Abstract
Myocardial infarction (MI) due to coronary atherosclerosis in young adults is uncommon; rare causes such as cocaine abuse, arterial dissection, and thromboembolism should be considered. A 21-year-old football player, and otherwise healthy African American man, developed chest pain during exercise while bench-pressing 400 lbs. Acute MI was diagnosed based on physical examination, electrocardiography findings, and elevated cardiac enzymes. Coronary arteriography showed a thrombus occluding the proximal left anterior descending artery (LAD). Aggressive antiplatelet therapy with aspirin, clopidogrel, and eptifibatide was pursued, in addition to standard post-MI care. This led to the successful resolution of symptoms and dissolution of the thrombus, demonstrated by repeat coronary arteriography. Five months later, he presented with similar symptoms during exercise after lifting heavy weights, and was found to have another acute MI. Coronary arteriography again showed a thrombus occluding the LAD. No evidence of coronary artery dissection or vasospasm was found. Only mild atherosclerotic plaque burden was observed on both occasions by intravascular ultrasound. A bare metal stent was placed at the site as it was thought this site had acted as a nidus for small plaque rupture and thrombus formation. Elevated serum factor VIII activity at 205% (reference range 60%–140%) was found, a rare cause of hypercoagulability. Further workup revealed a patent foramen ovale during a Valsalva maneuver by transesophageal echocardiography. Both events occurred during weight lifting, which can transiently increase right heart pressure in a similar way to the Valsalva maneuver. In light of all the findings, we concluded that an exercise-related increase in factor VIII activity led to coronary arterial thrombosis in the presence of a small ruptured plaque. Alternatively, venous clots may have traversed the patent foramen ovale and occluded the LAD. In addition to continuing aggressive risk factor modification, anticoagulation therapy with warfarin was initiated with close follow-up.
Introduction
Myocardial infarction (MI) in young adults is rare and only accounts for 3% of coronary heart disease cases; classification falls under atheromatous, nonatheromatous, substance abuse, and hypercoagulable states.Citation1 There are documented cases of early MI from atherosclerosis secondary to dyslipidemia, obesity, diabetes, smoking, and hyperhomocysteinemia; however, these cases generally occur in patients in their mid-30s at the earliest.Citation2
Alternatively, perhaps there is a nonatheromatous process occurring. The patient could have some coronary dissection or compression from previous trauma to the chest in light of a high-impact sport such as football. This has been documented in patients involved in motor vehicle accidents with blunt trauma to the chest.Citation3–Citation5 Perhaps there is a bridging of coronary vessels within actual myocardial tissue that becomes compressed during activity. Maybe there is an anomalous origin of the coronary arteries, resulting in chronic intermittent ischemia and leading to scarring and death with typical chest pain symptoms. Certainly, intense activity such as weight lifting may exacerbate such a condition.Citation6
Moreover, drug use and septic embolism, cocaine, amphetamines, tetrahydrocannabinol (THC), and binge drinking are known to precipitate these events via vasospasm, arrhythmia, and vessel rupture.Citation7 This is one of the more common causes of MI in young adults engaging in risky behaviors that include drug abuse. The final category, hypercoagulable state, is a feature of this case and will be discussed subsequently.Citation8–Citation10
Case presentation
A healthy 21-year-old male African American college football player, with a body mass index (BMI) of 39 and no additional risk factors for coronary artery disease, was admitted to the emergency department. The patient was complaining of a single episode of unrelenting sharp chest pain immediately after he had finished powerlifting 400 lbs in a bench press lift. The chest pain was largely typical: substernal, associated with shortness of breath, exertional, constant, and not musculoskeletal in nature.
The patient reported no known drug allergies and took no medications or supplements. He had no chronic medical problems and both parents were alive without chronic medical problems. There was no history of deep venous thrombosis (DVT), pulmonary embolism, or coronary heart disease in the family. A review of systems was only positive for the above. His vital statistics on admission were within normal limits. The physical exam was largely benign.
