Abstract
Granulocytic sarcoma (GS) is a rare extramedullary manifestation of acute myeloid leukemia (AML). It may also represent blastic transformation of myelodysplastic syndromes or myeloproliferative neoplasms. Although usually seen in the context of advanced and poorly controlled disease, it may also present as the first manifestation of illness, without concurrent bone marrow or blood involvement. In the medical literature, chloroma and GS are terms that have been used interchangeably with myeloid sarcoma. GS usually manifests as soft tissue or bony masses in several extracranial sites, such as bone, periosteum, and lymph nodes; involvement of the head and neck region is uncommon. We report a case of a woman with insidious onset of progressive nasal congestion and diminished hearing who was diagnosed with an isolated GS of the nasopharynx. With involved field radiotherapy, she achieved a complete remission of 12-months duration before being diagnosed with overt AML. She has remained disease-free for greater than 18 months following induction and consolidation chemotherapy. Through a MEDLINE®/PubMed® search we identified an additional 13 cases of nasopharyngeal GS. The median age was 37 years (range 1 to 81 years). The cases were equally distributed among the sexes. The most common presenting symptoms were conductive hearing loss and sinonasal congestion. Isolated GS was identified in six cases, and the median time from diagnosis of GS to AML was 12 months (range 3 to 48 months). The treatment varied, but responses were seen in all the patients who received chemotherapy with or without radiotherapy.
Introduction
Acute myeloid leukemia (AML) may occur in a variety of extramedullary (EM) tissues, with or without bone marrow disease. Two well-known EM manifestations of AML are granulocytic sarcoma (GS) and leukemia cutis. GS, also known as myeloid sarcoma or chloroma, is a rare EM tumor of immature myeloid cells.Citation1 The high expression of myeloperoxidase (MPO) makes these tumors appear green hence, the name “chloroma” (from the Greek “chloros,” meaning green).Citation2 GS can develop de novo or concurrently with AML, myeloproliferative neoplasms (MPNs) or myelodysplastic syndromes (MDSs).Citation1,Citation3–Citation6 Isolated GS defined by the absence of a history of AML, MDS, or MPN, and unremarkable blood and bone marrow analyses has been described, albeit sparsely.Citation7–Citation9 GS, most commonly see in the context of widespread and uncontrolled disease, may also be the first manifestation of AML, antedating it by months or years, or represent the initial manifestation of relapse in a patient previously treated for AML.Citation10,Citation11 The risk factors for GS include specific chromosomal abnormalities, such as translocations between chromosomes 8 and 21 (t[8;21]) and inversion of chromosome 16 (inv[16]), the expression of cell-surface markers (cluster of differentiation [CD]56, CD2, CD4, and CD7), and the French–American–British (FAB) classification M2, M4, and M5 leukemia subtypes. Additional risk factors include poor nutritional status, cellular immune dysfunction, high presenting leukocyte count, and decreased blast Auer rods.Citation12 GS is slightly more common in men than in women (male to female ratio, 1.42:1).Citation13 While various body sites have been associated with GS, the most common locations include soft tissue, bone, periosteum and the lymph nodes.Citation9,Citation12 Here, we describe the case of a female with GS manifesting as a nasopharyngeal mass that was treated with radiation therapy, after which AML manifested 12 months later. We also review the literature of previously reported cases of nasopharyngeal GS and focus on the clinical presentation, diagnosis, and the treatment of this disorder.
