Abstract
This case report describes the case of a female infant hospitalized for severe pneumonia. During the treatment process, various antibiotics are used to treat and prevent further infection. The child had a weak physical condition, combined with neuroblastoma, paraneoplastic syndrome, and low immune function, leading to Tsukamurella tyrosinosolvens infection. The treatment was eventually abandoned owing to poor prognosis. This study aims to through the medium, dyeing, electron microscope observation, 16s rRNA and high-throughput sequencing investigated the morphological characteristics, staining properties, electron microscope morphology, antibiotic resistance, and genomic characteristics of Tsukamurella tyrosinosolvens. The aim of the study is to provide data reference for clinical laboratory staff in bacteria identification research, and to provide relevant help for clinicians in diagnosis and treatment.
Introduction
Tsukamurella tyrosinosolvens is an aerobic, weakly acid-resistant, gram-positive, non-motile, rod-shaped bacterium that exists in various environments. It belongs to the order Actinomycetales and is a conditional pathogen that mainly infects patients with impaired immune functions. Human infections caused by this bacterium are less frequently reported and are often misdiagnosed as Mycobacterium tuberculosis infections. Matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF MS) and sequencing of 16S ribosomal RNA (16S rRNA) play important roles in identifying this bacterium. This unusual case deserves the attention of doctors who should pay more attention to patients with low immune function to prevent further infections and improve patient prognosis.
Case
A two-month-old girl was transferred from a local hospital to the pediatric ICU of a tertiary hospital because of cough and wheezing for 10 days, which worsened after six days. The child had a history of asphyxia and weak physical condition. She was diagnosed with bronchial pneumonia and was hospitalized for seven days at another hospital.
On admission, the infant had an acute facial appearance, with anhidrosis on the left side of the face, hyperhidrosis on the right side, tachypnea, and three depressions sign. After oral ibuprofen administration and physical cooling, the body temperature decreased slowly. When fever was high, the patient experienced convulsions, cough, wheezing, and diarrhea. Computed tomography (CT) performed at another hospital revealed a high-density shadow in the right lower lung and a large soft tissue mass in the posterior mediastinum. Enhanced CT tomography revealed a space-occupying lesion in the posterior mediastinum. Combined with the results for neuron-specific enolase (71.72 ng/mL), the possibility of neuroblastoma was considered. The patient subsequently underwent mediastinal tumor resection under general anesthesia, and the surgery was successful. The child had persistent symptoms, such as fever, convulsions, muscle spasms, and muscle weakness, postoperatively. After the relevant examinations, paraneoplastic syndrome was considered, and isotretinoin was administered orally to induce tumor differentiation.
The child had multiple infections during hospitalization. After admission, the result of the Mycoplasma pneumoniae nucleic acid test of the throat swab was 4.024×E5Copies/mL, indicating the presence of Mycoplasma pneumoniae infection. Subsequent bacterial culture of the tracheal tube tip suggested hemolytic Staphylococcus, indicating the presence of bacterial infection. TheCitation1–3 -β-D-glucan test was 160.79pg/mL, and Aspergillus galactomannan test was 0.35ug/L, indicating fungal infection. To control and prevent further infection, a variety of antibiotics, such as cefotaxime sodium, ceftriaxone, ceftazidime, cefoperazone sulbactam sodium, azithromycin, vancomycin, meropenem, linezolid, voriconazole, and metronidazole, are used throughout the hospital stay. During the third month of hospitalization, the patient’s sputum smear showed a large number of gram-positive bacilli outside the white blood cells on Gram staining () and weak acid-fast staining was positive (). The sputum culture grew dry, rough, branched, and slightly yellow-pigmented colonies on Columbia Blood Agar Base Medium ( and ). MALDI-TOF MS of Merieux VITEK MS identity the colonies as Tsukamurella species (), and the DNA (Ezup column bacterial genomic DNA extraction kit, sangon Biotech, Shanghai) of the bacterial strain was extracted for 16S rRNA sequencing (sangon Biotech, Shanghai), and the base pair matching of the sequencing results showed that the Query Cover 99%, E value 0.0, per.ident 99.80%. After sequencing and comparing the results, the colony from the sputum was determined to be Tsukamurella tyrosinosolvens, which was subsequently cultured from the tracheal apex catheter of the patient. MALDI-TOF MS compared with 16S rRNA, time and cost advantages, but can only be identified to species level, and there is a certain error detection rate.Citation4 Unfortunately, despite active treatments, such as anti-infection, immune regulation with gamma globulin, methylprednisolone pulse therapy, intravenous nutritional support, and invasive ventilator-assisted ventilation, the patient’s family required abandonment of treatment due to her critical condition and threatened life. The patient was discharged with a stomach tube during the third month of hospitalization.
