Abstract
Objective
The proportion of patients with systemic lupus erythematosus (SLE) who manifest retinal involvement increases many fold in patients with active systemic disease. The objective of this report is to stress upon the significance of comprehensive ophthalmic assessment of all SLE patients to prevent and manage blinding ocular manifestations of the disease.
Methods
Retrospective case review.
Results
Incidental retinal vascular complications seen in patients undergoing baseline hydroxychloroquine screening.
Conclusion
The purpose of comprehensive ophthalmic screening in SLE patients is twofold. It will aid in the diagnosis and treatment of blinding ocular complications of the disease and monitor hydroxychloroquine macular toxicity.
Introduction
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease. Based on studies published over the span of three decades (1965–1995), the mean estimates for SLE incidence and prevalence, which vary widely, were 7.3% and 23.8% respectively.Citation1–Citation3
Assessment of SLE is often challenging due to multi-organ involvement and varied physical manifestations. Ocular lesions in SLE, such as cotton-wool spots, disk hyperemia, and white retinal patches were first reported by Bergmeister in 1929.Citation4 While SLE can affect the ocular adnexa, and other areas in the central nervous system, the most common ocular manifestation in patients with SLE is dry eye syndrome. SLE can also cause severe vision loss as a result of vaso-occlusive insults to the retina or optic nerve. One of the most difficult assessments for physicians managing SLE patients is a thorough ocular examination. summarizes the findings in 1,433 SLE patients as presented in a previous study.Citation5
Table 1 Intraocular findings in 1,433 patients with systemic lupus erythematosusTable Footnote*
Hydroxychloroquine (HCQ) is one of the mainstay maintenance therapies for SLE patients, often used with other US Food and Drug Administration (FDA)-approved and non-approved treatments such as mycophenolate mofetil or azathioprine. HCQ can cause reversible, visually insignificant changes in the cornea (vortex keratopathy) and, more importantly, an irreversible sight-threatening maculopathy. Initial changes are subtle and often asymptomatic but can progress to a “bull’s eye” maculopathy and even generalized atrophy of the retina and optic nerve. This complication is very rare, but may be potentially severe, thus requiring regular screening. Besides severe allergic reactions, retinal toxicity remains the only absolute contraindication to HCQ use in adults with SLE.Citation6
HCQ screening
According to evidence-based, expert recommendations, long-term users of chloroquine or HCQ sulfate should undergo regular visits to eye care providers and diagnostic testing for maculopathy.Citation7
The American Academy of Ophthalmology has published revised screening guidelines for the monitoring of any patient taking chloroquine or HCQ.Citation7 This examination includes slit lamp exam, dilated fundus exam, fundus photography, automated 10-2 visual fields, and one or more of the recommended objective tests which include spectral domain optical coherence tomography (SD OCT), fundus auto fluorescence, and multifocal electroretinogram. Additionally, if a patient is seen to have any retinal vascular complications, they may be assessed with fundus fluorescein angiogram (FA).
The rationale for the initial HCQ screening eye examination within the 1st year of therapy is to serve as a reference point and to rule out maculopathy which might be a contraindication to the use of this medication. However, patients with lupus are also at risk for other ocular changes. For that reason, we recommend an ocular exam for all SLE patients to identify any pre-existing abnormalities. As a practical matter, to establish compliance and identify any changes early, our rheumatology patients typically receive more frequent screening, on a 6–12 month schedule. We herein present our experience with incidental findings during HCQ screening for SLE patients.
Case report
Patient 1
A 28-year-old African-American female with SLE and autoimmune lymphoproliferative syndrome was referred for baseline visual examination 3 months after starting HCQ 400 mg/day. She was 64 inches in height and weighed 56.69 kg. The calculated dose of HCQ was 7 mg/kg per day. The patient reported no vision changes since starting HCQ, but stated that she had always seen better out of her right eye. Her best-corrected visual acuity (BCVA) was 20/20 right eye (OD) and 20/25 left eye (OS) with normal pupil reactions, extra-ocular motility, and intraocular pressure (IOP). By confrontation method, she was noted to have a superior field defect OS.
