Abstract
Patients suffering from allergic rhinitis often attempt to self-manage their symptoms and may seek advice from pharmacists about nonprescription product choices. Several drug classes, both prescription and over-the-counter (OTC), are available, including intranasal corticosteroids (INCSs); oral, intranasal, and ocular antihistamines; leukotriene antagonists; and topical and systemic decongestants, as well as immunotherapies. Selection of the optimal treatment approach depends on the temporal pattern, frequency, and severity of symptoms as well as the patient’s age. Nasal congestion is typically the most bothersome symptom, although rhinorrhea, postnasal drip, and ocular symptoms are also problematic. Together, these symptoms may adversely impact the quality of life, work productivity, sleep quality, and the ability to perform daily activities, particularly when uncontrolled. Practice guidelines recognize that INCSs are the most effective medications for controlling allergic rhinitis symptoms, including nasal congestion. Available INCS products have comparable safety and efficacy profiles, but they differ in formulation characteristics and sensory attributes. Several barriers can impede the use of INCSs, including concerns about safety, misperceptions regarding the loss of response from frequent use, and undesirable sensations associated with intranasal administration. Given the increasing number of INCSs available OTC, pharmacists can help allay these concerns by discussing treatment expectations, recommending INCS products with favorable formulation characteristics, and reviewing proper use and technique for the administration of the selected product. These steps can help to foster a collaborative relationship between the patient and the pharmacist in the treatment of allergic rhinitis.
Introduction
Allergic rhinitis causes a variety of symptoms, including nasal congestion, sneezing, rhinorrhea, postnasal drip, nasal and ocular itching, and watery eyes.Citation1 Classification is based on the pattern (eg, seasonal, perennial, or episodic), frequency (intermittent or persistent), and severity (mild or moderate/severe) of symptoms.Citation1,Citation2 Among individuals with self-reported nasal symptoms,Citation3 the 12-month prevalence of allergic rhinitis was estimated at 30%, corresponding to ~90 million Americans. Notably, only 22% of individuals in this survey reported a physician diagnosis of allergic rhinitis, suggesting that the disorder is being largely self-managed without physician oversight.Citation3
National surveys evaluating the burden of allergic rhinitis on affected individuals have found that approximately three-quarters of respondents consider nasal congestion to be bothersome or extremely bothersome.Citation4,Citation5 Other highly bothersome symptoms included runny nose (by 69% of respondents), red, itchy eyes (68%), and postnasal drip (65%).Citation4,Citation5
As with the overall population of allergy sufferers, the majority of allergy symptoms experienced by children and adolescents are attributable to allergic rhinitis.Citation3 The symptom profile in children is comparable with that in adults, with both children and their parents reporting nasal congestion as the most common and the most bothersome symptom.Citation6 In the Pediatric Allergies in America survey,Citation6 parents of children with allergic rhinitis reported a 30% decrease in productivity at school and home when allergy symptoms were at their worst. Children with allergies were also significantly more likely to avoid school or social activities compared to children without allergies (P<0.001).
Nasal congestion and other allergy symptoms have a notable impact on the quality of life, work productivity, sleep quality, the ability to perform daily activities, and medical costs, particularly when uncontrolled.Citation7–Citation12 For example, employed individuals reported that their work productivity was reduced by ~25% on days when their symptoms were at their worst.Citation4,Citation5 Allergic rhinitis sufferers typically self-recognize their symptoms and then initiate management with over-the-counter (OTC) medications;Citation13 82% of those managing their allergic rhinitis symptoms with OTC medications report that they required minimal or no guidance from their physicians. Since most individuals are self-managing their symptoms outside of direct physician’s care, pharmacists are well positioned, when needed, to provide guidance and advice regarding the management and treatment of allergic rhinitis symptoms.
