Abstract
We report the case of a 53-year-old man who was diagnosed with Holmes tremor and underwent deep brain stimulation of the ventro-intermediate thalamic nucleus and posterior subthalamic area. We assessed the patients’ tremor with the Fahn-Tolosa-Marin Tremor Rating Scale at 1, 3, 6, 12 and 24 months after deep brain stimulation. Deep brain stimulation relieved the patient’s tremor during the 24-month follow-up period.
Introduction
Tremor caused by midbrain diseases was first reported by Benedikt in 1889. The first Holmes tremor (HT) case was described in detail by Holmes in 1904.Citation1 This tremor is often secondary to nervous system diseases, such as stroke, brain trauma, neurodegenerative diseases, and brain tumors, and the onset time is mostly 1~24 months after central nervous system injury. Because the onset is closely related to injury to the midbrain and thalamus, it is also called midbrain tremor and thalamus tremor. HTs are irregular often nonrhythmic and characterized by resting, postural, and intentional low-frequency (<4.5 Hz) tremors involving the proximal and distal parts of the extremities. HTs seriously affect the quality of life of patients, but the effect of drug treatment is not ideal. Most patients do not respond to medication; therefore, surgical procedures have become the main treatment option.Citation2 The posterior subthalamic (PSA) tract containing Zona incerta (Zi) and premature radiation (raprl) have been previously resected for tremor control. It is believed that the Cerebello-thalamic junction of the PSA is related to tremor, and the somatotopic organization of the PSA can especially improve the control of proximal tremors. In regard to stimulation, the results published in the literature show that the therapeutic effect on the PSA may be equivalent to that on the ventro-intermediate thalamus (VIM) or even better than that on the VIM. Testing the PSA is especially useful for case series of patients with severe proximal tremors and has been found to have beneficial effects. The VIM is located on the back side of the PSA, allowing access to two structures through a single electrode. In fact, a review of the relationship between the VIM lead position and tremor control showed that more ventral contact can better control tremors, which is more likely to stimulate the PSA and achieve better tremor control than does thalamic stimulation. HTs are rare, especially those that occur secondary to brainstem hemorrhage. The clinical data of a patient with HT secondary to brainstem hemorrhage who underwent deep brain stimulation (DBS) and experienced significantly relieved upper limb tremor and improved quality of life. This case can provide guidance for clinical workers. Thus, neurologists can consider DBS for patients with HT.
Case Report
A 53-year-old man with a 4-month history of tremor affecting mostly his left upper limb was referred to our department. He had developed left limb numbness secondary to hypertensive brainstem hemorrhage 1 year prior. At that time, brain computed tomography (CT) revealed acute hemorrhage in the right pons near the red nucleus ( and ). Two weeks after brainstem hemorrhage, magnetic resonance imaging (MRI) revealed that the brainstem hemorrhage was located in the right pontine arm and that it destroyed local brain tissue ( and ).
Figure 1 Image of a right pontine hemorrhage near the red nucleus. (A and B) Preoperative computed tomography image showing acute intracerebral hemorrhage in the right pons near the red nucleus. (C and D) MR image showing that the brainstem hemorrhage was located in the right pontine arm. (E and F) Preoperative T2-weighted and T1-weighted axial magnetic resonance images revealing the resolution of hemorrhage. Encephalomalatic changes, cystic degeneration, and hemosiderin around the lesion were also absorbed.
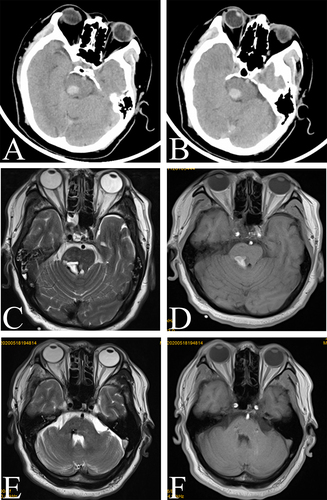
Approximately 5 months after conservative management and physical therapy, the patient was able to care for and feed himself. After approximately 6 months, the tremor gradually returned to the left upper limb and progressively worsened. At that time, axial T2-weighted MR images revealed encephalomalatic changes, cystic degeneration, and hemosiderin around the lesion ( and ).
