Abstract
Patients may refuse, be unable to use, or show nonresponse to conventional steroid treatment of multiple sclerosis (MS) exacerbation. Adrenocorticotropic hormone (ACTH), one of several melanocortin peptides with mechanisms of action beyond steroidogenesis, should be reconsidered in the treatment of MS exacerbations. The current case report presents the treatment outcome of a patient with new-onset MS exacerbation treated with ACTH following lack of response to steroid treatment. A 49-year-old female presented with slurred speech, blurry vision, off-balance feeling, and possible left-sided mild internuclear ophthalmoplegia. Magnetic resonance imaging showed findings typical for primary demyelinating disease. Despite 5-day high-dose intravenous methylprednisolone treatment, the patient’s symptoms worsened, including right-sided facial weakness, gait instability that required unilateral support, drooling, and new dorsal pontine white matter lesion on magnetic resonance imaging. Treatment with ACTH gel 80 U for 5 consecutive days resulted in patient functional improvement, including vision and gait. ACTH gel treatment stabilized disease progression, allowing the initiation of long-term disease-modifying treatment with monthly intravenous natalizumab. Effects of melanocortin signaling on immune function and inflammation beyond steroidogenesis provide a basis for understanding the clinical experience with ACTH gel treatment in patients with MS exacerbation.
Introduction
Multiple sclerosis (MS) is a chronic demyelinating and degenerative autoimmune disease of the central nervous system (CNS) resulting from genetic and environmental risk factors. Proposed etiologies include autoimmunity against the CNS, an infectious agent, and a primary degenerative process targeting oligodendrocytes.Citation1 Relapsing-remitting MS (RRMS), the most common subtype, involves episodes of clinical symptom exacerbation separated by periods of full or partial recovery. A relapse is defined as a sustained new or worsening neurologic symptom, in the absence of fever or infection, lasting greater than 24 hours and potentially lasting for days to weeks or even months at a time.Citation2,Citation3 Effective relapse treatment remains a challenge, yet is critical as patients’ level of disability may continue to increase rather than return to baseline following each relapse.Citation4 It is believed that by limiting the severity and frequency of relapses over time, one may also prevent severe disability and disease progression over time.Citation3
To treat the acute disability seen in RRMS as well as hasten recovery from a relapse, MS practitioners have long used corticosteroid therapy. The most common treatment is a 3- to 5-day course of corticosteroids, primarily intravenous (IV) methylprednisolone (MP) or dexamethasone, with or without an oral steroid taper over 1 to 2 weeks.Citation5 High-dose oral prednisone also is sometimes used. These medications can cause deleterious side effects that may limit their use, including stomach irritation, elevation of blood sugar, water retention, restlessness, insomnia, and mood swings, and long-term use is not recommended due to the potential for serious side effects, including stomach ulcers, weight gain, acne, cataracts, osteoporosis, and diabetes. Additionally, at times the patient’s relapse recovery response may not be adequate following treatment with steroids. Treatment with adrenocorticotropic hormone (ACTH) gel, Food and Drug Administration (FDA)-approved to treat MS relapse, is an option for patients who are unable to tolerate the side effects of high-dose IVMP or oral prednisone, do not adequately respond to corticosteroid treatment, do not have access to IV therapy, or who have difficulty accessing veins and receiving IV medication.
ACTH gel (HP Acthar® Gel, repository corticotropin injection; Questcor Pharmaceuticals, Inc., Hayward, CA, USA) is a long-acting formulation of the full sequence ACTH(1–39) that includes other proopiomelanocortin peptides.Citation6,Citation7 In 2010, the FDA reviewed and modernized the entire ACTH gel label alongside granting a new indication for infantile spasms, and following reexamination of the study data submitted in 1978, the MS exacerbation indication was reapproved for treatment of acute exacerbations in adults.Citation7 ACTH gel has been used for several decades to treat acute exacerbations in MSCitation8–Citation12 and has an established safety profile. Randomized, controlled clinical trials examining ACTH gel treatment in acute exacerbations of MS (total of 298 subjects) include two placebo-controlled, double-blind studies and one double-blind comparator trial versus IVMP.Citation8–Citation10 Early placebo-controlled studies of ACTH gel treatment of MS exacerbation demonstrated faster recovery from rapidly worsening MS compared with placebo, and the comparator study with IVMP showed equivalent, marked improvement outcomes.Citation8–Citation10
Interestingly, the use of corticosteroids as the common therapy to treat MS relapse is derived from these early randomized, placebo-controlled studies of ACTH treatment showing rapid improvement of relapse.Citation8,Citation9 The belief that the mechanisms of action and treatment effects of ACTH and high-dose corticosteroids are relatively equivalent in treating MS acute exacerbations is longstanding, with the assumption that the efficacy of ACTH is due to its corticosteroid effects on immune function and inflammation.Citation10,Citation11,Citation13 However, evidence suggests ACTH may have actions independent of steroidogenesis, such as in the treatment of infantile spasms, in which ACTH has been shown effective and corticosteroid treatment has had limited effectiveness.Citation14–Citation17 ACTH is one of several melanocortin peptides, and the emerging characterization of the melanocortin system and receptor functions beyond steroidogenesis indicates the need to reconsider ACTH in the treatment of acute MS exacerbations.Citation7,Citation18
The clinical case presented below describes a patient who, following nonresponse to a trial of IVMP for new-onset MS exacerbation, was effectively stabilized with ACTH gel treatment. Stabilization of the disease exacerbation then allowed the initiation of long-term disease-modifying therapy with monthly IV natalizumab.