Pathophysiology of allergic rhinitis
Allergic rhinitis is caused by immunoglobulin E (IgE)-mediated responses to inhaled allergens, which trigger a series of immunological and biochemical events that produce the clinical symptoms characteristic of the disorder ().Citation14 The process of allergic sensitization involves uptake of the allergens by antigen-presenting cells in nasal tissues, subsequent presentation to other immune response cells, and production of allergen-specific IgE. The IgE binds to high-affinity FcεRI receptors on the surface of nasal mast cells and circulating basophils, thereby sensitizing them to the offending allergen but not yet causing any symptoms.Citation14–Citation16 Upon re-exposure, the offending allergen is recognized by IgE on sensitized mast cells and basophils, which induce early- and late-phase responses that lead to the clinical symptomatology of allergic rhinitis. Within minutes, the allergen–IgE interaction causes degranulation of the sensitized cells, leading to the release/production of mediators, including histamine, tryptase, leu-kotrienes, and prostaglandins. Histamine activates H1 receptors on sensory nerve endings to cause sneezing and nasal secretion, as well as both H1 and H2 receptors on mucosal blood vessels to cause nasal congestion. The leukotrienes act on receptors located on blood vessels and mucus glands to induce nasal congestion and mucus secretion. The symptoms produced by this immediate hypersensitivity reaction typically last for ~1 hour and then dissipate.Citation14,Citation15 Approximately 50% of patients with allergic rhinitis then experience a late-phase response that may persist for hours after allergen exposure.Citation14 The late-phase response is characterized by the influx and activation of multiple inflammatory cells, including T cells, eosinophils, basophils, neutrophils, and monocytes, as well as by an increase in mast cell number in the nasal submucosa and epithelium, and is thought to be mediated by cytokines and chemokines released by the local activation of Th2 cells. Nasal congestion is the most prominent symptom during the late-phase response.Citation14–Citation16
Figure 1 Pathophysiological steps leading to allergic rhinitis symptoms.
Abbreviations: EOS, eosinophil; GM-CSF, granulocyte-macrophage colony-stimulating factor; IgE, immunoglobulin E; IL, interleukin; Th2, helper T-cell type 2.
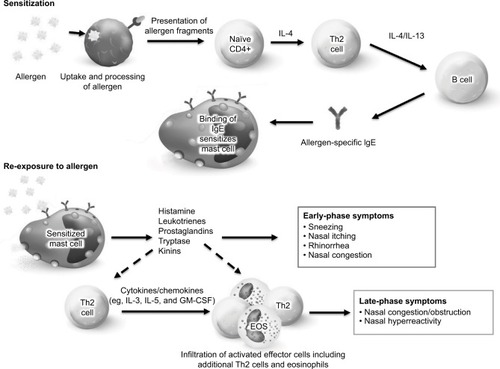
Figure 2 Treatment recommendations for the self-care of allergic rhinitis.
Abbreviations: ADR, adverse drug reactions; AH, antihistamine; AR, allergic rhinitis; HCP, healthcare provider; INCS, intranasal corticosteroid; Rx, prescription.
Allergic rhinitis can be classified as intermittent (≤4 days/week or ≤4 weeks/year) or persistent (>4 days/week and >4 weeks/year) and as mild (normal sleep, no impairment of daily activities, normal work and school, and no troublesome symptoms) or moderate to severe (abnormal sleep, impairment of daily activities, impairment of work and school activities, or troublesome symptoms).Citation2 Symptoms also may be classified according to their temporal pattern as seasonal, perennial/year-round, or episodic (eg, following exposure to a home with pets).Citation1 These classifications can help to identify the most appropriate treatment options on an individual basis.
