Abstract
The world’s population is ageing, with the number of those over 60 years expected to represent a fifth of the total population by 2050. Increases in chronic long-term health conditions (LTCs) associated with ageing, and requiring regular but often avoidable medical intervention, are pressurising already overloaded, health and social care systems. Atrial fibrillation (AF) is an LTC, which is most frequently diagnosed in the elderly. An often, asymptomatic condition, AF is associated with a 3- to 5-fold increased risk of severe ischemic stroke. Stroke prevention, with risk-stratified oral anticoagulants (OACs) is the standard recommended care for patients with AF. Stroke avoidance is, however, dependent on persistent adherence to OAC medication, with an adherence rate of >80% considered necessary to achieve optimal health outcomes. Suboptimal adherence to OACs is common, with a third of all AF patients not taking their medication as prescribed. This combined with the short half-life of OACs can result in poor clinical outcomes for patients. Policy makers now consider improving adherence to prescribed medicines for LTCs, a public health priority, to ensure better health outcomes for patients, whilst minimising unnecessary health system costs. Prescribing medicines to treat LTCs, such as AF, is not enough, particularly when the patient may not experience any measurable benefit to the treatment and may instead, experience medication-associated adverse events, including a risk of bleeding. Pharmacists who are experts in medicines management are ideally placed to support medication adherence, to educate, and to improve health outcomes for patients with AF. In this review, I will consider the evidence for poor medication adherence in LTCs and in particular adherence to OACs in patients with AF and highlight the role that pharmacists can play in ensuring optimal adherence and showcase pharmacist-led interventions that effectively address this problem.
Introduction
Ageing and Chronic Long-Term Conditions
The world’s population is ageing. The global life expectancy is now estimated at 72.3 years, rising to over 80 years in developed countries.Citation1,Citation2 In 2050, it is predicted that a fifth of the population worldwide will be aged 60 years and older, with 400 million of those being 80 years or older, and residing mainly in low- to middle-income countries.Citation3 Longer life expectancy, however, can more often than not coincide with a growing risk of developing chronic long-term disease conditions (LTCs) and as a consequence experiencing a deleterious decline in physical and mental capacity ().Citation4
Figure 1 Relationship between ageing and chronic long-term conditions. Created with BioRender.com.
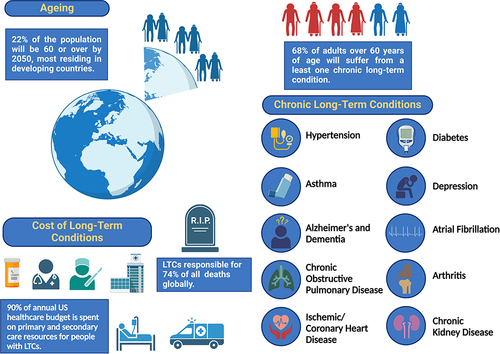
In 2020, “The UN Decade of Healthy Ageing” was proclaimed by the World Health Organization (WHO) and the United Nations (UN). Its purpose to develop strategies, as a global multisectoral collaboration, to promote healthy ageing in older people.Citation5,Citation6 Chronic LTCs, such as diabetes, hypertension, chronic obstructive pulmonary disease, chronic kidney disease, ischemic/cardiovascular disease, osteoarthritis, asthma, depression, atrial fibrillation, Alzheimer’s and dementia, which are managed with drugs and therapies, are more prevalent in the older population. Almost two thirds of people suffering from chronic, LTCs will be over 60 years old, compared to only 14% in those under the age of 40.Citation4 As well as increasing the prevalence of LTCs, age also increases the number, with 25% of the over 60’s suffering from two or more LTCs.Citation4 In addition to age, lifestyle is also another major risk factor in developing LTCs, with those from disadvantaged communities with a poorer quality of life, having the likelihood of developing multiple LTCs, 10–15 years earlier than those living in affluent areas.Citation7 Chronic LTCs are responsible for 74% of all deaths globally, with over three-quarters of these occurring in low- and middle-income countries.Citation8 People with LTCs are intensive users of health and social care, with the average cost for treatment of an LTC being much higher than for those without one. It is estimated for every £10 of the UK healthcare budget, £7 is spent on people with LTCs, due to the concomitant demands on primary and secondary care resources,Citation4 whilst in the US, 90% of the annual healthcare budget is spent on chronic LTCs.Citation9 For the patient, LTCs can mean managing complex health regimes and adhering to prescribed medicines, every day, often for the duration of their life.