Figure 1 (A) The Gram stain of the specimen was a cluster of blue-purple, indicating that the organism was Gram-positive bacilli. (B) Weak acid-fast staining was red, indicating that the bacteria was positive for weak acid-fast staining. (C) Dry, rough, and branched bacterial colonies with a little yellowish pigment appeared on the blood agar plate. (D)Under the microscope, the colony morphology was clear.
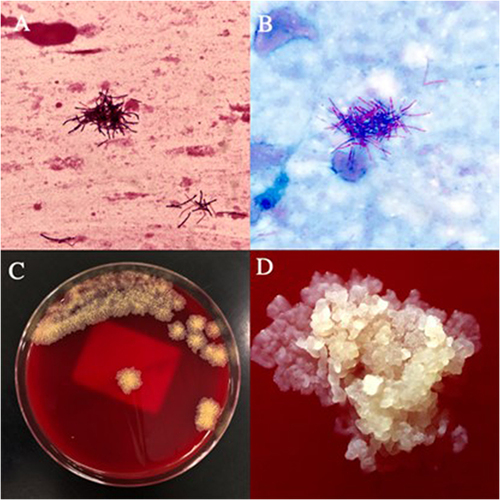
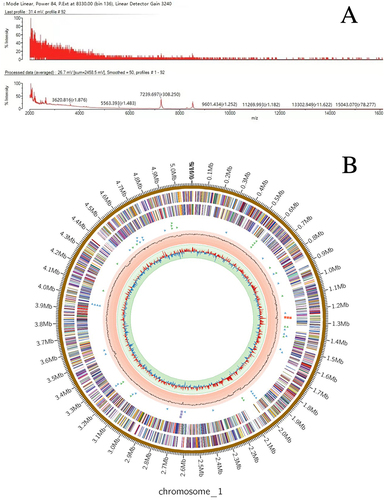
Figure 2 (A) The MALDI-TOF MS spectra. (B) Genome circle map: The first circle shows the size of the genome. The second circle shows the genes predicted by the Forward strand (+) of the genome and the third circle shows the genes predicted by the Reverse strand (-) of the genome. The fourth circle triangle shows the tRNA (the forward and reverse strands are green and blue, respectively), and square represents rRNA (the forward and reverse strands are red and purple, respectively). The fifth circle represents GC Content, and the sixth circle represents GC skew.
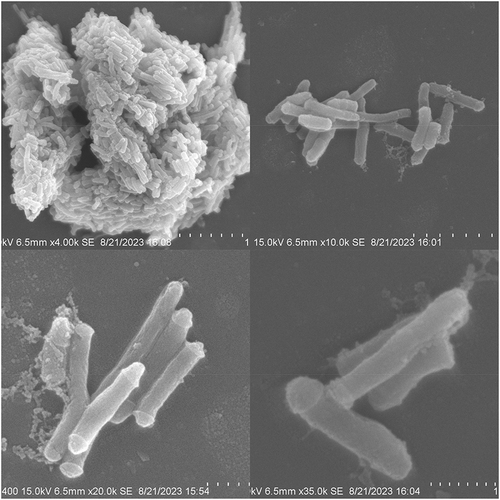
Tsukamurella tyrosinosolvens is an aerobic, weakly acid-fast, non-motile, gram-positive bacterium that colonizes various environments and can infect immunocompromised patients. Confusions are easily made when distinguishing between Actinomycetes such as Mycobacterium tuberculosis and Tsukamurella tyrosinosolvens. After culturing pure colonies of this strain, antimicrobial susceptibility testing and the gradient diffusion method were performed (). DNA of the strain was extracted and sent for high-throughput sequencing (Uniteomics, Tianjin). Basic genomic information was also analyzed. Based on the genomic information, a constructed genome circle map described the relational and multidimensional data (). The pure colonies were subjected to scanning electron microscopy to further explore their morphology characteristics () (Xiangya Medical College of Central South University).