Case report
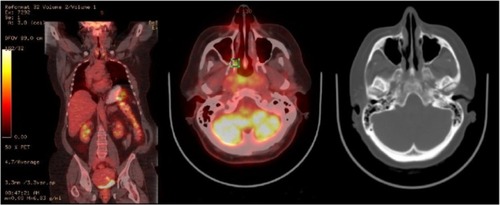
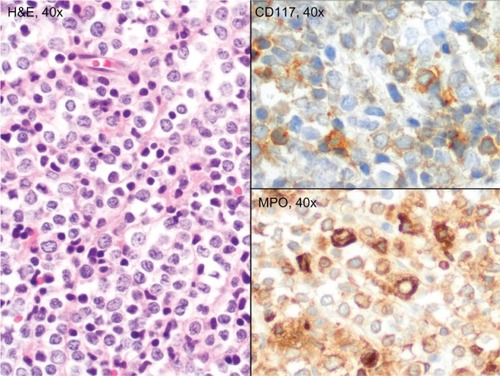
A 63-year-old Caucasian female sought evaluation in the Otolaryngology clinic (Virginia Mason Medical Center, Seattle, WA, USA) due to decreased hearing in her left ear and progressive nasal congestion. Prior to that visit, she had been treated empirically for several months with various antibiotics and decongestants and briefly with intranasal steroids. Her chronic medical problems were notable for nonallergic rhinitis, hypertension, hyperlipidemia, and morbid obesity. Her general and cranial nerve exam were unremarkable, but she was identified as having conductive hearing loss due to a left middle ear effusion. On sinonasal endoscopy, an excoriated mass was also identified, involving the posterior nasopharynx and nasal turbinates. A biopsy of the mass revealed intermediate-sized blastic cells that stained positive for MPO, CD68 (dim), CD99, and CD117 (dim), consistent with involvement by GS (). The results of the laboratory studies included a normal complete blood count (CBC) as well as unremarkable serum chemistry and liver enzyme levels. A flow cytometry study of the circulating white cells was also unremarkable, as were the polymerase chain reaction (PCR) studies of peripheral blood for the breakpoint cluster region (BCR)-Abelson (ABL) gene rearrangements and Janus kinase 2 (JAK-2) mutations. A bone marrow biopsy showed no evidence of AML or features of an MPN; normal trilineage hematopoiesis was present. A chromosome analyses showed an XX karyotype. An integrated whole body positron emission tomography–computed tomography (PET-CT) scan showed only modest inflammation in the nasopharynx (). Because of her age and comorbid medical conditions, we elected to treat her GS with involved-field radiation therapy to the nasopharynx, 30 Gy delivered in 15 daily fractions. She tolerated the radiation therapy well, with alopecia involving the vertex of her scalp and mild mucositis, fatigue, and dysgeusia. Subsequently, she was monitored closely, with exams of her nasopharynx coupled with blood studies every 2 to 3 months. Twelve months after her initial diagnosis of isolated GS, the patient presented to clinic with fever and cough of a week’s duration and complained of recrudescent fatigue and dyspnea on exertion. On exam, she appeared acutely ill, dehydrated, febrile, and diaphoretic, and with diminished breath sounds and crackles at the left lung base. A chest radiograph showed findings consistent with left lower lobe pneumonia. Her CBC consisted of a white blood count (WBC) of 11.8 × 109/L with 62% myeloid blasts, hematocrit of 23%, and platelets of 117 × 109/L. The flow cytometric analysis of peripheral blood revealed an abnormal population of blasts (CD45-dim) that expressed CD117 (moderate) and bright uniform CD33; this population comprised approximately 80% of the circulating WBCs. The tumor cells were negative for human leukocyte antigen (HLA)-DR, CD34, CD13, CD14, CD15, CD16, CD64, CD2, CD3, CD4, CD5, CD7, CD56, CD71, and CD38. The phenotype was similar to the phenotype of the myeloid blasts identified in her prior nasopharyngeal biopsy. Band karyotyping of the peripheral blood blasts showed normal (46, XX) cytogenetics. Induction chemotherapy was prescribed, consisting of cytarabine (100 mg/m2/day, days 1–7) and idarubicin (13 mg/m2/day, days 1–3).Citation14 Her clinical course was notable for mild mucositis, fluctuating blood sugars, and culture-negative neutropenic fever. A bone marrow aspirate and biopsy obtained on day 14 was markedly hypocellular; a repeat bone marrow biopsy on day 28 was normocellular, with maturing trilineage hematopoiesis and no residual leukemia. She went on to receive an additional three cycles of consolidation therapy, with continuous infusion cytarabine and bolus idarubicin (5+2 regimen).Citation14 She remains in remission, with a normal CBC at the 18-month follow-up visit.