Overview of current treatment guidelines
Multiple drug classes are available by prescription or OTC for the treatment of allergic rhinitis. To help guide the selection of appropriate medications, several professional organizations have issued practice guidelines, including the Allergic Rhinitis and its Impact on Asthma (ARIA) working group,Citation2,Citation17 the American Academy of Allergy, Asthma & Immunology (AAAAI) and American College of Allergy, Asthma & Immunology (ACAAI) jointly,Citation18 and the American Academy of Otolaryngology–Head and Neck Surgery Foundation (AAO-HNSF).Citation1
Each set of practice guidelines recognizes that intranasal corticosteroids (INCSs) are the most effective medication class for controlling allergic rhinitis symptoms ().Citation1,Citation2,Citation18 The high efficacy of INCSs is attributed to their ability to modulate the pathophysiology of allergic rhinitis, including the release of multiple mediators and cytokines as well as the recruitment and infiltration of activated inflammatory cells to the nasal mucosa.Citation1 Accordingly, INCSs are effective at attenuating both early- and late-phase symptoms, including nasal congestion and hyperresponsiveness. The onset of action usually occurs within 12 hours of administration but may commence as early as 3–4 hours after use,Citation18 which is slower than the onset of antihistamines.Citation2,Citation18 Continued daily use of INCSs may be needed in order to achieve maximum efficacy; although as-needed INCS dosing is less effective than continuous dosing, it may be an appropriate option for some patients with intermittent symptoms.Citation1 Local INCS side effects include nasal irritation/dryness, epistaxis, taste and smell disturbances, and, rarely, nasal septal perforation, which can be minimized with proper technique during administration.Citation1,Citation18–Citation24 Headache is a common systemic side effect with INCSs; cataracts and/or glaucoma may occur but are rare.Citation1,Citation19–Citation23 In general, the second-generation INCSs, including mometasone furoate, fluticasone propionate, ciclesonide, and fluticasone furoate, have very low (<1%) systemic bioavailability, which is postulated to limit the risk for clinically significant systemic side effects.Citation24 Evidence regarding potential effects on growth suppression with INCSs continues to evolve with the availability of data from more robust study designs, which has underscored the need for a thorough evaluation of safety and the balance of benefits and risks when used in children.Citation25 Additional long-term studies are needed to determine the effect of INCSs during childhood on final adult height.Citation25
Table 1 Relative efficacy of medication classes by allergic rhinitis symptom, symptom frequency, and symptom severity
Oral antihistamines are effective against histamine-mediated allergic rhinitis symptoms, including rhinorrhea, sneezing, nasal itching, and ocular symptoms.Citation2 Agents in this class are less effective against nasal congestion (). Oral antihistamines can be categorized into first-generation and second-generation agents. Use of the former (eg, diphenhydramine and chlorpheniramine) may be limited by sedation and mucosal dryness reflecting their ability to cross the blood–brain barrier and their anticholinergic effects, whereas second-generation agents (eg, fexofenadine, cetirizine, levocetirizine, loratadine, and desloratadine) exhibit selectivity for the H1 receptor and minimal penetration across the blood–brain barrier.Citation1 Although not as effective as the INCSs, an oral antihistamine may be sufficient for patients with mild-to-moderate symptoms of allergic rhinitis or intermittent symptoms, offering the advantages of lower cost and a more rapid onset of action.Citation1 When indicated, each set of guidelines recommends a second-generation oral antihistamine in order to minimize the risk of sedation, performance impairment, and anticholinergic side effects.Citation1,Citation17,Citation18
Intranasal antihistamines are more effective than oral antihistamines for nasal congestion and at least as effective in controlling other allergic rhinitis symptomsCitation1,Citation18 but, again, not as effective as INCSsCitation1,Citation18 in providing relief of nasal symptoms.Citation18,Citation26 Agents in this class may benefit patients who fail oral antihistamines or cannot otherwise tolerate them. Advantages include a rapid onset of action within 15–30 minutes and targeted delivery of higher antihistamine dosages to nasal tissues while minimizing systemic side effects. The most common side effects are bitter taste, epistaxis, headache, somnolence, and nasal burning.Citation1
Allergic rhinitis sufferers may experience ocular symptoms in addition to nasal congestion, as previously described. Ocular antihistamines and mast cell stabilizers are also available and may be used to alleviate concomitant symptoms of allergic conjunctivitis.Citation27
The orally administered leukotriene antagonist (LTRA), montelukast, is the only US Food and Drug Administration-approved LTRA for the treatment of allergic rhinitis in adults and children; other available LTRAs have not been adequately studied in allergic rhinitis.Citation1 Limitations compared with oral antihistamines include higher cost, prescription-only availability, and potential for side effects including headache and rare neuropsychiatric events.Citation1,Citation11 Although LTRAs are not recommended as primary therapy for allergic rhinitis, they may be beneficial for patents with both allergic rhinitis and asthma.Citation1,Citation18
Combination therapy may be suggested when monotherapy does not adequately control allergic rhinitis symptoms.Citation1 For patients already on an INCS, an intranasal antihistamine can be added, or, alternatively, treatment can be switched to a combination product containing both medications, such as azelastine/fluticasone propionate. Similarly, temporary addition of an intranasal decongestant (eg, oxymetazoline), with use limited to 3–5 days in order to avoid rebound nasal congestion, represents another therapeutic approach.Citation1,Citation17 An oral antihistamine or LTRA should not be added to an INCS, as clinical trials have shown no benefit from these combinations. Options for patients already using an oral antihistamine include switching to an INCS or an intranasal antihistamine, or adding an oral decongestant. The latter approach, however, is associated with an increased risk of side effects. Similarly, an INCS should not be added to an oral antihistamine because large clinical trials have not demonstrated a benefit of an INCS with an oral antihistamine compared to an INCS alone.Citation1 For patients inadequately controlled by an intranasal antihistamine, the addition of an INCS (a combination product currently available only via prescription) is the only recommended option based on current evidence.