He was treated with several medications, including levodopa/benazepine and trihexyphenidyl, but none of them were effective. He had no history of using neuroleptics or tremor-inducing drugs prior to or after brainstem hemorrhage and had no family history of movement disorders. Quantitatively analyzed the results of the Fahn-Tolosa-Marin Tremor Rating Scale (FTMTRS) to assess the degree of tremor. The FTMTRS is a widely used tremor assessment tool that assesses tremor position (Part A), movement (Part B) and function (Part C). The patient’s FTMTRS score was 56, and he could not draw the spiral of Archimedes.
According to the imaging examination, signs and symptoms, and medical history, the patient was initially diagnosed with HT.
We decided to use the VIM+PSA as a double target. Needle design: Through the planning system, the puncture path through the VIM nucleus and PSA was designed, so that the electrode is placed at the PSA area as the target point and passes through the VIM nucleus at the same time.
Surgical Procedure
Unilateral electrode implantation for DBS was performed under local anesthesia using a Leksell stereotactic frame and computed tomography/magnetic resonance imaging fusion-guided targeting with a Leksell SurgiPlan (ELEKTA Instruments AB, Stockholm, Sweden). A permanent quadripolar DBS electrode (Model 3387s, Medtronic, Minneapolis, MN, USA) was implanted.
The electrode was implanted under local anesthesia. During the operation, a microelectrode recording needle was used to record the electrical signal at 10 mm above the nucleus of the VIM. When it reached the VIM nucleus, the typical tremor cell discharge was recorded, and when it passed through the VIM nucleus and continued to travel deep, no signal area was recorded. After satisfactory positioning, the microelectrode recording needle was removed, the stimulation electrode was implanted, and temporary tests were carried out during the operation to ensure that the front two contacts of the stimulation electrode were located in the PSA and that the other two contacts were located in the VIM nucleus. We identified the ventral thalamic border and tremor cells inside the VIM. After confirming that the tip of the microelectrode reached the right PSA target and passed through the VIM, the microelectrode was removed, the permanent electrode (Model 3387S, Medtronic, Minneapolis, MN, USA) was placed, and intraoperative test stimulation was performed to verify tremor control in the absence of stimulation-induced side effects. During the operation, the tremor symptoms in the patient’s left upper limb were obviously relieved without obvious side effects. Postoperative CT was performed, and the images were merged with the preoperative MR images to verify the location of the electrode. The results showed that the electrode position was accurate, and the electrode passed through both the VIM and PSA simultaneously (see ).
Figure 2 (A–C) The fusion results of preoperative MR and postoperative CT show that the position of the deep brain stimulation electrode was accurate, and the electrode passed through both the VIM and PSA simultaneously.
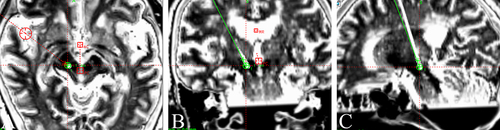
Furthermore, in merging with the atlas, we confirmed that the distal electrodes (contacts 0 and 1) were positioned at the PSA and the proximal electrodes (contacts 2 and 3) at the VIM. The DBS electrode was connected subcutaneously to a pulse generator (IPG, Activa RC, Medtronic, Minneapolis, MN, USA) that was implanted in the infraclavicular area under general anesthesia.
Clinical Outcomes
The patient experienced a remarkable reduction in tremor from the microlesion effect. Stimulation was initiated 1 month after surgery. The parameters were titrated to provide optimal tremor control without side effects. The initial DBS setting had an amplitude of 1.9 V, a pulse width of 90 μs, and a frequency of 130 Hz with the electrode configuration using contact 0-negative and C+ positive. When stimulation was applied through the most effective contact, the FTMTRS score decreased to 11. The patient did not exhibit any stimulation-induced side effects at the time of discharge. Due to the patient’s gradual tolerance to stimulation, in the next 24 months, the severity of tremor decreased and then slightly increased. During the follow-up period, the FTMTRS score only increased to 21 points, with an amplitude of 2.4 V, a pulse width of 90 μs and a frequency of 160 Hz.