A 30-degree automated visual field testing confirmed non-specific scattered changes OD, and a superior scotoma OS (). On dilated fundus exam, there was evidence of an old branch retinal vein occlusion (BRVO) with vascular collaterals OS (). FA confirmed non-perfusion inferotemporally and a low grade trickle of collaterals without macular leakage (). SD OCT revealed a normal foveal contour both eyes (OU) and inner retinal thinning OS in area of BRVO ().
Figure 1 Visual field and fundus imaging of the patient with BRVO.
Abbreviations: BRVO, branch retinal vein occlusion; FA, fluorescein angiogram; OD, right eye; OS, left eye; SD OCT, spectral domain optical coherence tomography.
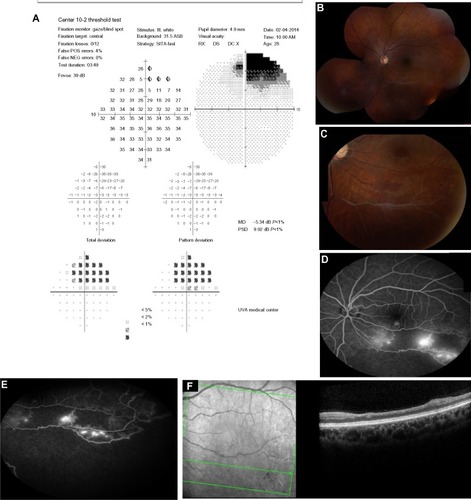
The patient was diagnosed with BRVO OS and associated collateral formation without macular edema or neovascularization. There was no evidence of HCQ toxicity. The ocular findings were discussed with the patient along with the potential need for sectoral laser in the future. Given the chronicity of the ocular changes and likely remote occlusion, the patient opted to undergo observation. We recommended HCQ dose less than 6.5 mg/kg per day with follow-up every 3–4 months.
During her most recent follow-up, 3 months after the patient’s initial diagnosis, the patient’s vision was stable at 20/20 OD and 20/25 OS with a normal IOP. Ophthalmic examination and repeat OCT showed no further changes or progression.
Patient 2
A 20-year-old female with a history of SLE presented for HCQ screening. She had been on HCQ therapy for 5 months at 400 mg per day. Her height was 68 inches and she weighed: 89.086 kg. The calculated dose of HCQ was 4.5 mg/kg per day. She noted visual loss OS for 5 months a few days prior to starting HCQ, while she was hospitalized with SLE flare. Her BCVA was 20/20 OD and 1/200 OS with normal eye pressures and an afferent pupil defect OS. The OD examination and FA was unremarkable (). Dilated fundus examination OS showed marked vascular attenuation, macular exudates along with neovascularization of the disk (NVD), neovascularization peripherally/elsewhere (NVE), and vitreous heme (). FA OS demonstrated diffuse vascular blockage, retinal non-perfusion, and leakage from NVE (). SD OCT of macula showed thinning of retinal layers OS with central macular thickness 227 μ OD and 128 μ OS (). Automated visual field testing revealed severe constriction of visual field OS ().
Figure 2 Fundus imaging and visual field analysis of the patient with ophthalmic artery occlusion.
Abbreviations: FA, Fluorescein angiogram; HVF, Humphrey visual field; NVD, neovascularization of the disk; OD, right eye; OS, left eye; PRP, pan-retinal photocoagulation; SD OCT, spectral domain optical coherence tomography.
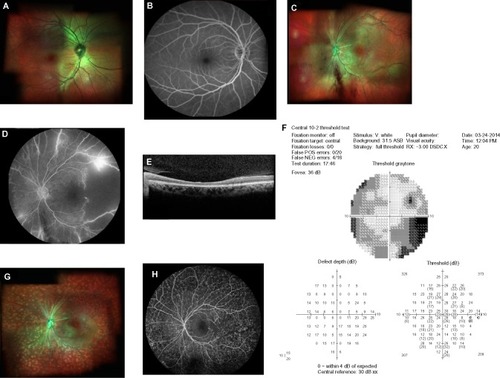
The patient was diagnosed with central retinal artery occlusion/ophthalmic artery occlusion (CRAO/OAO) with neovascularization. She received pan-retinal photocoagulation and an intravitreal injection of 0.05 cc (1.25 mg) bevacizumab OS. She was already on oral 81 mg aspirin daily as she was positive for lupus anticoagulant factor.