Imaging results obtained included chest radiography that only showed cardiomegaly, consistent with an athletic heart. An electrocardiogram showed a sinus rhythm with a right bundle branch block (RBBB) (). A urine toxicity screen was negative for cocaine and central nervous system stimulants. The lipid profile was within normal limits. At the emergency room, blood tests showed elevated serum levels of cardiac enzymes, with troponin at a level of 0.53, which peaked at 10.43 the next day (). Non-ST elevation myocardial infarction was diagnosed and the patient received medical treatment as per acute coronary syndrome protocol, which includes aspirin, a statin, a high intensity intravenous heparin protocol, an angiotensin converting enzyme (ACE) inhibitor, and a beta blocker.
Figure 1 EKG initial presentation.
Abbreviation: EKG, electrocardiogram.
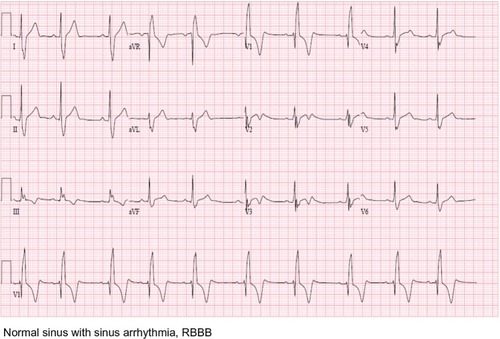
Table 1 Cardiac enzymes
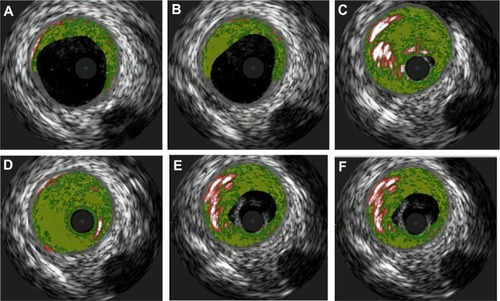
Transthoracic echocardiography (TTE) was performed in the emergency room and was unremarkable. A computerized tomography (CT) angiogram of the chest was negative for aortic dissection. A transesophageal echocardiogram (TEE) showed a very minor patent foramen ovale (PFO) only with a Valsalva maneuver and was not apparent on a Doppler view (). During the Valsalva maneuver, a single bubble became apparent (). There was no aortic dissection. A coronary angiography showed a thrombus in the proximal to mid left anterior descending artery (LAD) in otherwise normal coronary arteries (). Intravascular ultrasound showed evidence of thrombotic material in the mid LAD in addition to mild plaque disease, and no evidence of dissection in the left main, proximal, or mid LAD (). The patient received Integrilin® after coronary angiography. One week after the onset of chest pain, another coronary angiography showed resolution of the LAD thrombus (). The patient was then discharged with aspirin (81 mg daily), clopidogrel (75 mg daily), and atorvastatin (80 mg daily), with advice to avoid strenuous activities such as heavy weight lifting. The patient received six sessions in the cardiopulmonary rehabilitation program before volitional discontinuation.
Figure 2 Echocardiogram TEE PFO.
Abbreviations: PFO, patent foramen ovale; TEE, transesophageal echocardiogram.
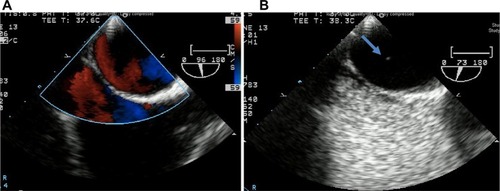
Figure 3 Left heart catheterization.
Abbreviation: LAD, left anterior descending artery.