Allergen-specific immunotherapy should be considered for patients who respond inadequately to available pharmacologic options.Citation1,Citation18 This approach aims to increase immune tolerance through repetitive, controlled exposure to the offending allergen(s) and may be considered for patients with persistent symptoms despite pharmacological therapy.Citation1
Barriers to the use of INCS
Although current practice guidelines recognize that INCSs are the most effective agents available for the treatment of allergic rhinitis, a large online survey found that only 30% of patients in the USA with severe nasal congestion were actually receiving INCS therapy.Citation7 However, it is important to note that the survey was conducted in 2004, prior to the OTC availability of INCSs in the USA (the first of which became available in 2014) and before the most recent treatment guidelines emphasizing their use. At present, multiple INCS products (including several OTC options) that are comparable in efficacy are available ().Citation19–Citation23,Citation28–Citation33 Nevertheless, patient perceptions, beliefs, and preferences as well as formulation characteristics and cost may be barriers to the initiation of and adherence to INCS therapy. Fear of side effects has been reported more often for INCSs than for oral antihistamines (48 vs 33%) among patients with allergic rhinitis. The most common specific fears with INCSs were habituation (ie, loss of response due to frequent use), damage to mucous membranes, and side effects on other organs, whereas the most common fear with oral antihistamines was fatigue.Citation34 In another survey, few respondents thought that INCSs were unsafe but most did not use their INCSs because they feared a loss of effectiveness if used too much.Citation35
Table 2 Intranasal corticosteroids approved for allergic rhinitisTable Footnotea
Sensory attributes of an intranasal spray – including scent/odor, immediate taste, aftertaste, throat rundown, nose runout, burning, and feel of the spray in the nose and throat – may influence patient adherence. Among individuals with allergic rhinitis in US allergy/immunology clinics,Citation36 patient preference for an INCS decreased with increasing intensity of each of these sensory attributes, with the most important attributes identified as aftertaste, immediate taste, throat rundown, and nose runout. Notably, 77% of the respondents indicated that they would be able to adhere to a daily regimen for 3 months if the INCS had the lowest level of each sensory attribute, compared to only 4% of respondents if given an INCS with moderate levels of the sensory attributes (P<0.01).Citation36 Moreover, patients indicated a willingness to pay more to avoid sensory attributes of INCS sprays, particularly aftertaste, throat rundown, and nose runout.Citation37
Numerous studies of sensory perceptions and patient preferences for INCS products ()Citation38–Citation46 have illustrated that patients can detect significant differences in sensory attributes and specify preference for one product over another. High preference was shown across studies for several products, including fluticasone furoate, mometasone furoate, and triamcinolone acetonide aqueous spray. Fluticasone furoate was preferred over mometasone furoate after treatment for 2 weeks,Citation38 and triamcinolone acetonide was preferred over mometasone furoate in several single-dose studies.Citation42,Citation45,Citation46 Notably, fluticasone furoate was preferred over fluticasone propionate in terms of having less odor/scent, causing less nose runout/throat rundown, and having less aftertaste.Citation39 Thus, even though a pat ent may not have liked the sensory attributes of fluticasone propionate in the past, this should not preclude the use of fluticasone furoate given the differences in sensory perception. Finally, practice guidelines recognize that patient preference should be considered when recommending an INCS product.Citation1,Citation2
Table 3 Comparison of sensory attributes and patient preferences among INCS products
Role of the pharmacist in the self-management of allergic rhinitis symptoms
