitreous hemorrhage in children was even rarer.Citation3
Although retinal laser photocoagulation was the preferred treatment for proliferative sickle retinopathy in adult sickle cell patients, the decision for treatment is challenged by the fact that seafans lesions may undergo spontaneous auto-infarction in as high as 60% of cases.Citation4,Citation5 Herein, paediatric patients with sickle retinopathy were often left untreated and deemed unnecessary.Citation5 Hence information and treatment guidance on paediatric sickle retinopathy remain lacking. We reported 2 paediatric sickle patients presented with severe sickle retinopathy, described details of their disease progression and treatments over 2–3 years, along with the challenges faced when managing this particular group of young age sickle cell patients. Our report follows the Goldberg Classifications for proliferative sickle retinopathy severity ().Citation6
Table 1 Goldberg Proliferative Sickle Retinopathy Staging (PSR) to Categorize Severity of Sickle Retinopathy
Cases Presentation
Patient 1 is known to have sickle genotype SC; at aged 13 was first seen by optician and referred with queried temporal retina lesions and hazy views in both eyes. First clinic attendance was 8 months later, confirmed good vision of Snellen 6/6 each eye but history of right eye frequent floaters. Initial examination confirmed existing advanced sickle retinopathy in peripheral retinae of both eyes, with main concern of large fibrosed seafan retinal neovascularization already on traction but flat retina (). There was no evidence of vitreous hemorrhage in either eye, hence no treatment was offered at this initial stage. Maculae were also normal.
Figure 1 (A) Patient 1, first seen by optician at aged 13 who queried temporal retina lesions and hazy view in both eyes. First clinic attendance in 2022 August, confirmed good vision of 6/6 each eye but history of Right eye frequent floaters. Visible in these photos are large auto-infarcted seafan fibrotic scars on traction in peripheral supero-temporal retinae (white arrows) in both eyes; other smaller pre-retina fibrosis (black arrows), ghost vessels (red arrow), flat retina and no evidence of vitreous hemorrhage in either eye. No treatment was offered. (B) By 2023 June, patient 1 re-presented in eye casualty with Right eye vitreous hemorrhage (red arrows) with flat retina and reduced vision of 6/60. Previous supero-temporal large seafan fibrotic scar was also enveloped with hemorrhage (white arrow). Left eye status unchanged, good vision 6/6. Patient was referred to specialist sickle eye clinic for further management. (C) By 2023 September, Right eye vision improved to 6/7 as vitreous hemorrhage gravitated, revealing more auto-infarcted seafan scar on traction in supero-nasal peripheral retina (white arrow), previous blood-enveloped seafan site had part altered blood still masking any underlying reactivation of seafan (double white arrows). Left eye good vision, no vitreous hemorrhage but small “reactivated tips” were noted in previous auto-infarcted seafan scar (red arrow). With the clearing view, patient 1 was advised and received same day localized barrier laser to Right eye, to prevent retinal detachment with future traction bleed. Left eye was to be observed. (D) By 2023 December, Patient 1 maintained good vision 6/9 Right, 6/7 Left. Assessment showed no fresh/added vitreous hemorrhage in Right eye, previous barrier laser appeared inadequate (white arrow). Left eye however had worsened features of reactivation of some seafans on traction (red arrows), with evidence of gravitated vitreous hemorrhage (new). Patient was advised and received further laser top-up to Right eye, barrier laser to left eye. (E) By 2024 April, patient 1 reported no episodes of new floaters, vision remained good and unchanged. Assessment confirmed no clinical evidence of new added vitreous hemorrhage in either eye, previously treated seafans and traction scars (white arrows) were less aggressive and altered blood resolved. Left eye had a new sprouting seafan (not in photo view). Patient received additional sector laser to Left eye new seafan; scheduled for routine review in 6 months, earlier if experienced episode of unsettling floaters.