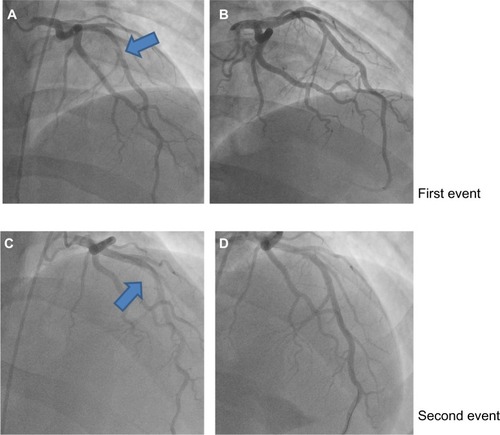
Figure 4A–F Coronary ultrasound showing vessel walls with mild plaque disease and thrombus lodged in vessel walls of LAD appreciated especially in (C) and (D).
Abbreviation: LAD, left anterior descending artery.
Five months later, the patient had another episode of chest pain after heavy weight lifting. This episode was similar to the last, but had less intense, substernal nonradiating pressure without shortness of breath or lightheadedness. Pain was relieved with aspirin, nitroglycerin, and morphine. His vital statistics on admission this time showed some stage II hypertension.
He admitted not taking his medication for several days to a week before this episode of chest pain. The physical exam was again benign. Cardiac enzymes were elevated, with electrocardiography showing marked sinus bradycardia with PAC and existing T wave inversions in the inferior leads ( and ). The patient had a coronary angiography on the day of symptom onset. A thrombus was again found in the mid LAD, but was longer than the thrombus found 5 months previously. It was thought that this site had provided a nidus for thrombus formation via recurrent plaque rupture and previous vessel injury. Hence, a bare metal stent was placed on this occasion, as the patient had a previous thrombus that had recurred at the same location. The rest of the coronary arteries were normal by angiography. Due to the PFO, a hypercoagulable workup was performed. A lower extremity ultrasound did not show any DVT. Results of laboratory studies showed elevated factor VIII activity at 205% (). A decision to put the patient on anticoagulation therapy was made in light of the likely arterial embolus versus paradoxical embolism. He was discharged home with aspirin (81 mg daily), prasugrel (10 mg daily), atorvastatin (80 mg daily), and warfarin (5 mg daily with enoxaparin bridging). The ACE inhibitor was temporarily held because of mild acute kidney injury that was more likely a falsely elevated creatinine secondary to increased muscle mass. A beta blocker was stopped secondary to bradycardia.
Figure 5 EKG second event.
Abbreviations: EKG, electrocardiogram; PAC, premature atrial complex.
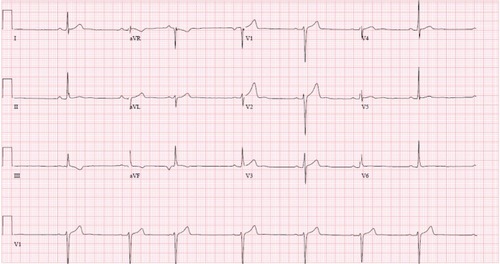
Table 2 Hypercoagulability results
Discussion
Factor VIII is a nonenzymatic plasma protein necessary for blood coagulation to proceed. Deficiency of factor VIII results in a condition named hemophilia A that affects 1/5,000 males. The opposite condition, where there is too much factor VIII available, results in increased risk for thrombosis. Hemophilia A is inherited as an X-linked recessive disease expressed in males, with females as asymptomatic carriers. Factor VIII serves as a cofactor to enzyme 9a in the activation of factor X. Factor VIII must be activated before it can support factor IXa as an effective cofactor. The factor VIII protein contains over 2,300 amino acids and is approximately 330 kDa, preceded by a 19 residue hydrophobic signal peptide that is important for secretion.Citation11
Just as there is a genetic basis to the absence of factor VIII, there is also a genetic component to having increased activity and levels. One study was able to localize high factor VIII levels to genes found on chromosome 5 and chromosome 11.Citation12 Another quantitative trait locus was discovered on chromosome 18.Citation13 Factor VIII is often found in a complex with von Willebrand factor (vWF) in the body. Hence, high factor VIII levels may also be caused by a rise in vWF levels; this could be from either increased synthesis or decreased clearance of vWF.Citation14 As further investigation continues, it will become interesting to determine if these chromosomal locations are involved in the regulation of vWF as well. The genetic basis for increased factor VIII levels is also greatly contingent on blood group types, as blood group non-O has higher levels of vWF and factor VIII than those with group O.Citation15 Our patient has not pursued these genetic tests as he has other risk factors that can elevate factor VIII levels.