Figure 1 Biopsy of the nasopharyngeal mass, showing sheets of intermediate-sized blasts with round nuclei, dispersed chromatin, distinct nucleoli, and small amounts of cytoplasm. The tumor cells stained positive for myeloperoxidase and weakly for CD117.
Discussion and literature review
GS is an infrequently diagnosed condition. It is reported in about 2.5%–9.1% of patients with AML.Citation15–Citation17 While it can be solitary or multifocal, most cases manifest as isolated single lesions.Citation1 The most common sites of EM involvement of AML include the skin (leukemia cutis), lymph node, and bone, but other sites, including the female reproductive tract, breast, gut, and testis have been reported. In the head and neck region, GS will usually involve the orbits, and only rarely will it affect the nasopharynx.Citation18 Utilizing a MEDLINE®/PubMed® search, with the key words “granulocytic/myeloid sarcoma,” “chloroma,” “nasopharynx,” and “acute myeloid leukemia” and restricting our search to the English language reports between the years 2000–2013, we identified 13 additional patients between the ages of 1 to 81 years (median age of 37 years) with GS involving the nasopharynx ().Citation4,Citation19–Citation30 The male to female ratio within the group was 1:1. Sinonasal congestion or hearing loss was the reason why most patients sought medical attention. The longest reported disease-free interval from diagnosis was 36 months.Citation4,Citation22 Among the seven cases where the disease-free interval was reported, the median was 18 months (range, 4 to 36 months).
Table 1 Case reports of granulocytic sarcoma involving the nasopharynx
In the absence of circulating myeloblasts, GS is seldom considered in the initial differential diagnosis of a soft tissue mass.Citation10,Citation12 Its similarity, both radiographically and histopathologically, to other small round cell tumors, such as lymphoblastic leukemia, melanoma, Ewing sarcoma, blastic plasmacytoid dendritic cell neoplasm, as well as certain forms of non-Hodgkin lymphoma and benign EM hematopoiesis means that there may be a delay in diagnosis while the clinician waits for supportive diagnostic tests from the pathologist.Citation31 GS typically consists of a diffuse and infiltrative population of myeloblasts and granulocytic cell types. Infrequently, tumors can occur in the setting of trilineage hematopoiesis or erythroid or megakaryocytic precursors, particularly in cases of transformation from MPN. Immunohistochemistry is useful for establishing the diagnosis of GS and can be easier to perform than flow cytometry, which requires fresh tissue.Citation32 Immunohistochemical markers, such as CD68, MPO, lysozyme, and CD43, can assist in differentiating between myeloid and nonmyeloid cells.Citation1 Myelocytic differentiation may also be confirmed by Leder stain (chloroacetate esterase).Citation17 CD117, CD34, and terminal deoxynucleotidyl transferase (TdT) are useful as markers of immaturity. When GS is found in an EM location and in the absence of circulating blasts, a bone marrow biopsy is necessary to rule out concurrent marrow involvement. Cytogenetic abnormalities are seen in roughly 50% of cases and include monosomy 7, trisomy 8, inv(16), t(9;11), deletion (del)(16q), t(8;16), and t(1;11).Citation1,Citation32–Citation37Nucleophosmin (NPM1) mutations have been reported in 15% and FMS-related tyrosine kinase 3 (FLT 3) gene mutations in 20%–30% of GS cases;Citation38,Citation39 however, the clinical significance of NPM1 and FLT3 mutation in this clinical context remains uncertain.Citation38,Citation39