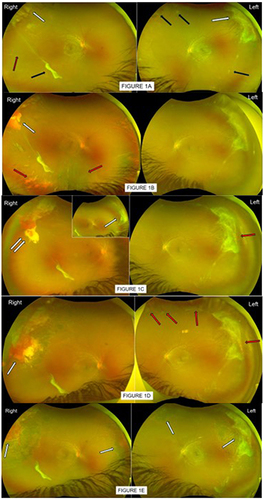
Over the course of 1 year, patient experienced recurrence vitreous hemorrhage in his right eye from existing traction scars with no retinal detachment and no new active seafan, worse recorded vision over this period had been 6/60 ( and ). Left eye vitreous hemorrhage happened later, bled from traction as well as from new active seafans ( and ). Barrier laser photocoagulations were first performed to each tractional seafan fibrotic scars in supero-temporal peripheral, to reduce the risk of retinal detachment in future traction bleeds. At the last clinic review seven months following the initial laser treatment, although old vitreous hemorrhage still to resolve, but patient reported no further re-bleeds in his right eye, vision recovered well and maintained at Snellen 6/7 in each eye. Additional laser treatment was repetitively performed at each clinic visit to any observed new seafans or residual activeness of treated seafans.
Patient 2 has sickle genotype SC, at aged 12 and asymptomatic, optician referred with queried right eye retinal detachment and left eye retinal lesions. Initial assessment confirmed excellent Snellen vision at 6/5 despite evidence of advanced fibrosed seafan neovascularization on traction and significant vitreous hemorrhage in his Right eye. His left eye had less severe tractional seafan neovascularization, a salmon patch but no vitreous hemorrhage (). Maculae were normal. With some obscured view from vitreous bleed, limited barrier laser treatment was nevertheless performed on the tractional seafans in Right eye.
Figure 2 (A) Patient 2 first attended eye casualty in 2020 December, aged 12 having referred by optician for queried Right eye retinal detachment, left odd retinal lesion. Patient was asymptomatic. Assessment confirmed excellent vision 6/5 each eye, both eyes had auto-infarcted seafans scars on traction (white arrows). Right eye had vitreous hemorrhage (red arrows), flat retina. Left eye lesion previously queried by optician was a salmon patch in mid-temporal retina (black arrow). Patient was referred to specialist sickle eye clinic for management. (B) In 2021 April clinic assessment, vision remains good at 6/5. Both eyes had auto-infarcted seafans on traction as previously seen but also numerous smaller new sprouts of seafans (white arrows, better seen on slit-lamp). Right eye still limited view but clearer as old vitreous hemorrhage gravitated, flat retina, no fresh bleed. Left eye had no vitreous hemorrhage, previous salmon patch in mid-temporal had resolved, seen as a round shadowy mark (black arrow), revealing no underlying seafan. There were other smaller salmon patches in resolution (grey arrows). Patient was advised and received limited barrier laser to Right eye tractional seafan (supero-temporal) to reduce risk of retinal detachment in future traction bleeds, and additional sector laser around new sprouting seafans. Left eye was to be observed. (C) Patient 2 reattended 4 months later with fresh bleed in Right eye giving hazy fundus view, localized supero-temporal traction bleed was heavier (red arrow). Both eyes maintained good vision 6/6 as center macula was clear, retinae were also flat. Left eye numerous small seafans on traction, some were larger in size, but no evidence of vitreous hemorrhage. Patient was advised and received sector/barrier laser treatment to Left eye seafans but additional laser treatment to Right eye was not possible at this visit due to poor view. (D) Having missed a few appointments, patient was re-examined 10 months later (2022 June), maintained good vision 6/6 and improved Right eye floaters. Fundoscopy of Right eye however remained a struggle although improved; previous supero-temporal tractional seafan scar appeared more extensive and elevated (white arrow), another resolved salmon patch shadow (black arrow) was seen in nasal retina. Left eye had new active seafans, not yet elevated (white arrows) around previous resolved salmon patch; previous treated lesions appeared adequate, no evidence of vitreous hemorrhage yet in this eye. Left eye received further sector laser; Right eye was to await for further vitreous hemorrhage clearing to allow any possible effective laser top-up. (E) Patient re-attended clinic in 2023 September, having missed a few appointments, attended eye casualty once in 2023 June with further Right eye bleed. Vision was still good 6/7 Right, 6/5 Left. Right eye status relatively unchanged with some new bleed, old bleed slow to resolve; previous supero-temporal traction was mimicking localized retinal detachment but no subretinal fluid and not advancing. Left eye continued to have few more new seafans and residual active tip of an inadequately lasered seafan (white arrow), other adequately treated seafans resolved, no vitreous hemorrhage. Left eye received further sector laser. Plan for Right eye was to perform heavier barrier laser to supero-temporal traction as soon as view was clearer. Consideration for vitrectomy was discussed if rebleed and tractional detachment worsen.