On follow-up, she had BCVA 20/20 OD and counting finger OS with normal IOP. Fundus examination showed regression of NVD, macular exudates, and vascular attenuation with pan-retinal photocoagulation scars (). FA revealed resolving vascular leakage OS without macular edema ().
Discussion
Ocular involvement in SLE is well-described, but the ability of non-ophthalmologists to screen for retinal vascular disease is limited. Ideally, clinicians should conduct a fundoscopic examination to look for any vascular lesions. In common clinical practice, however, fundoscopic examination is often difficult to perform, and in any case does not permit a full view of the retina.
The cases discussed in this paper highlight the importance of scheduling SLE patients with an experienced ophthalmology team as more than HCQ toxicity may be diagnosed by the ophthalmologist and managed accordingly. Ocular vascular involvement may be an indication of active systemic disease which may require systemic anticoagulation and refined treatment.
Treatment for such findings would be selected based on disease activity; as such findings may represent old ocular damage which would not be amenable to systemic therapy. For active systemic disease, therapy typically begins with systemic corticosteroids, and early introduction of intravenous cyclophosphamide, or oral mycophenolate mofetil. In the cases reported here the initial onset was remote, and disease activity was limited, so the primary therapy was HCQ. Additionally, since the second patient with CRAO had a positive test for lupus anticoagulant in her initial workup, she had been started on aspirin as prophylactic anticoagulant, before the first ophthalmologic exam. Because there had been no history of clot or miscarriage, and she reported distant onset of the CRAO/OAO, there was no indication for more aggressive anticoagulation or immunosuppression. In addition, if the patient develops neovascularization definitive treatment is retinal laser photo-coagulation, which one of the patients received.
The proportion of patients with SLE who manifest retinal involvement ranges from 3% in well-controlled patients to 29% in patients with more active systemic disease.Citation8–Citation11
Retinal vascular changes correlate with the degree of systemic disease activity. Stafford-Brady et al reported that 88% of patients with retinopathy had active systemic disease and 73% had central nervous system involvement.Citation12 Furthermore, they suggested that the patients with retinopathy had a lower overall rate of survival compared to individuals without retinopathy. Notably, severe vaso-occlusive retinopathy is a rare but well-described entity that is associated with widespread retinal capillary non-perfusion, multiple branch retinal artery occlusions, ocular neovascularization, vitreous hemorrhage, and significant resultant visual loss.Citation13,Citation14 ().
Table 2 Signs of lupus retinopathy
A study by Jabs et alCitation11 showed that 55% of eyes in lupus patients with severe retinal vaso-occlusive disease suffered vision loss, often with visual acuity worse than 20/200. The authors also observed more frequent central nervous system involvement in lupus patients with marked retinal vascular changes. Furthermore, central retinal vein or artery occlusions may occur, either independently or together, and may be unilateral or bilateral.Citation15–Citation17
While central retinal artery occlusion is rare, it is considered an ocular emergency and can cause permanent visual loss due to widespread retinal ischemia. It is often characterized by rapid, painless visual loss, a Marcus-Gunn afferent pupil defect, arterial attenuation, macular edema, and cherry red spot of fovea.Citation18 Sudden visual loss with central retinal artery occlusion in a young patient should prompt the clinician to include SLE and other collagen diseases in the differential diagnosis.
Central or BRVO is a less common manifestation of lupus retinopathy as the disease largely manifests as arteritis, but can still be a cause of permanent visual loss.Citation19,Citation20
The recently conducted National Ambulatory Medical Care Survey indicated that many patients at high risk for chloroquine or HCQ maculopathy are not undergoing routine monitoring for this serious adverse event. These findings underscore the need for physician education related to potentially devastating effects on the eye and an understanding of factors that may contribute to suboptimal adherence to expert guidelines.Citation21
Conclusion
SLE patients are at a high risk of retinal vascular events that correlate with systemic disease activity. A comprehensive ophthalmological exam is recommended in all SLE patients, not only for HCQ screening, but also to ensure prompt diagnosis and management of blinding ocular complications related to the disease. Also, anti-platelet therapy may be considered for those at high risk for developing retinal vascular occlusion.