A CT scan is best for evaluating soft tissue GS, and magnetic resonance imaging (MRI) with gadolinium is preferred when GS involves the central nervous system.Citation40,Citation41 A PET scan may be helpful for detecting additional sites of EM AML and also for planning local (eg, radiotherapy) treatment and/or systemic chemotherapy.Citation42 It can also be used for monitoring the response to treatment.Citation32 For patients who achieve a complete remission with treatment, the role of routine monitoring with radiographs has not been established.Citation32
The treatment of GS depends upon patient and disease characteristics. The patient’s age, associated medical comorbidities, clinical symptoms, tumor karyotype, stage, and the extent of disease are important factors to be considered before planning treatment. There are no randomized studies to help guide the treatment choices, and the GS location does not appear to impact survival. Chemotherapy (both systemic and intrathecal), radiation therapy, surgical extirpation, or any combination of these interventions have all been employed (). Remission-induction chemotherapy similar to that used for AML is favored for either isolated GS or GS presenting with concomitant AML.Citation43,Citation44 Significantly longer disease-free intervals without leukemia have been achieved in patients with isolated GS who received systemic chemotherapy rather than surgery or radiation therapy, belying the fact that this is inevitably the first manifestation of a systematic disease.Citation45,Citation46 Surgery is generally reserved for cases with acute symptoms (eg, pain, acute nerve compression), and at times, to obtain an adequate tissue sample following a nondiagnostic fine-needle aspiration.
Table 2 Treatment approaches for granulocytic sarcoma
The role of allogeneic hematopoietic stem cell transplantation (AHSCT) in patients with GS has not been studied prospectively, but improved outcomes with AHSCT have been reported in repeated retrospective analyses.Citation1,Citation47 In a retrospective study of 51 patients with GS, either isolated or associated with AML, the 5-year overall survival was 48%, with no significant differences in outcomes between the two presentations.Citation48 For those patients with GS and graft-versus-host disease, there was a trend toward improved overall survival.
Patients with a history of GS are at risk for relapse, and careful assessments at follow-up appointments are needed as early isolated GS can be asymptomatic.Citation49 A biopsy of any new or suspicious soft tissue or skin abnormality should be performed, and if no circulating blasts are present, a bone marrow biopsy is necessary.Citation32 For patients who relapse after chemotherapy, reinduction chemotherapy may be an option if there has been a long disease-free interval after the initial treatment. For those individuals who are deemed hardy enough and who have an identified HLA match, AHSCT is the preferred consolidative approach. When bone marrow and EM relapse occurs synchronously after AHSCT, survival is very poor and no standard treatment has proven effective, although donor lymphocyte infusions, tapering of immunosuppression, or investigational agents may be considered.Citation50,Citation51
Disclosure
The authors report no conflicts of interest in this work.
References
- PileriSAAscaniSCoxMCMyeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patientsLeukemia200721234035017170724
- KingAA case of chloromaMonthly J Med18531797
- KasaharaSTsurumiHHaraTGotoHMoriwakiHIdiopathic myelofibrosis developing isolated granulocytic sarcoma with der (1;7)(q10;p10) after splenectomy and finally transforming to acute myelogenous leukemiaLeuk Lymphoma2000393–442743311342325