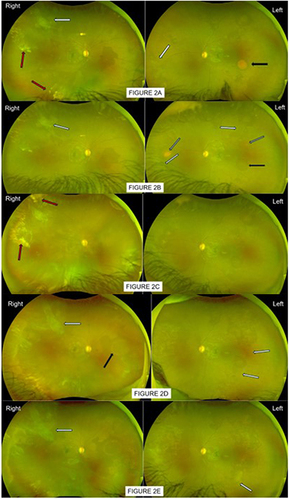
The salmon patch in left eye resolved in 4 months, with more new salmon patches developed in different parts of the retina of the same eye (). Over the period of 2.5 years, patient 2 frequently missed scheduled clinic visits (attendance rate = 45%). There were recurrent vitreous hemorrhage in Right eye with worst recorded visual level of counting fingers from his right eye when he eventually re-attended eye casualty. However, patient’s right eye vision did improve to normal at 6/6 at most clinic visits despite dense vitreous hemorrhage which was slow to clear obscuring most of peripheral retina view ( and ). Additional laser to Right eye was hence not possible due to non-clearing peripheral hemorrhage. However, barrier laser/sector laser were performed more easily to Left eye when new sprouting seafans were found at each clinic visit, before any vitreous hemorrhage happened (). Keeping good vision of 6/7 right, 6/5 left, lacking window opportunity for effective laser treatment through the unclearing peripheral vitreous hemorrhage, patient eventually developed a localized peripheral tractional retinal detachment in his right eye, which had enough surrounding barrier laser to be secured and non-progressive at the last clinic visit ().
Discussion
Previous published literature confirmed that children with SCD could develop proliferative sickle retinopathy but severe blinding disease is rarely reported in children with sickle cell.Citation5,Citation7 Our case reports confirmed that sickle cell children as young as age 12 could have severe advanced sickle retinopathy. Indeed, the severe retinopathy was already manifested in the first clinic examination, indicating that retinopathy development must have started years before. Visual level is a poor indication to reflect severity of sickle retinopathy. Majority of sickle cell patients (both adults and children) have good vision with no symptoms until retinopathy is too advanced causing symptoms of floaters or visual loss due to vitreous hemorrhage as in our case reports. This is due to sickle retinopathy disease clinical signs and features manifest predominantly in the far peripheral retina, hence often undetected by standard imagings used by optician. With the availability of advanced fundus imagings in recent years, in particular, the non-invasive wide-field fundus photography provides easy and clearer detection of peripheral sickle retinopathy disease.Citation8 We therefore speculate the prevalence of severe sickle retinopathy in children could be much higher than previously reported.Citation7,Citation9
Decision to treat sickle retinopathy is perplexing for clinicians due to lack of published literature and lack of clinical intervention treatment trials. Most published studies on treatments were based on sickle adult patients but not on sickle paediatric cohort. Although laser photocoagulation was regarded as the preferred choice to treat sickle retinopathy, guidance is unclear on applying the many different types/patterns of laser photocoagulation described.Citation5 Although seafans could undergo auto-infarction and become inactive, their localized fibrosis often lead to tractional scars; traction bleeds contribute towards recurrent vitreous hemorrhage and the risk of retinal detachment. We described effective laser treatment to paediatric sickle retinopathy as demonstrated in our patients: “sectorial laser” is best applied when active seafan lesion is still small and not yet elevated, sector laser application is a ring of confluent laser around a seafan lesion (similar to delivering an effective “retinopexy” laser treatment for a retinal hole) (); “barrier laser” is best applied at the border of an elevated fibrosed seafan scar on traction (), to hope to reduce risk of retinal detachment with future traction bleeds.
Decision on optimal effective treatment in young patients remain a challenge due to few factors. Delivering effective laser required good cooperation of patients in which case, both our 2 school boys coped very well with the out-patient slit-lamp lasering procedures at their young age of 13. Understandably, attending scheduled clinic visits would be unlikely priorities in their busy schooling activities, when they also benefited more time of good vision than period affected by bad vision as vitreous hemorrhage seem to clear faster in center than in peripheral. In addition, good vision and painless nature of sickle retinopathy often render patients unaware of seriousness of the eye disease, subsequently denying the opportunity of optimal treatment window and eventuality of disease progression. We nevertheless had clinic reviews arranged during school holiday weeks to encourage needed attendances.