In addition, there are several acquired reasons for having high factor VIII levels, and some can be modified by lifestyle. Studies have found that a higher BMI positively correlated with higher factor VIII levels, as well as African descent that is associated with a 15%–18% increase of factor VIII levels above those of Caucasian descent.Citation16 Our patient had a BMI of 39 and is of African descent; hence this is likely to increase his risk. Moreover, factor VIII levels increase with age, with an average rise of 5–6 IU/dL per decade. Exercise can transiently increase factor VIII levels, which are thought to be related to adrenalin and β2 adrenoreceptor stimulation.Citation17 Factor VIII levels are known to increase up to 300% in normal individuals during certain exercise.Citation18 The weight lifting with extreme loads probably transiently increased the patient’s factor VIII levels above his already high baseline. However, it is unclear whether the intensity of the exercise is correlated to the degree of factor VIII released. A rise in factor VIII can be seen in pregnancy, surgery, chronic inflammation, malignancy, liver disease, hyperthyroidism, intravascular hemolysis, and renal disease.Citation19 Females generally have higher factor VIII levels for unknown reasons.Citation20 Higher factor VIII levels were found in postmenopausal women compared to premenopausal.Citation21 Furthermore, vasopressin enhances plasma factor VIII levels indirectly or directly via the v2 receptor.Citation22 Thus, it is likely a good idea for postmenopausal women to remain euvolemic.
Factor VIII is known to increase a patient’s risk for hypercoagulability. One study examined how factor VIII influences risk for MI among young Mexican patients with acute MI. It was again noted that younger patients <45 years accounted for only 5%–10% of acute MI cases. Twenty-five percent of patients had increased factor VIII compared with 8.8% of control subjects. Mean levels for patients and controls were 134 mg/dL versus 118 mg/dL.Citation23 Hence, even slightly elevated factor VIII levels contribute to MI, whereas higher levels are found in the more rare cases of young individuals having coronary events. Another report describes a 27-year-old female that had an MI and was found to have elevated factor VIII without any other hypercoagulability. There was a 90% stenosis of the proximal LAD. The level of her factor VIII was 3.03 IU/mL; the normal range is 0.45–1.58 IU/mL.Citation24
The previously considered idiopathic cases of venous thromboembolism are now having correlations with elevated factor VIII levels. There was a dose-response relationship between subjects with factor VIII levels above 150% (150 IU/dL) and an adjusted odds ratio for venous thromboembolism of 4.8.Citation13 It is also known that there is a 33% prevalence of factor VIII activity above 175% (90th percentile) in recurrent venous thromboembolism and is dose-related.Citation25 Those above the 90th percentile for factor VIII had a 6.7-fold relative risk of recurrent events compared with lower levels.Citation25 It is known that factor VIII levels above the normal 150 IU/dL can be found in 11% of the adult population.Citation26 It is also concerning that there are additive effects to factor VIII and other hypercoagulable states. For instance, oral contraceptives in women with high factor VIII levels increased the risk of venous thromboembolism (VTE) greater than in women taking oral contraceptives alone.Citation10
PFO is a common congenital cardiac anomaly that is estimated to be present in more than 25% of the general population.Citation27 It is an independent risk factor for cerebrovascular events among young adults with cryptogenic stroke.Citation28 Embolic events occurring through the PFO can affect all regions including the brain, extremities, and coronary circulation, depending on which branch of the aorta or sinus a thrombus may enter. Paradoxical coronary artery embolism causing MI with factor V Leiden thrombophilia in a young woman has been documented.Citation9 There have also been associations with PFOs and MI for patients in hypercoagulable states, such as peripartum women.Citation29 A girl as young as 8 years had a PFO combined with a heritable thrombophilia that resulted in MI.Citation8 It is unclear where the initial thrombus in our patient developed – whether in the arterial circulation or the venous circulation with a translocation via a PFO. However, the venous Doppler of lower extremities was negative for DVT.