- ImamuraTMatsuoSYoshiharaTGranulocytic sarcoma presenting with severe adenopathy (cervical lymph nodes, tonsils, and adenoids) in a child with juvenile myelomonocytic leukemia and successful treatment with allogeneic bone marrow transplantationInt J Hematol200480218618915481450
- HancockJCPrchalJTBennettJMListinskyCMTrilineage extramedullary myeloid cell tumor in myelodysplastic syndromeArch Pathol Lab Med199712155205239167610
- Cho-VegaJHMedeirosLJPrietoVGVegaFLeukemia cutisAm J Clin Pathol2008129113014218089498
- EshghabadiMShojaniaAMCarrIIsolated granulocytic sarcoma: report of a case and review of the literatureJ Clin Oncol1986469129172423654
- MeisJMButlerJJOsborneBMManningJTGranulocytic sarcoma in nonleukemic patientsCancer19865812269727093465429
- PaydasSZorludemirSErginMGranulocytic sarcoma: 32 cases and review of the literatureLeuk Lymphoma200647122527254117169797
- KrauseJRGranulocytic sarcoma preceding acute leukemia: a report of six casesCancer197944310171021383264
- OhanianMBorthakurGQuintas-CardamaAOcular granulocytic sarcoma: a case report and literature review of ocular extramedullary acute myeloid leukemiaClin Lymphoma Myeloma Leuk2013131939623017332
- ByrdJCEdenfieldWJShieldsDJDawsonNAExtramedullary myeloid cell tumors in acute nonlymphocytic leukemia: a clinical reviewJ Clin Oncol1995137180018167602369
- García-ArpaMRodríguez-VázquezMMurillo LázaroCCalle PrimoCMyeloid sarcoma in the area of a skin flapActas Dermosifiliogr20111029737739 Spanish21640961
- WiernikPHBanksPLCaseDCJrCytarabine plus idarubicin or daunorubicin as induction and consolidation therapy for previously untreated adult patients with acute myeloid leukemiaBlood19927923133191730080
- WiernikPHSerpickAAGranulocytic sarcoma (chloroma)Blood19703533613694908654
- LiuPIIshimaruTMcGregorDHOkadaHSteerAAutopsy study of granulocytic sarcoma (chloroma) in patients with myelogenous leukemia, Hiroshima-Nagasaki 1949–1969Cancer19733149489554513297
- NeimanRSBarcosMBerardCGranulocytic sarcoma: a clinicopathologic study of 61 biopsied casesCancer1981486142614377023656
- NohBWParkSWChunJEKimJHKimHJLimMKGranulocytic sarcoma in the head and neck: CT and MR imaging findingsClin Exp Otorhinolaryngol200922667119565030
- SelvarajanSSubramanianSThulkarSKumarLGranulocytic sarcoma of nasopharynx with perineural spread along the trigeminal nerveNeurol India200856221021218688157
- MeiKDLinYSChangSLMyeloid sarcoma of the cheek and the maxillary sinus regionsJ Chin Med Assoc201376423523823557893
- BassichisBMcClayJWiatrakBChloroma of the masseteric muscleInt J Pediatr Otorhinolaryngol2000531576110862926
- AuWYKwongYLHoWKShekTWPrimary granulocytic sarcoma of the nasopharynxAm J Hematol200167427327411443644
- NayakDRBalakrishnanRRajGPillaiSRaoLManoharCGranulocytic sarcoma of the head and neck: a case reportAm J Otolaryngol2001221808311172221
- GeisseMMallGFritzeDGartenschlägerMGranulocytic sarcoma of the tonsils associated with myelodysplastic syndromeDtsch Med Wochenschr20021275026732676 German12481238
- PradesJMAlaaniAMosnierJFDumollardJMMartinCGranulocytic sarcoma of the nasal cavityRhinology200240315916112357718
- OzçelikTAliROzkalemkaşFA case of granulocytic sarcoma during complete remission of acute myeloid leukemia with multiple masses involving the larynx and nasopharynxKulak Burun Bogaz Ihtis Derg200311618318815567934
- SugimotoYNishiiKSakakuraMAcute myeloid leukemia with t(8;21)(q22;q22) manifesting as granulocytic sarcomas in the rhinopharynx and external acoustic meatus at relapse after high-dose cytarabine: case report and review of the literatureHematol J200451848914745436
- FerriEMinottoCIannielloFCavaleriSArmatoECapuzzoPMaxillo-ethmoidal chloroma in acute myeloid leukaemia: case reportActa Otorhinolaryngol Ital200525319519916450777