Discussion
We reported a meaningful case of HT treated with double-target DBS, hoping to enlighten neurologists. HT is an extrapyramidal disease that is mostly caused by pathological changes in the brainstem, cerebellum and thalamus, among which the thalamus is the most frequently involved region.Citation3 Its pathogenesis is still unclear, and it is generally believed that the cerebellum-red nucleus-thalamus pathway and substantia nigra-striatum pathway are damaged.Citation4 The diagnostic criteria refer to the tremor consensus formulated by the International Association for Dyskinesia in 1998:Citation5 1) static and intentional tremor, often accompanied by postural tremor; 2) usually lower than 4.5 Hz; and 3) late onset, more than 1~24 months after the primary disease. It has the following characteristics:Citation6 1) it mostly involves the proximal muscles of limbs; 2) it cannot be relieved by itself, and levodopa or receptor agonists may be effective; 3) the primary lesion is located between the thalamus and midbrain; and 4) PET-CT may show a decrease in 18F-DOPA uptake in the ipsilateral putamen and caudate nucleus. In addition, HT is a manifestation of midbrain red nucleus syndrome, which is often complicated by limb hemiplegia, oculomotor nerve paralysis, and hypertrophic olivary nucleus degeneration.Citation7
The main internal medicine used for treating HT is drug therapy, including levodopa, decarboxylation of peripheral DOPA, enzyme inhibitors, benserazide, dopamine agonists, piribedil, antiepileptic drugs, clonazepam, central cholinergic receptor blockers, phenoxide, and botulinum toxin injection.Citation8
The correct selection and location of DBS targets is the key to successful operation.Citation9 At present, the commonly used targets for DBS are the STN, GPi and VIM. Among them, STN and Gpi mainly improve the clinical symptoms (resting tremor, myotonia and bradykinesia) of PD patients.Citation10
The VIM is invisible on the image, and it is mainly located by coordinates.Citation11 X: 12–14 mm, 11 mm beside the wall of the third ventricle. Y: PC point 5–6 mm forward, 1/5th point behind the AC-PC connection. Z: 1–2 mm under AC‒PC. Imaging positioning of the PSA:Citation12 The first horizontal line was constructed at the horizontal center of the maximum diameter of the red nucleus, the second line (STN axis) was drawn from the foremost inner side to the rearmost outer side of the STN, and the midpoint between the lateral margin of the red nucleus and the medial margin of the STN was determined. The empirical target coordinates of the PSA were as follows:Citation13 X: 10.8–13.4 mm from the midline. Y: 4.7–7.9 mm after MCP; Z: 0.7–5.3 mm after MCP.
VIM has the most obvious therapeutic effect on tremor.Citation14 However, clinically, we found that the curative effect of VIM-DBS on tremor decreased over time:Citation15 1) resistance to electrical stimulation; 2) non-benign progression; and 3) side effects such as balance problems and dysarthria.
The anatomical structure of the posterior subthalamic region (PSA) includes the following:Citation16 the caudal Zi (cZi), the pallidothalamic white matter, and anterior radiation (Raprl). Therapeutic effect of PSA-DBS:Citation17 PSA can improve tremor, rigidity, bradykinesia, a stiff pace and abnormal posture in PD patients and can also significantly relieve symptoms such as movement fluctuations. The side effects of PSA-DBS,Citation18 including dysarthria (cerebellopontine thalamic fiber involvement), paresthesia (medial colliculus), balance disorder, ataxia, muscle contraction (suspected internal capsule involvement, abnormal muscle tone), and transient or short-term side effects, are mostly avoidable by program control.
According to many references, from three aspects,Citation19 namely, effective control of tremor, fewer side effects, such as balance, dysarthria, etc., and long-term effectiveness, we finally decided to adopt VIM+PSA double targets. Needle design:Citation20 Through the planning system, the VIM nucleus and PSA are designed at the same time, and then the electrode puncture path is designed so that the electrode passes through the VIM nucleus and the PSA at the same time.