The other challenging factor is getting children to recognize their visual symptoms. Children are less likely to report to their parents or teachers unless the disease causes symptoms in both eyes; unilateral visual changes is often ignored. Herein, retinopathy screening at an early age could help eliminate late assessment from delayed reporting of symptoms. Published literature had suggested retinopathy screening to start as young as age 9 in children with SCD.Citation3,Citation9 Our case reports would support this concept as our 2 patients had already presented with advanced tractional scars at age 12 and 13, hence speculating proliferative sickle retinopathy development would indeed have started at a much earlier age.
Conclusion
In summary, children as young as age 12 can progress to develop advance sickle retinopathy if untreated. As in the adults cohort, severe sickle retinopathy in children is preventable if there is an early window of recognition and optimal treatment opportunity. Our case reports detailed management and laser treatment for paediatric sickle retinopathy which is very much lacking in available literature. Optimal management plan for this young age group remains a challenge, taking into consideration of schooling timing and cooperation of individual child for laser treatment. Our case-reports nevertheless provide some evidence of the need to start sickle retinopathy screening programme at earlier school age, perhaps inco-operating into the existing visual screening programme for childhood amblyopia/childhood squints (at around school age of 5 years old). With the easily available, non-invasive advanced investigational tool such as ultra-widefield fundus photography, sickle retinopathy screening programme at younger age could indeed be very feasible and effective. This prospect also leads us to the much needed research and studies that can better understand sickle retinopathy treatment choices and effectiveness in both adults and paediatrics.
Ethics Approval and Consents for Publication
This ethical approval for this retrospective case series report was granted from the Institutional Review Board (Sandwell and West Birmingham Research and Development review board) in accordance with the “Good Clinical Practice” regulations in the United Kingdom and adhered to the tenets of the Declaration of Helsinki. Informed written parental consents were obtained from all paediatric patients for investigations/treatment procedures as part of the routine and standard clinical care in our real-world clinical practice. Relevant fundus photography and angiographic images were anonymized with patients’ consents/approvals to publish.
We also obtained from parents of our two paediatric patients on specific consents/approvals for this article and relevant fundus photography images to be used for publication.
Disclosure
The authors received no funding for this work, and declared no conflicts of interest related to this work.
References
- Van Meurs JC. Relationship between peripheral vascular closure and proliferative retinopathy in sickle cell disease. Graefes Arch Clin Exp Ophthalmol. 1991;229(6):543–548. doi:10.1007/BF00203319
- Downes SM, Hambleton IR, Chuang EL, et al. Incidence and natural history of proliferative sickle cell retinopathy: observations from a cohort study. Ophthalmology. 2005;112:1869–1875. doi:10.1016/j.ophtha.2005.05.026
- Babalola OE, Wambebe CO. When should children and young adults with sickle cell disease be referred for eye assessment? Afr J Med Med Sci. 2001;30(4):261–263.
- Penman AD, Serjeant GR. Recent advances in the treatment of proliferative sickle cell retinopathy. Curr Opin Ophthalmol. 1992;3(3):379–388. doi:10.1097/00055735-199206000-00013
- Myint KT, Sahoo S, Thein AW, Moe S, Ni H. Laser therapy for retinopathy in sickle cell disease. Cochrane Database Syst Rev. 2015;2015(10):CD010790. doi:10.1002/14651858.CD010790.pub2
- Goldberg MF. Classification and pathogenesis of proliferative sickle cell retinopathy. Am J Ophthalmol. 1971;71(3):649–665. doi:10.1016/0002-9394(71)90429-6
- Children With Sickle Cell Disease Appear to Suffer Eye Complications at Same Rate as Adults, American Academy of Ophthalmology, Available from: https://www.aao.org/newsroom/news-releases/detail/children-with-sickle-cell-disease-appear-to-suffer. Accessed April 18, 2024.
- Pahl DA, Green NS, Bhatia M, Chen RWS. New ways to detect pediatric sickle cell retinopathy: a comprehensive review. J Pediatr Hematol Oncol. 2017;39(8):618–625. doi:10.1097/MPH.0000000000000919
- Li J, Bender L, Shaffer J, Cohen D, Ying GS, Binenbaum G. Prevalence and onset of pediatric sickle cell retinopathy. Ophthalmology. 2019;126(7):1000–1006. doi:10.1016/j.ophtha.2019.02.023