ACTH gel patient case
Demographics and medical history
The patient was a 49-year-old right-handed female who worked as a dental hygienist. She presented with a 1-day history of blurry vision, off-balance feeling, and slurred speech. She started having double vision when looking toward the left, stated that “everything seemed fuzzy,” and said she felt as if she needed to hold onto something in order to keep her balance. The patient had no past medical or surgical history and no family history for MS. Diagnosis of new-onset MS exacerbation followed a neurological exam and finding of cerebral spinal fluid positive for six oligoclonal bands, comprehensive metabolic and blood panels to rule out alternative diagnoses, and magnetic resonance imaging (MRI) at baseline and post-IVMP treatment consistent with McDonald 2010 criteria of dissemination of lesions in space (T2 callosal and spinal cord lesions on initial MRI) and time (new T2 dorsal pontine lesion at second MRI).Citation2
Physical and neurological exam
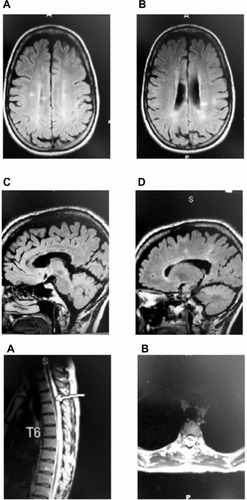
The exam of cranial nerves II–XII showed possible left-sided, mild internuclear ophthalmoplegia (INO). Her gait showed slight ataxia when walking, with some difficulties with heel-toe walking after three steps. MRI testing of the patient’s brain without enhancement showed findings typical for primary demyelinating disease, with several callosal plaques, right greater than left (). No changes were seen in the brainstem or at the cervical level. MRI testing at the thoracic level showed possible demyelinating plaque at T3–T4.
Figure 1 Pretreatment MRI.
Abbreviations: MRI, magnetic resonance imaging; FLAIR, fluid-attenuated inversion recovery.
Treatment
The patient was admitted to the hospital for 5 days of IVMP at 1,000 mg/day. After 3 days, she still had persistent left INO with nystagmus; however, her gait was slightly improved. The patient requested an additional 2 days of treatment as an outpatient, and she was discharged with physical therapy and neurology follow-up within 7 days.
Follow-up neurological exam
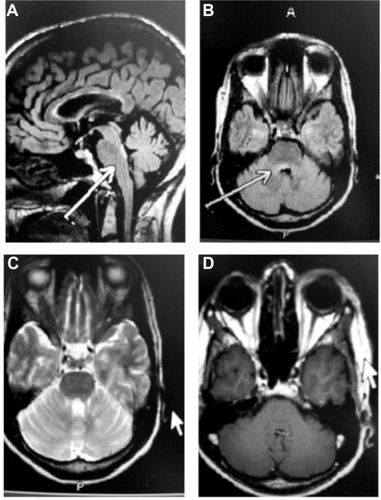
The patient was seen within 7 days of discharge, and despite 5 days of IVMP, she presented to the outpatient neurology MS center with new-onset right facial weakness. The patient also complained of drooling and gait instability. Neurologic exam at this time showed mild bilateral INO and mild-to-moderate right facial weakness affecting the forehead, with slight Bell’s phenomenon. Coordination testing showed mild finger-to-nose ataxia bilaterally, and gait testing showed mild gait instability with difficulty in tandem, and equivocal toes. Herpes simplex virus evaluation was negative. The patient received an urgent MRI scan and spinal fluid analysis. The MRI revealed a new dorsal pontine white matter lesion located slightly eccentric to the right that was consistent with the clinical hypothesis of a demyelinating lesion affecting cranial nerve VII (). The lesion did not show abnormal enhancement, but the radiologist commented that the lesion possibly showed restricted diffusion. The remainder of the brain was unchanged.