Many patients with allergic rhinitis attempt to self-manage their symptoms, and some will seek advice from pharmacists about choosing appropriate OTC products. Pharmacists should ask the patient to describe his/her symptoms in order to confirm that the patient is suffering from allergic rhinitis, including whether a medical diagnosis of hay fever, allergic rhinitis, or asthma has previously been identified. Establishing the history of symptoms (including the onset and temporal pattern, frequency, severity, and duration) and evaluating the exacerbating or mitigating factors and the therapies that have already been tried are critical factors in helping the patient select the proper treatment for their symptoms.Citation47 In addition, inquiring about other medical comorbidities or the use of other medications may help to identify which treatment approach is optimal for a particular patient.Citation2 The presence of two or more of the following symptoms lasting >1 hour on most days is suggestive of allergic rhinitis: watery anterior rhinorrhea, sneezing, nasal congestion, and nasal itching; conjunctivitis may also be present. The presence of symptoms in only one nostril, nasal congestion without other symptoms, mucopurulent rhinorrhea, postnasal drip with thick mucus and/or no anterior rhinorrhea, pain, recurrent epistaxis, and loss of the sense of smell are not usually associated with allergic rhinitis. Such patients should be referred to a physician for further evaluation and treatment.Citation1,Citation2,Citation18
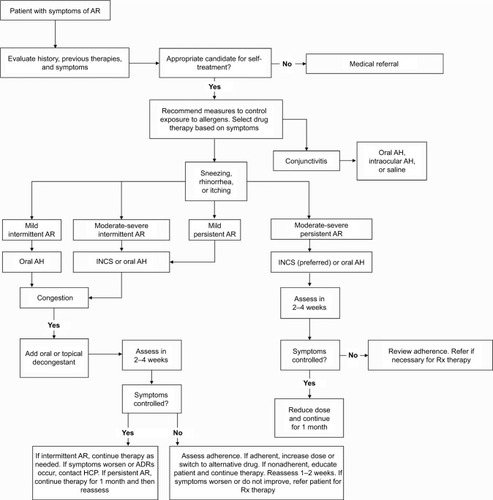
The American Pharmacists Association algorithm for the self-care of allergic rhinitis ()Citation48 outlines a suggested approach to treatment recommendations for individuals with symptoms consistent with intermittent or persistent allergic rhinitis who are appropriate candidates for self-treatment. Exclusions for self-treatment include the presence of symptoms of nonallergic rhinitis; otitis media, sinusitis, bronchitis, or other infection; undiagnosed or uncontrolled asthma (eg, wheezing and shortness of breath); chronic obstructive pulmonary disease; or other lower respiratory disorder, and those who have experienced severe or unacceptable side effects of treatment. For children younger than 12 years and pregnant or lactating women, self-treatment is acceptable only if a physician has diagnosed allergic rhinitis and approved the use of OTC treatment.
When possible, pharmacists should advise the use of nonpharmacological measures for the avoidance of known allergens and environmental control.Citation1,Citation18,Citation47 Patients should be encouraged to avoid or minimize exposure to allergens that trigger allergic rhinitis symptoms. For severe seasonal symptoms caused by outdoor allergens, such as pollen, measures might include staying inside air-conditioned buildings with windows and doors closed, particularly on sunny, windy days with low humidity.Citation18 For indoor allergens (dust mites and mold), multifaceted environmental controls (eg, reduction of moisture, use of protective bed covers, washing of bedding and soft toys, use of acaricides, and removal of carpets) may be beneficial where practicable. For pet dander, measures include the avoidance or removal of animal allergens from the household.Citation18
Pharmacists are often the primary source to provide medication counseling to patients with allergic rhinitis.Citation49 To guide treatment recommendations, it is important to discuss patient preferences and goals of treatment, which may include providing symptom relief, preventing symptom recurrence, and improving or restoring the quality of life and ability to function.Citation18 Based on this information along with the symptom profile, including frequency and severity, the pharmacist should provide counseling regarding available OTC treatment options.
Several OTC INCS products are available, differing in terms of formulation, number of sprays required per dose, age range approved for use, and, to some extent, dosing frequency (). The characteristics of nasal spray formulations may influence patient preferences. Additives and preservatives can irritate nasal mucosal membranes, thereby influencing comfort of use, and can confer an unpleasant odor or taste. Formulations containing phenylethyl alcohol may have a strong odorCitation43 and cause a feeling of dryness after administration.Citation46 Formulations containing benzalkonium chloride as a preservative may have a bitter taste.Citation43 Formulations delivering smaller volumes may have less nose runout and throat rundown.