Table 1 Results of Pathogen Susceptibility Test (Gradient Diffusion Method)
Discussion
Tsukamurella species are strictly aerobic, weakly acid-fast, and non-spore-forming Gram-positive non-motile bacilli that do not produce aerial hyphae.Citation1 These are saprophytic bacteria that belong to the order Actinomycetes and exist in various environments such as soil, water, and sludge.Citation2 As opportunistic pathogens, human infections with this bacterium are rare. It infects immunocompromised patients and is associated with immunocompromised diseases (malignant tumors, post-chemotherapy, AIDS, and chronic renal failure) and intravascular catheters.Citation3 It has been isolated from human specimens and is associated with pneumonia, catheter-related blood infections, eye infections, meningitis, peritonitis, skin infections, bacteremia, and septicemia.Citation3,Citation5
Tsukamurella species are often confused with other actinomycetes, especially Mycobacterium tuberculosis, because of their similarities. It is difficult to accurately identify Tsukamurella species using phenotypic methods only, Standard laboratory methods are also hard for identifying Tsukamurella species from others.Citation5 Therefore, infections caused by Tsukamurella species are underestimated in China, the second-largest TB country in the world.Citation6,Citation7 The initial positive result of weak acid-fast staining and microscopic observation of a rod-shaped organism with short branches before MALDI-TOF MS spectrometry or sequencing may be helpful in the identification of Tsukamurella species.Citation8 Sequencing of 16S ribosomal RNA (16S rRNA), the essential secretory protein-encoding gene secA (the secretion ATPase), rpoB (beta-subunit of DNA-dependent RNA polymerase), groEL (60 kDa chaperonin and heat shock protein), and the transfer messenger RNA coding gene ssrA (stable small RNA) can be used to assist in the rapid and reliable identification of Tsukamura species.Citation4 Metagenomic next-generation sequencing (mNGS) as a new type of pathogen detection method in the complicated and plays an important role in a rare infectious diseases. The mNGS sequencing can also be considered if conditions permit, which can comprehensively analyze the pathogens in vivo and improve the diagnostic rate.Citation9
In this study, the E-test method was used to evaluate the sensitivity of Tsukamurella tyrosinosolvens. Interpretation of the CLSI M24-A2 guidelineCitation10 for nocardia and other aerobic actinomycetes interprets that amoxicillin-clavulanic acid, tobramycin and doxycycline were intermediary; Amikacin, ceftriaxone, ciprofloxacin, clarithromycin, imipenem, linezolid, minocycline, moxifloxacin, trimethoprim-sulfamethoxazole, cefepime, and cefotaxime were susceptible (). According to a recent drug susceptibility study of multiple strains of Tsukamurella species, Tsukamurella species isolates in vitro showed high sensitivity to quinolones, trimethoprim/sulfamethoxazole, amikacin, minocycline, linezolid, and tigecycline.Citation4
Another study showed that imipenem, amikacin, ciprofloxacin, and cotrimoxazole appear to be the most effective antibiotics in vitro, but as a opportunistic pathogen, the generally accepted optimal treatment of Tsukamurella species infections has not been established, mainly caused by the rare infectious cases and the lack of standard drug susceptibility methods for Tsukamurella species isolates.Citation11
High-throughput sequencing was performed using Tsukamurella tyrosinosolvens. Genomic analysis was performed after the sequencing results qualified for assembly quality control. The assembled genome size of this strain was 5,100,639 bp (G+C% 70.9). Prokka software was used to predict genes from the assembly results of the samples. Prodigal predicted 5089 CDS, Aragorn predicted 59 tRNA, and Barrnap predicted six rRNA. Research on drug resistance genes in the genome of Tsukamurella tyrosinosolvens using ResFinder has shown no drug resistance-related genes, and there are no conclusive reports or literature indicating the presence of any specific virulence factor in this genus.Citation5
In summary, this study reports a case of pulmonary infection caused by Tsukamurella tyrosinosolvens in an infant who eventually withdrew treatment. Molecular methods can accurately identify this pathogen and avoid the misdiagnosis of Mycobacterium tuberculosis and other actinomycete infections. Drug susceptibility testing is helpful in the treatment of Tsukamurella infections, and improve patient prognosis. In this case, the patient was treated with a variety of antibiotics for infection prevention during hospitalization, but she was still infected with Tsukamurella tyrosinosolvens strain in the later stage. As opportunistic pathogenic bacteria are sensitive to antibiotics, the mechanism of infection is worth further exploration. We consider it maybe because the body dysbacteriosis caused by long-term use of antibiotics, and then infected with the bacteria. Whether there are some differences in the drug sensitivity of Tsukamurella tyrosinosolvens in vivo and in vitro maybe needs to be further explored. This unusual case is worthy of clinicians’ attention, and clinicians should pay more attention to patients with weakened immunity to avoid further infection as much as possible.