Figure 2 MRI following treatment with IVMP for 5 days.
Abbreviations: IVMP, intravenous methylprednisolone; MRI, magnetic resonance imaging.
At a follow-up visit 4 days later, the patient complained of worsening symptoms of right-sided facial weakness, gait instability, and drooling. She now required assistance at times, to walk. The diagnosis given was MS exacerbation. Treatment considerations included the severity of the exacerbation, the brainstem location, and the patient’s acute worsening despite high-dose IVMP. The treatment plan options included natalizumab injection, pulse IV cyclophosphamide, plasma exchange, and ACTH gel. The treatment recommendation was to treat the patient with ACTH gel 80 U subcutaneous injection for 5 consecutive days with the goal to rapidly stabilize disease.
At the follow-up visit 7 days later, the patient had completed her course of ACTH gel and reported improvement in her vision and gait. She reported feeling more steady on her feet but said she still required some walking assistance at times. The patient’s facial weakness was no longer a complete Bell’s-like phenomenon and was significantly improved. The neurologic exam at this time showed mild residual bilateral INO, improved right facial weakness with improved forehead and frontalis musculature strength, and improved asymmetry. The patient had mild finger-to-nose ataxia and some slight gait instability with some difficulty in tandem. The patient showed stabilization of disease exacerbation in response to ACTH gel, despite being an IVMP nonresponder. The patient was in the most severe form of an MS exacerbation, with worsening brainstem presentation. Treatment with ACTH gel stabilized the continued worsening of the patient’s persistent disease progression and actually improved some of the patient’s brainstem symptoms, such as facial weakness and eye movement weakness. The patient was subsequently started on monthly IV natalizumab, approximately 1 to 2 weeks following cessation of ACTH gel, for long-term immune-modulating MS treatment.
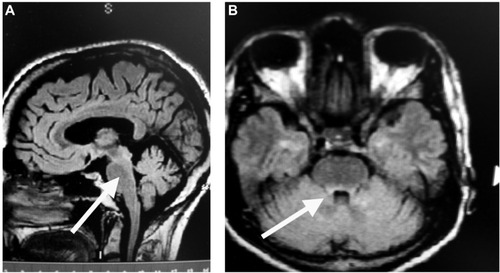
At 5 months follow-up, the patient had returned to baseline function and had been working for 3 months. The patient was doing very well except for some mild loss of energy, and the neurologic exam was normal. MRI testing of the brain revealed findings compatible with the patient’s known history of demyelinating disease; however, the previously described lesion in the dorsal pons was less prominent ().
Figure 3 MRI 5 months following treatment with ACTH gel for 5 days, followed by monthly IV natalizumab.
Abbreviations: ACTH, adrenocorticotropic hormone; IV, intravenous; MRI, magnetic resonance imaging; FLAIR, fluid-attenuated inversion recovery.
Conclusion
Effects of melanocortin signaling on immune function and inflammation provide a basis for understanding the clinical experience with ACTH gel in the treatment of patients with MS relapse.Citation7,Citation18,Citation19 Emerging understanding of the melanocortin system and interaction of ACTH with widespread melanocortin receptors strongly suggests that the mechanisms of action of ACTH gel in MS are not limited to steroidogenesis effects.Citation20,Citation21 Although no studies are currently available examining ACTH gel efficacy in patients with MS exacerbation who are refractory to steroids and other therapies, this has been shown in other disorders, including infantile spasms.Citation14–Citation17 Consistent with the emerging understanding of ACTH gel mechanisms of action, ACTH gel has been proposed within a relapse management treatment algorithm for MS, as a second-line treatment for patients who do not improve or are unable to tolerate steroid treatment.Citation22 In the clinical case that was presented, the patient experienced severe progression of new-onset MS exacerbation with brainstem presentation. Despite 5 days of high-dose IVMP, the patient’s symptoms actually worsened. ACTH gel stabilized the progressive course of the MS exacerbation, was associated with some improvement in vision and gait, and provided valuable time needed to initiate long-term disease-modifying therapy with natalizumab. Patients with RRMS who show nonresponse to conventional IV steroid formulations, or who are unable to tolerate associated side effects, are an important subgroup of patients for consideration of treatment with ACTH gel. Thus, the awareness of a treatment alternative such as ACTH gel, with demonstrated efficacy and suggested mechanisms of action beyond steroidogenesis, is important.