- TeramotoHMiwaHPatelVGene expression changes in a patient presenting nonleukaemic nasal granulocytic sarcoma to acute myelogenous leukaemia using 40 K cDNA microarrayClin Lab Haematol200628426226616898967
- ChoSFLiuYCTsaiHJLinSFMyeloid sarcoma mimicking nasopharyngeal carcinomaJ Clin Oncol20112925e706e70821747080
- NguIWSinclairECGreenawaySGreenbergMLUnusual presentation of granulocytic sarcoma in the breast: a case report and review of the literatureDiagn Cytopathol2001241535711135470
- BakstRLTallmanMSDouerDYahalomJHow I treat extramedullary acute myeloid leukemiaBlood2011118143785379321795742
- SwerdlowSHCampoEHarrisNLWHO Classification of Tumours of Haematopoietic and Lymphoid Tissues4th edLyonIARC Press20082
- ZhangXHZhangRLiYGranulocytic sarcoma of abdomen in acute myeloid leukemia patient with inv(16) and t(6;17) abnormal chromosome: case report and review of literatureLeuk Res201034795896120116851
- HagemeijerAHählenKSizooWAbelsJTranslocation (9;11) (p21;q23) in three cases of acute monoblastic leukemiaCancer Genet Cytogenet198252951056950808
- DacharyDBernardPLacombeFAcute myeloid leukemia with marrow hypereosinophilia and chromosome 16 abnormalityCancer Genet Cytogenet1986203–42412463455867
- BernsteinRPintoMRSpectorIMacdougallLGA unique 8; 16 translocation in two infants with poorly differentiated monoblastic leukemiaCancer Genet Cytogenet19872422132203466674
- FaliniBLenzeDHasserjianRCytoplasmic mutated nucleophosmin (NPM) defines the molecular status of a significant fraction of myeloid sarcomasLeukemia20072171566157017443224
- Ansari-LariMAYangCFTinawi-AljundiRFLT3 mutations in myeloid sarcomaBr J Haematol2004126678579115352981
- FritzJVogelWBaresRHorgerMRadiologic spectrum of extramedullary relapse of myelogenous leukemia in adultsAJR Am J Roentgenol2007189120921817579173
- KookHHwangTJKangHKKimSHKimJHSpinal intramedullary granulocytic sarcoma: magnetic resonance imagingMagn Reson Imaging19931111351378423716
- StölzelFRölligCRadkeJ18F-FDG-PET/CT for detection of extramedullary acute myeloid leukemiaHaematologica201196101552155621685468
- TsimberidouAMKantarjianHMEsteyEOutcome in patients with nonleukemic granulocytic sarcoma treated with chemotherapy with or without radiotherapyLeukemia20031761100110312764375
- LanTYLinDTTienHFYangRSChenCYWuKPrognostic factors of treatment outcomes in patients with granulocytic sarcomaActa Haematol2009122423824619887783
- YamauchiKYasudaMComparison in treatments of nonleukemic granulocytic sarcoma: report of two cases and a review of 72 cases in the literatureCancer20029461739174611920536
- ImrieKRKovacsMJSelbyDIsolated chloroma: the effect of early antileukemic therapyAnn Intern Med199512353513537625623
- AvniBRundDLevinMClinical implications of acute myeloid leukemia presenting as myeloid sarcomaHematol Oncol2012301344021638303
- ChevallierPMohtyMLioureBAllogeneic hematopoietic stem-cell transplantation for myeloid sarcoma: a retrospective study from the SFGM-TCJ Clin Oncol200826304940494318606981
- MichelGBouladFSmallTNRisk of extramedullary relapse following allogeneic bone marrow transplantation for acute myelogenous leukemia with leukemia cutisBone Marrow Transplant19972021071129244412
- BékássyANHermansJGorinNCGratwohlAGranulocytic sarcoma after allogeneic bone marrow transplantation: a retrospective European multicenter survey. Acute and Chronic Leukemia Working Parties of the European Group for Blood and Marrow TransplantationBone Marrow Transplant19961758018088733701
- SzerJThe prevalent predicament of relapsed acute myeloid leukemiaHematology Am Soc Hematol Educ Program20122012434823233559