The pharmacist should clarify any potential misperceptions about INCSs that could be a barrier to their appropriate use when indicated and, after recommending a product, provide counseling regarding its proper use, treatment expectations, and instructions on when to consult a physician. With INCSs, some benefit may be achieved within 3–4 hours,Citation18 but these medications provide optimal symptom control when used continually for several days. Moreover, patients should be instructed to continue using the INCS to maintain symptom control and not simply resort to a use-as-needed approach. For seasonal sufferers, treatment should be maintained during the allergy season.
Counseling regarding proper medication self-administration, including priming the device and using proper spray technique, in turn, may improve adherence and facilitate better symptom control.Citation29,Citation33,Citation50 Although the directions differ somewhat for each product, in general, the recommended technique involves several steps to ensure proper administration ().Citation48,Citation51 Each OTC product includes specific step-by-step directions for dosing and administration; these should be reviewed with the patient.Citation29,Citation33,Citation50
Figure 3 General instructions for the use of intranasal corticosteroid sprays.

A number of prescription-to-OTC switches have occurred in the INCS category over the past several years, providing the general public with greater access to these products. Additional prescription-to-OTC switches may occur in the future, which would expand access to options that allow easier or more preferable drug dosage delivery and self-administration. For example, fluticasone furoate, which was recently approved for OTC treatment of seasonal and perennial allergic rhinitis in the USA,Citation52 is a scent- and alcohol-free formulation with minimal throat/nose drip provided in a nasal device that contains a side-actuated mist-release button with a cap that prevents the button from being pressed accidentally.Citation32 The OTC product (FLONASE® Sensimist™ Allergy Relief; GlaxoSmithKline Consumer Healthcare, Research Triangle Park, NC, USA) is expected to become available in the USA in 2017.Citation52
Conclusion
Given the prevalence of allergic rhinitis and symptom burden associated with the condition, many patients will opt for self-management and seek advice from pharmacists. Pharmacists, in turn, must keep abreast of the latest clinical evidence related to the prevention and treatment of symptoms, including product efficacy and nuances in product formulation.
In addition to suggesting strategies for avoiding exposure to allergens and irritants, pharmacists are often asked for recommendations regarding which OTC products to use. Current practice guidelines recognize that INCSs are the most effective medications for controlling allergic rhinitis symptoms including nasal congestion,Citation1,Citation2,Citation18 which is consistently identified in national surveys as the most bothersome symptom.Citation4,Citation5 By asking a series of questions, pharmacists can establish if the patient has allergic rhinitis and identify the temporal pattern, frequency, and severity of symptoms.Citation2 This information is important for identifying when an INCS is the best choice; examples include patients with persistent and moderate-to-severe symptoms, patients suffering from predominantly nasal congestion, or patients whose symptoms disrupt their sleep or interfere with their work or school activities. To implement successful INCS use, pharmacists may have to address and help resolve several barriers, including concerns about safety or loss of response due to frequent use, and recognize that patient preferences and formulation characteristics are important considerations. All available INCS products have comparable efficacy and safety;Citation1 however, differences in sensory attributes, formulation characteristics, or spray bottle features may be important factors that influence patient adherence to therapy.
By educating and collaborating with patients to set appropriate treatment goals, pharmacists can play an important role in improving symptom control and quality of life in patients with allergic rhinitis.
Acknowledgments
Medical writing assistance was provided by Barry M Weichman, PhD, and Diane Sloan, PharmD, of Peloton Advantage and was funded by GlaxoSmithKline Consumer Healthcare. GlaxoSmithKline Consumer Healthcare provided a full review of the article.
Disclosure
The author reports no conflicts of interest in this work.