The results of using VIM DBS alone in HT patients are not satisfactory.Citation21 For HT patients, larger-area stimulation or multilead implantation may be necessary.Citation22
In the process of double-target design, attention should be given to avoiding the sulcus, ventricle and intracranial blood vessels.Citation23 It is not necessary to force two targets to pass through at the same time during the operation, so PSA is recommended as the first choice.Citation21 Intraoperative electrophysiological monitoring and intraoperative temporary testing are effective ways to determine the target.Citation24 In the next 24 months, the tremor severity in our patient decreased and then slightly increased.
Conclusions
PSA-DBS is an effective way to treat HT. Neurologists may consider PSA-DBS for HT. Through the double-target surgical design, there may be more contact options during postoperative program control.
Ethics Statement
Because of intractable tremor, the patient’s hand tremors are severe, and he is unable to hold objects and signs. Written informed consent was obtained from the patient’s family for publication of this case report. All procedures were performed in accordance with the Helsinki Declaration. This case report was approved by the Institutional Ethics Committee of Shengli Oilfield Central Hospital (approval number: Q/ZXYY-ZY-YWB-LL202205), and the paper submission was approved by Shengli Oilfield Central Hospital.
Disclosure
Zonglei Chong and Xiaoqian Yang are co-first authors for this study. Hongxing Li and Yilei Xiao are co-correspondence authors for this study. The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Shimada T, Uchida W, Shindo A, Kamagata K, Hattori N, Tsunemi T. Delayed-onset motor aphasia succeeds Holmes’ tremor and neuropathic pain after left thalamic hemorrhage. J Neurol Sci. 2021;423:117367. doi:10.1016/j.jns.2021.117367
- Woo JH, Hong BY, Kim JS, et al. Holmes tremor after brainstem hemorrhage, treated with levodopa. Ann Rehabil Med. 2013;37(4):591–594. doi:10.5535/arm.2013.37.4.591
- Maesawa S, Torii J, Nakatsubo D, et al. A case report: dual-lead deep brain stimulation of the posterior subthalamic area and the thalamus was effective for Holmes tremor after unsuccessful focused ultrasound thalamotomy. Front Human Neurosci. 2022;16:1065459. doi:10.3389/fnhum.2022.1065459
- Zhao H, Shi M, Yang F, Yang D, Hou X, Yang X. Damage to the central nucleus of the thalamus via atypical Holmes tremor: a case report. J Int Med Res. 2021;49(4):300060521999567. doi:10.1177/0300060521999567
- Wang Q, Aljassar M, Bhagwat N, et al. Reproducibility of cerebellar involvement as quantified by consensus structural MRI biomarkers in advanced essential tremor. Sci Rep. 2023;13(1):581. doi:10.1038/s41598-022-25306-y
- Latorre A, Hallett M, Deuschl G, Bhatia KP. The MDS consensus tremor classification: the best way to classify patients with tremor at present. J Neurol Sci. 2022;435:120191. doi:10.1016/j.jns.2022.120191
- Schaller-Paule MA, Baumgarten P, Seifert V, et al. A paravermal trans-cerebellar approach to the posterior fossa tumor causes hypertrophic olivary degeneration by dentate nucleus injury. Cancers. 2021;13(2):258. doi:10.3390/cancers13020258
- Patil S, Mishra VS, Yadav N, Reddy PC, Lochab B. Dendrimer-functionalized nanodiamonds as safe and efficient drug carriers for cancer therapy: nucleus penetrating nanoparticles. ACS Appl. Bio Mater. 2022;5(7):3438–3451. doi:10.1021/acsabm.2c00373
- Gouveia FV, Diwan M, Martinez RCR, Giacobbe P, Lipsman N, Hamani C. Reduction of aggressive behaviour following hypothalamic deep brain stimulation: involvement of 5-HT(1A) and testosterone. Neurobiol Dis. 2023;183:106179. doi:10.1016/j.nbd.2023.106179