Our patient was found to have a RBBB on initial presentation. RBBB quite frequently occurs with PFO in healthy people and can even be used as a marker for PFO.Citation30 PFO can be a problem for other recreational sports as well, such as recreational diving, which is becoming more and more popular, and also requires a Valsalva maneuver. Counseling should be provided to adults and adolescents with RBBB when they start high-risk sports or during qualifications for some types of employment.
One last point, we would like to make is that instrumentation poses its own risks to the development of atherosclerosis and plaque rupture, and that further investigation should be performed into preventing this remodeling effect. In previous experiments, substances such as the antioxidant hydrogen sulfide have been used as a means to reduce this remodeling effect.Citation31
Conclusion
We must never discount the cause of chest pain being MI in young adults as there are distinctive mechanisms by which these rare events can and do occur. The question then becomes how to prevent such rare cases as these. Strenuous exercise may possibly be a risk factor for coronary thrombosis because of transiently elevated factor VIII levels. It may be important to further risk stratify patients for physical activity using such factors as BMI, sex, descent, comorbidities, and hypercoagulability studies. As our genetic testing for hypercoagulability becomes both cheaper and more sensitive, it may be reasonable to put into action regular screening against these thrombotic disorders as a means of primary prevention before events occur. In light of the great prevalence of PFO and risks associated with fixing these genetic defects, fixing them should be discussed on an individual basis. Due to these existing limitations, an event tends to occur before this kind of extensive investigation is performed.
Disclosure
The authors report no conflicts of interest in this work.
References
- EgredMViswanathanGDavisGKMyocardial infarction in young adultsPostgrad Med J20058196274174516344295
- DavidsonLWilcoxJKimDBentonSFrediJVaughanDClinical features of precocious acute coronary syndromeAm J Med2014127214014424332726
- CarboneIFranconeMGaleaNBenedettiGFrustaciAImages in cardiology. Computed-tomography and magnetic resonance imaging assessment of traumatic left anterior descending coronary dissection causing acute myocardial infarctionJ Am Coll Cardiol2011572e321211681
- MastrorobertoPDi MizioGColosimoFRicciPOcclusion of left and right coronary arteries and coronary sinus following blunt chest traumaJ Forensic Sci20115651349135121644989
- Al-AqeediRFAliWMAl-AniFAbdulrahmanYSAlnabtiAA blunt chest trauma causing left anterior descending artery dissection and acute myocardial infarction treated by deferred angioplastyHeart Views2011122717322121464
- BassoCMaronBJCorradoDThieneGClinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletesJ Am Coll Cardiol20003561493150110807452
- KandelDBMurphyDKarusDCocaine use in young adulthood: patterns of use and psychosocial correlatesNIDA Res Monogr198561761103932882
- CaranoNAgnettiAHaglerDJTchanaBSquarciaUBernasconiSAcute myocardial infarction in a child: possible pathogenic role of patent foramen ovale associated with heritable thrombophiliaPediatrics20041142e255e25815286265
- CroftAPKhanJNChittariMVVarmaCParadoxical coronary artery embolism causing acute myocardial infarction in a young woman with factor V Leiden thrombophilliaJ R Coll Physicians Edinb201242321822022953315