Acknowledgments
The author thanks Lynanne McGuire, PhD, of MedVal Scientific Information Services, LLC, for providing medical writing and editorial assistance. This manuscript was prepared according to the International Society for Medical Publication Professionals’ “Good Publication Practice for Communicating Company-Sponsored Medical Research: The GPP2 Guidelines.”
Funding to support preparation of this manuscript was provided by Questcor Pharmaceuticals.
Disclosure
SN has had speaking and consultancy relationships with the following entities: Questcor Pharmaceuticals, Teva Neuroscience, Biogen, Pfizer, Accorda, Serono, and Novartis. The author reports no other conflicts of interest.
References
- BoppanaSHuangHItoKDhib-JalbutSImmunologic aspects of multiple sclerosisMt Sinai J Med201178220722021425265
- PolmanCHReingoldSCBanwellBDiagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteriaAnn Neurol201169229230221387374
- VollmerTThe natural history of relapses in multiple sclerosisJ Neurol Sci2007256Suppl 1S5S1317346747
- LublinFDBaierMCutterGEffect of relapses on development of residual deficit in multiple sclerosisNeurology200361111528153214663037
- National Clinical Advisory Board (National Multiple Sclerosis Society)Recommendations Regarding Corticosteroids in the Management of Multiple SclerosisNew York, NYNational Multiple Sclerosis Society2008
- HP Acthar® Gel (repository corticotropin) injection [prescribing information]Hayward, CAQuestcor Pharmaceuticals, Inc2012
- ArnasonBGBerkovichRCataniaALisakRPZaidiMMechanisms of action of adrenocorticotropic hormone and other melanocortins relevant to the clinical management of patients with multiple sclerosisMult Scler201319213013623034287
- MillerHNewellDJRidleyAMultiple sclerosis. Treatment of acute exacerbations with corticotrophin (ACTH)Lancet196127872121120112214474011
- RoseASKuzmaJWKurtzkeJFNamerowNSSibleyWATourtellotteWWCooperative study in the evaluation of therapy in multiple sclerosis. ACTH vs placebo – final reportNeurology19702051594314823
- ThompsonAJKennardCSwashMRelative efficacy of intravenous methylprednisolone and ACTH in the treatment of acute relapse in MSNeurology19893979699712544829
- FilippiniGBrusaferriFSibleyWACorticosteroids or ACTH for acute exacerbations in multiple sclerosisCochrane Database Syst Rev2000CD00133111034713
- SimsarianJPSaundersCSmithDMFive-day regimen of intramuscular or subcutaneous self-administered adrenocorticotropic hormone gel for acute exacerbations of multiple sclerosis: a prospective, randomized, open-label pilot trialDrug Des Devel Ther20115381389
- SibleyWASpotlight series: pivotal trials through today’s knowledge – adrenocorticotrophic hormoneInt MS J2009162424619671367
- StafstromCEArnasonBGBaramTZTreatment of infantile spasms: emerging insights from clinical and basic science perspectivesJ Child Neurol201126111411142121719797
- BaramTZMitchellWGTournayASneadOCHansonRAHortonEJHigh-dose corticotropin (ACTH) versus prednisone for infantile spasms: a prospective, randomized, blinded studyPediatrics19969733753798604274
- MackayMTWeissSKAdams-WebberTAmerican Academy of NeurologyChild Neurology SocietyPractice parameter: medical treatment of infantile spasms: report of the American Academy of Neurology and the Child Neurology SocietyNeurology200462101668168115159460
- GoCYMackayMTWeissSKChild Neurology SocietyAmerican Academy of NeurologyEvidence-based guideline update: medical treatment of infantile spasms. Report of the Guideline Development Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology SocietyNeurology201278241974198022689735
- RossAPBen-ZachariaAHarrisCSmrtkaJMultiple sclerosis, relapses, and the mechanism of action of adrenocorticotropic hormoneFront Neurol201342111223355832
- CataniaAGattiSColomboGLiptonJMTargeting melanocortin receptors as a novel strategy to control inflammationPharmacol Rev200456112915001661
- LugerTABrzoskaTalpha-MSH related peptides: a new class of anti-inflammatory and immunomodulating drugsAnn Rheum Dis200766Suppl 3iii52iii5517934097
- BrzoskaTLugerTAMaaserCAbelsCBöhmMAlpha-melanocyte-stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseasesEndocr Rev200829558160218612139
- BerkovichRTreatment of acute relapses in multiple sclerosisNeurotherapeutics20131019710523229226