Acknowledgments
We are thankful to Rabia Aman for her valuable contribution to this paper with editing and collection of figures.
Disclosure
None of the authors have any conflicts of interest to disclose.
References
- McCartyDJManziSMedsgerTAJrRamsey-GoldmanRLaPorteREKwohCKIncidence of systemic lupus erythematosus: race and gender differencesArthritis Rheum1995389126012707575721
- FeldmanCHHirakiLTLiuJEpidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000–2004Arthritis Rheum201365375376323203603
- JacobsonDLGangeSJRoseNRGrahamNMEpidemiology and estimated population burden of selected autoimmune diseases in the United StatesClin Immunol Immunopathol19978432232439281381
- BergmeisterRUber primare and miliare Tuberkulose der Retina [About primary and miliary tuberculosis of the retina]Wien Med Schnschr19297911161119 German
- GoldDHMorrisDAHenkindPOcular findings in systemic lupus erythematosusBr J Ophthalmol197256118008044647128
- BajwaAFosterCSOcular Manifestations of Systemic Lupus Erythematosus, Review articleJ Clin Cell Immunol20145191
- MarmorMFKellnerULaiTYAmerican Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathyOphthalmology2011118241542221292109
- MontehermosoACerveraRFontJAssociation of antiphospholipid antibodies with retinal vascular disease in systemic lupus erythematosusSemin Arthritis Rheum199928532633210342390
- KlinkhoffAVBeattieCWChalmersARetinopathy in systemic lupus erythematosus: relationship to disease activityArthritis Rheum1986299115211563753541
- UshiyamaOUshiyamaKKoaradaSRetinal disease in patients with systemic lupus erythematosusAnn Rheum Dis200059970570810976084
- JabsDAFineSLHochbergMCNewmanSAHeinerGGStevensMBSevere retinal vaso-occlusive disease in systemic lupus erythematosusArch Ophthalmol198610445585633954661
- Stafford-BradyFJUrowitzMBGladmanDDEasterbrookMLupus retinopathy. Patterns, associations, and prognosisArthritis Rheum1988319110511103422014
- AuAO’DayJReview of severe vaso-occlusive retinopathy in systemic lupus erythematosus and the antiphospholipid syndrome: associations, visual outcomes, complications and treatmentClin Experiment Ophthalmol20043218710014746601
- ReadRWChongLPRaoNAOcclusive retinal vasculitis associated with systemic lupus erythematosusArch Ophthalmol2000118458858910766154
- LeibovitchIGoldsteinMLoewensteinABarakACombined central retinal artery and vein occlusion in a patient with systemic lupus erythematosusRheumatology (Oxford)200140101195119611600756
- DurukanAHAkarYBayraktarMZDincASahinOFCombined retinal artery and vein occlusion in a patient with systemic lupus erythematosus and antiphospholipid syndromeCan J Ophthalmol2005401878915825539
- MendrinosEMavrakanasNKielRPournarasCJBilateral combined central retinal artery and vein occlusion in systemic lupus erythematosus resulting in complete blindnessEye (Lond)20092351231123218535593
- VarmaDDCugatiSLeeAWChenCSA review of central retinal artery occlusion: clinical presentation and managementEye (Lond)201327668869723470793
- SilvermanMLubeckMJBrineyWGCentral retinal vein occlusion complicating systemic lupus erythematosusArthritis Rheum1987217839843697953
- EllisCJHamerDBHuntRWMedical Investigation of Retinal Vascular OcclusionBr Med J1964254171093109820790334
- SugaiDYGustafsonCJDe LucaJFTrends in the outpatient medication management of lupus erythematosus in the United StatesJ Drugs Dermatol201413554555224809877