References
- SeidmanMDGurgelRKLinSYGuideline Otolaryngology Development GroupAAO-HNSF. Clinical practice guideline: allergic rhinitisOtolaryngol Head Neck Surg20151521 supplS1S43
- Members of the WorkshopsARIA in the pharmacy: management of allergic rhinitis symptoms in the pharmacy. Allergic rhinitis and its impact on asthmaAllergy200459437338715005760
- NathanRAMeltzerEODereberyJThe prevalence of nasal symptoms attributed to allergies in the United States: findings from the burden of rhinitis in an America surveyAllergy Asthma Proc200829660060819173786
- BieloryLSkonerDPBlaissMSOcular and nasal allergy symptom burden in America: the allergies, immunotherapy, and rhinoconjunctivitis (AIRS) surveysAllergy Asthma Proc201435321121824801463
- Allergies in America: a landmark survey of nasal allergy sufferers. Executive summaryHealthSTAR Communications, Inc, in partnership with Schulman, Ronca & Bucuvalas, Inc145 Available from: http://www.worldallergy.org/UserFiles/file/Allergies%20in%20America%20(AIA)%20-%20Adult%20Executive%20Summary.pdfAccessed March 30, 2017
- MeltzerEOBlaissMSJenniferDMBurden of allergic rhinitis: results from the pediatric allergies in America surveyJ Allergy Clin Immunol2009124suppl 3S43S7019592081
- SheddenAImpact of nasal congestion on quality of life and work productivity in allergic rhinitis: findings from a large online surveyTreat Respir Med20054643944616336028
- BlaissMSMeltzerEODereberyMJBoyleJMPatient and healthcare-provider perspectives on the burden of allergic rhinitisAllergy Asthma Proc200728suppl 1S4S1018307838
- CanonicaGWMullolJPradalierADidierAPatient perceptions of allergic rhinitis and quality of life: findings from a survey conducted in Europe and the United StatesWorld Allergy Organ J20081913814423282577
- BlaissMSAllergic rhinitis: direct and indirect costsAllergy Asthma Proc201031537538020929603
- GreinerANHellingsPWRotirotiGScaddingGKAllergic rhinitisLancet201137898092112212221783242
- ThompsonASardanaNCraigTJSleep impairment and daytime sleepiness in patients with allergic rhinitis: the role of congestion and inflammationAnn Allergy Asthma Immunol2013111644645124267356
- FromerLMBlaissMSJacob-NaraJALongRMMannionKMLauersenLACurrent allergic rhinitis experiences survey (CARES): consumers’ awareness, attitudes and practicesAllergy Asthma Proc201435430731524992550
- SinBTogiasAPathophysiology of allergic and nonallergic rhinitisProc Am Thorac Soc20118110611421364228
- BernsteinDISchwartzGBernsteinJAAllergic rhinitis: mechanisms and treatmentImmunol Allergy Clin North Am201636226127827083101
- WheatleyLMTogiasAClinical practice. Allergic rhinitisN Engl J Med2015372545646325629743
- BrozekJLBousquetJBaena-CagnaniCEGlobal Allergy and Asthma European NetworkGrading of Recommendations Assessment, Development and Evaluation Working GroupAllergic rhinitis and its impact on asthma (ARIA) guidelines: 2010 revisionJ Allergy Clin Immunol2010126346647620816182
- WallaceDVDykewiczMSBernsteinDIThe diagnosis and management of rhinitis: an updated practice parameterJ Allergy Clin Immunol20081222 supplS1S8418662584
- Beconase AQ [package insert]Research Triangle Park, NCGlaxo-SmithKline2015
- Nasonex [package insert] Whitehouse Station, NJMerck & Co, Inc.2013
- Rhinocort Aqua [package insert]Wilmington, DEAstraZeneca2010
- Flixonase [summary of product characteristics France]Cedex, FranceGlaxoSmithKline Laboratory2013
- Nasacort [summary of product characteristics France]Gentilly, FranceSanofi Aventis France2016
- SastreJMosgesRLocal and systemic safety of intranasal corticosteroidsJ Investig Allergol Clin Immunol2012221112
- SkonerDPThe tall and the short: repainting the landscape about the growth effects of inhaled and intranasal corticosteroidsAllergy Asthma Proc201637318019126935232
- YanezARodrigoGJIntranasal corticosteroids versus topical H1 receptor antagonists for the treatment of allergic rhinitis: a systematic review with meta-analysisAnn Allergy Asthma Immunol200289547948412452206
- MounseyALGrayRETopical antihistamines and mast cell stabilizers for treating allergic conjunctivitisAm Fam Physician2016931191591627281835
- QNASL [package insert]Horsham, PATeva Respiratory, LLC2014