- BloemenkampKWHelmerhorstFMRosendaalFRVandenbrouckeJPVenous thrombosis, oral contraceptives and high factor VIII levelsThromb Haemost19998231024102710494758
- RotblatFO’BrienDPO’BrienFJGoodallAHTuddenhamEGPurification of human factor VIII:C and its characterization by Western blotting using monoclonal antibodiesBiochemistry19852416429443002413885
- BergerMMattheisenMKulleBHigh factor VIII levels in venous thromboembolism show linkage to imprinted loci on chromosomes 5 and 11Blood2005105263864415353485
- KosterTBlannADBriëtEVandenbrouckeJPRosendaalFRRole of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosisLancet199534589431521557823669
- KamphuisenPWEikenboomJCBertinaRMElevated factor VIII levels and the risk of thrombosisArterioscler Thromb Vasc Biol200121573173811348867
- JeremicMWeisertOGedde-DahlTWFactor VIII (AHG) levels in 1016 regular blood donors. The effects of age, sex, and ABO blood groupsScand J Clin Lab Invest1976365461466981956
- ConlanMGFolsomARFinchAAssociations of factor VIII and von Willebrand factor with age, race, sex, and risk factors for atherosclerosis. The Atherosclerosis Risk in Communities (ARIC) StudyThromb Haemost19937033803858259533
- SmallMTweddelACRankinACLoweGDPrenticeCRForbesCDBlood coagulation and platelet function following maximal exercise: effects of beta-adrenoceptor blockadeHaemostasis19841432622686147300
- RockGTittleyPPipeACoagulation factor changes following endurance exerciseClin J Sport Med19977294999113424
- BloomALThe biosynthesis of factor VIIIClin Haematol1979815377367666
- WellsPSLangloisNJWebsterMAJaffeyJAndersonJAElevated factor VIII is a risk factor for idiopathic venous thromboembolism in Canada – is it necessary to define a new upper reference range for factor VIII?Thromb Haemost200593584284615886797
- BalleisenLAssmannGBaileyJEppingPHSchulteHvan de LooJEpidemiological study on factor VII, factor VIII and fibrinogen in an industrial population – II. Baseline data on the relation to blood pressure, blood glucose, uric acid, and lipid fractionsThromb Haemost19855437217233937270
- KaufmannJEOkscheAWollheimCBGüntherGRosenthalWVischerUMVasopressin-induced von Willebrand factor secretion from endothelial cells involves V2 receptors and cAMPJ Clin Invest2000106110711610880054
- Majluf-CruzAMoreno-HernándezMMartínez-EsquivelNFactor VIII activity among young Mexican patients with acute myocardial infarctionGac Med Mex20081443199206 Spanish18714587
- GorogDARakhitRParumsDLaffanMDaviesGJRaised factor VIII is associated with coronary thrombotic eventsHeart19988044154179875127
- KraaijenhagenRAin’t AnkerPSKoopmanMMHigh plasma concentration of factor VIIIc is a major risk factor for venous thromboembolismThromb Haemost20008315910669145
- BénardELafumaARavaudPEpidemiology of venous thromboembolic diseasePresse Med2005346415419 French15902870
- HagenPTScholzDGEdwardsWDIncidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal heartsMayo Clin Proc198459117206694427
- WebsterMWChancellorAMSmithHJPatent foramen ovale in young stroke patientsLancet19882860111122898621
- RamineniRDanielGKAssociation of a patent foramen ovale with myocardial infarction and pulmonary emboli in a peripartum womanAm J Med Sci2010340432632820827172
- BakalliAKoçinajDGeorgievska-IsmailLBekteshiTPllanaESejdiuBRight bundle branch block as a marker for interatrial septal abnormalitiesCardiol Young2012221182521729501
- VacekTPGillespieWTyagiNVacekJCTyagiSCHydrogen sulfide protects against vascular remodeling from endothelial damageAmino Acids20103951161116920352463