Conclusion
Tsukamurella species are weakly acid-fast gram-positive bacteria that mainly infect immunocompromised patients. However, its prevalence is underestimated in China because it is easily confused with other Actinomycetes, especially Mycobacterium tuberculosis. The special growth morphology on the culture medium and the weak acid-fast staining positive can indicate the next direction. The early detection of pathogens can be determined by MALDI-TOF MS and 16S rRNA sequencing analysis, and mNGS sequencing can also be considered if conditions permit. No drug resistance genes have been identified, and the bacterium is sensitive to a variety of antibiotics. However, the generally accepted optimal treatment of Tsukamurella species infections has not been established.
Ethical Consideration
The paper is a study of medical records and biological specimens obtained in the course of previous clinical diagnosis and treatment, and it is difficult to obtain informed consent. The paper does not involve the privacy and personal identity information of patients, and will not affect the health and rights of patients. We have applied to the Medical Ethics Committee of Hunan Provincial People’s Hospital for exemption of informed consent, and we have obtained ethical approval from the Medical Ethics Committee of Hunan Provincial People’s Hospital for the publication of this case. In the absence of patient informed consent to publish the case details, the Editor-in-Chief has reviewed the manuscript and waived this requirement.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas, they took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Data Sharing Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, accession number PP916630 (16S rRNA), PRJNA1126077 (High-throughput sequencing).
Additional information
Funding
References
- Schwartz MA, Tabet SR, Collier AC, et al. Central venous catheter-related bacteremia due to Tsukamurella species in the immunocompromised host: a case series and review of the literature. Clin Infect Dis. 2002;35(7):e72–e77. doi:10.1086/342561
- Harrington SM, Bell M, Bernard K, et al. Novel fastidious, partially acid-fast, anaerobic Gram-positive bacillus associated with abscess formation and recovered from multiple medical centers. J Clin Microbiol. 2013;51(11):3903–3907. doi:10.1128/JCM.01497-13
- Usuda D, Tanaka R, Suzuki M, et al. Obligate aerobic, gram-positive, weak acid-fast, nonmotile bacilli, Tsukamurella tyrosinosolvens: minireview of a rare opportunistic pathogen. World J Clin Cas. 2022;10(24):8443–8449. doi:10.12998/wjcc.v10.i24.8443
- Yu S, Ding X, Hua K, et al. Systematic investigation of the emerging pathogen of Tsukamurella species in a Chinese tertiary teaching hospital. Microbiol Spect. 2023;11(6):e0164423. doi:10.1128/spectrum.01644-23
- Safaei S, Fatahi-Bafghi M, Pouresmaeil O. Role of Tsukamurella species in human infections: first literature review. New Microb New Infect. 2017;22:6–12. doi:10.1016/j.nmni.2017.10.002
- Dong Z, Wang QQ, Yu SC, et al. Age-period-cohort analysis of pulmonary tuberculosis reported incidence, China, 2006-2020. Infect Diseases Poverty. 2022;11(1):85. doi:10.1186/s40249-022-01009-4
- Yang L, Cao Y, Dan Z, Wang Z, Wang X. Community-acquired Tsukamurella pneumonia in a young immunocompetent adult: a case misdiagnosed as pulmonary tuberculosis and literature review. Postgraduate Med. 2017;129(6):563–566. doi:10.1080/00325481.2017.1344513
- Liu X, Shi J, Wang X, Chen Y, Zheng L, Tsukamurella pneumonia misdiagnosed as pulmonary tuberculosis. Lancet. 2022;22(7):1090. doi:10.1016/S1473-3099(22)00134-7
- Fourgeaud J, Regnault B, Ok V, et al. Performance of clinical metagenomics in France: a prospective observational study. Lancet Microbe. 2024;5(1):e52–e61. doi:10.1016/S2666-5247(23)00244-6
- Woods GL, Brown-Elliott BA, Conville PS, et al. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes. 2nd ed. Clinical and Laboratory Standards Institute; 2011.
- Leroy AG, Persyn E, Guillouzouic A, et al. Catheter-related bloodstream infection due to Tsukamurella pulmonis identified by MALDI-TOF spectrometry, 16S rRNA gene sequencing, and secA1 gene sequencing in an immunocompromised child: a case report and literature review. Diagn Microbiol Infect Dis. 2020;97(3):115052. doi:10.1016/j.diagmicrobio.2020.115052