- Fernandez-Delgado E, De la cruz-martinez F, Galan C, et al. Pt(II) and Pd(II) complexes with a thiazoline derivative ligand: synthesis, structural characterization, antiproliferative activity and evaluation of pro-apoptotic ability in tumor cell lines HT-29 and U-937. J Inorg Biochem. 2020;202:110870. doi:10.1016/j.jinorgbio.2019.110870
- Yin Z, Ma Z, Wang S, et al. Expression and tissue distribution analysis of VIM and TTR proteins associated with coat colors in sheep (Ovis aries). Animal Biosci. 2023;36(9):1367–1375. doi:10.5713/ab.23.0111
- Hara T, Terakawa T, Okamura Y, et al. Real-world analysis of metastatic prostate cancer demonstrates increased frequency of PSA-imaging discordance with visceral metastases and upfront ARAT/docetaxel therapy. Prostate. 2023;83(13):1270–1278. doi:10.1002/pros.24588
- Li H, Cai Q, Wang J, Jie G. Versatile FeMoOv nanozyme bipolar electrode electrochemiluminescence biosensing and imaging platform for detection of H(2)O(2) and PSA. Biosens Bioelectron. 2023;232:115315. doi:10.1016/j.bios.2023.115315
- Lueckel JM, Upadhyay N, Purrer V, et al. Whole-brain network transitions within the framework of ignition and transfer entropy following VIM-MRgFUS in essential tremor patients. Brain Stimulation. 2023;16(3):879–888. doi:10.1016/j.brs.2023.05.006
- Paoli D, Mills R, Brechany U, Pavese N, Nicholson C. DBS in tremor with dystonia: VIM, GPi or both? A review of the literature and considerations from a single-center experience. J Neurol. 2023;270(4):2217–2229. doi:10.1007/s00415-023-11569-6
- Nkwoada AU, Onyedika G, Oguzie E, Ogwuegbu M. Development of PSA@PS-TiO(2) nanocomposite photocatalyst: structure, mechanism, and application using response surface designs and molecular modeling. Wat Sci Technol. 2023;87(11):2701–2726. doi:10.2166/wst.2023.148
- Liote F, Constantin A, Dahan E, Quiniou JB, Frazier A, Sibilia J. A prospective survey on therapeutic inertia in psoriatic arthritis (OPTI’PsA). Rheumatology. 2023;2:1.
- Rombach I, Tillett W, Jadon D, et al. Treating to target in psoriatic arthritis: assessing real-world outcomes and optimising therapeutic strategy for adults with psoriatic arthritis-study protocol for the MONITOR-PsA study, a trials within cohorts study design. Trials. 2021;22(1):185. doi:10.1186/s13063-021-05142-7
- Okinaka Y, Kageyama S, Nishizawa K, et al. Clinical, pathological, and therapeutic features of newly diagnosed prostate cancer predominantly detected by opportunistic PSA screening: a survey of Shiga Prefecture, Japan. Prostate. 2021;81(15):1172–1178. doi:10.1002/pros.24212
- Saini V, Kaur T, Kalotra S, Kaur G. The neuroplasticity marker PSA-NCAM: insights into new therapeutic avenues for promoting neuroregeneration. Pharmacol Res. 2020;160:105186. doi:10.1016/j.phrs.2020.105186
- Kamo H, Oyama G, Ito M, Iwamuro H, Umemura A, Hattori N. Deep brain stimulation in posterior subthalamic area for Holmes tremor: case reports with review of the literature. Front Neurol. 2023;14:1139477. doi:10.3389/fneur.2023.1139477
- Pozzilli V, Marano M, Magliozzi A, et al. Deep brain stimulation of the dentato-rubro-thalamic tract in a case of Holmes tremor: a constrained spherical deconvolution (CSD)-guided procedure. Neurol Sci. 2023;44(1):411–415. doi:10.1007/s10072-022-06514-w
- Shen J, Marsili L, Dwivedi AK, et al. Does head tremor predict postural instability after bilateral thalamic stimulation in essential tremor? Cerebellum. 2022;22(5):1039–1044. doi:10.1007/s12311-022-01477-2
- Hou X, Mo Y, Zhu Z, et al. Technical issues of Vim-PSA double-target DBS for essential tremor. Brain Sci. 2023;13(4):566. doi:10.3390/brainsci13040566