- FDARhinocort Allergy (OTC Product) Approved Label 2016 Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/020746Orig1s034lbl.pdfAccessed May 25, 2016
- Omnaris [package insert]Marlborough, MASunovion Pharmaceuticals Inc2013
- Zetonna [package insert]Marlborough, MASunovion Pharmaceuticals Inc2014
- Veramyst [package insert]Research Triangle Park, NCGlaxoSmithKline2015
- FDANasacort Allergy 24HR (OTC product) Approved Label2013 Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020468Orig1s035lbl.pdfAccessed May 25, 2016
- HellingsPWDobbelsFDenhaerynckKPiessensMCeuppensJLDe GeestSExplorative study on patient’s perceived knowledge level, expectations, preferences and fear of side effects for treatment for allergic rhinitisClin Transl Allergy201221922643067
- ValovirtaERyanDPatient adherence to allergic rhinitis treatment: results from patient surveysMedscape J Med2008101024719099041
- MahadeviaPJShahSLeibmanCKleinmanLO’DowdLPatient preferences for sensory attributes of intranasal corticosteroids and willingness to adhere to prescribed therapy for allergic rhinitis: a conjoint analysisAnn Allergy Asthma Immunol200493434535015521370
- MahadeviaPShahSMannixSWillingness to pay for sensory attributes of intranasal corticosteroids among patients with allergic rhinitisJ Manag Care Pharm200612214315116515372
- YonezakiMAkiyamaKKarakiMPreference evaluation and perceived sensory comparison of fluticasone furoate and mometasone furoate intranasal sprays in allergic rhinitisAuris Nasus Larynx201643329229726498699
- MeltzerEOAndrewsCJourneayGEComparison of patient preference for sensory attributes of fluticasone furoate or fluticasone propionate in adults with seasonal allergic rhinitis: a randomized, placebo-controlled, double-blind studyAnn Allergy Asthma Immunol2010104433133820408344
- KhannaPShahAAssessment of sensory perceptions and patient preference for intranasal corticosteroid sprays in allergic rhinitisAm J Rhinol200519331632116011141
- MeltzerEOBardelasJGoldsobelAKaiserHA preference evaluation study comparing the sensory attributes of mometasone furoate and fluticasone propionate nasal sprays by patients with allergic rhinitisTreat Respir Med20054428929616086602
- StokesMAmorosiSLThompsonDDupclayLGarciaJGeorgesGEvaluation of patients’ preferences for triamcinolone acetonide aqueous, fluticasone propionate, and mometasone furoate nasal sprays in patients with allergic rhinitisOtolaryngol Head Neck Surg2004131322523115365540
- ShahSRMillerCPethickNUryniakTJonesMKO’DowdLTwo multicenter, randomized, single-blind, single-dose, crossover studies of specific sensory attributes of budesonide aqueous nasal spray and fluticasone propionate nasal sprayClin Ther20032582198221414512128
- LumryWHampelFLaForceCKiechelFel-AkkadTMurrayJJA comparison of once-daily triamcinolone acetonide aqueous and twice-daily beclomethasone dipropionate aqueous nasal sprays in the treatment of seasonal allergic rhinitisAllergy Asthma Proc200324320321012866325
- BunnagCSuprihatiDWangDYPatient preference and sensory perception of three intranasal corticosteroids for allergic rhinitisClin Drug Investig20032313944
- BachertCEl-AkkadTPatient preferences and sensory comparisons of three intranasal corticosteroids for the treatment of allergic rhinitisAnn Allergy Asthma Immunol200289329229712269650
- BousquetJKhaltaevNCruzAAAllergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen)Allergy200863suppl 86816018331513
- ScolaroKLColds and allergyKrinskyDLFerrariSPHemstreetBHandbook of Nonprescription Drugs: An Interactive Approach to Self-Care18th edWashington, DCAmerican Pharmacists Association2015171196
- KuehlBLAbdulnourSO’DellMKyleTKUnderstanding the role of the healthcare professional in patient self-management of allergic rhinitisSAGE Open Med20153 2050312115595822
- FDAFlonase Allergy Relief (OTC Product) Approved Label2014 Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205434Orig1s000Lbl.pdfAccessed May 25, 2016
- BenningerMSHadleyJAOsguthorpeJDTechniques of intranasal steroid useOtolaryngol Head Neck Surg2004130152414726906
- GSKFDA Approves Flonase ® Sensimist™ Allergy Relief [press release]2016 Available at: https://us.gsk.com/en-us/media/press-releases/2016/fda-approves-flonase-sensimist-allergy-relief/Accessed: September 8, 2016