Medication Adherence
Adherence to medicines can be described simply as taking a prescribed medicine, at the right time, in the right way and at the recommended frequency.Citation10 Medications in LTCs and particularly in the elderly are prescribed to mitigate disease, extend life-expectancy, and improve quality of life. Despite the proven efficacy of medications for treating LTCs, the full benefits to the patient are often not realised, with approximately 50% of the patients not taking their long-term medications as prescribedCitation10 when typically taking medication for all (100%) or for most of the time (>80%) is required to achieve optimal therapeutic efficacy.Citation11 This 80% adherent cut off point, however, is not without ambiguity, with the evidence for cardiovascular disease (CVD) suggesting a much higher cut-off rate being required to ensure good health outcomes.Citation12,Citation13 None or partial adherence to medications (<80%) has been shown to correlate with poor health outcomes, particularly in the elderly population, for whom the risk of hospitalisation, nursing home admission, and mortality increases.Citation14,Citation15 Non- or low adherence is not just a clinical problem, however, but is associated with huge economic costs, due to increased healthcare resource usage. Billions are lost annually in wasted medication prescriptions and avoidable inpatient and outpatient healthcare costs,Citation16,Citation17 not to mention, in the European Union, almost 200,000 preventable deaths every year.Citation18,Citation19 The elderly in particular are huge consumers of prescription medications and for whom non-adherence is common.Citation20 This population alone accounts for 61.6% of all prescribed medicines in the UK.Citation21 There is a myriad of reasons for non-adherence in this particular population and not all can be attributed solely to the patient but instead a complex network involving the patient, the prescriber, and the healthcare system.Citation10 Interestingly, but perhaps not surprisingly, most medication non-adherence is intentional,Citation22,Citation23 with patients actively making a conscious decision, either from prior knowledge, experience, or beliefs, not to take their medicine as prescribed.Citation24 Reasons for non-adherence can be related to a number of factors and not always in isolation. The World Health Organisation identified five interacting factors of adherence:Citation10 Patient-related; Medication-related; Condition-related; Health-care system-related; Social- and economic-related ().
Figure 2 Factors identified for medication non-adherence. Created with BioRender.com.
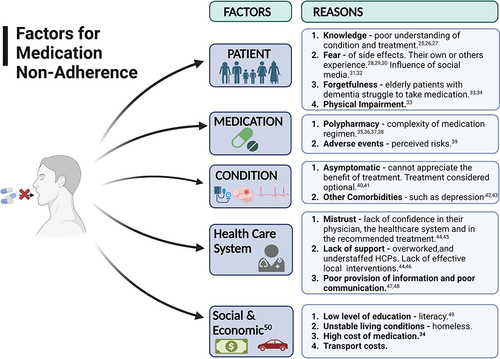
Taking these reasons for medication non-adherence into account when designing interventions to overcome them, is thought to improve the likelihood of patient adherence.Citation30 In addition, it is reported that effective interventions have to involve sustained behavioural changes such as changing misguided beliefs about the medication, improving patient-provider relationships, designing acceptable regimens, and focusing on self-management.Citation46,Citation51 Policy makers, healthcare managers, and healthcare professionals (HCPs) have realised that improving patients’ adherence to their medication is a public health issue and key to improving patient clinical outcomes, reducing medication waste, and improving the financial sustainability of healthcare systems and stretched medical resources.Citation52 This and the WHO’s statement that “increasing the effectiveness of adherence may have far greater impact on the health of the population than any improvement in specific medical treatments”Citation10 has meant that adherence is now a key component of several European Union (EU) policies, including legislation of pharmacovigilance,Citation53 health literacy; patient safety and quality of care; and the European innovation partnership on Healthy and Active Ageing.Citation54
The aim of this review is to focus on aspects of medication adherence for one chronic LTC, atrial fibrillation, examining known determinants of patient adherence and non-adherence to oral anticoagulant therapy, required to mitigate the risk of stroke. Additionally, the review will showcase some reported examples of pharmacist-led interventions aimed at improving anticoagulant adherence and clinical outcomes for patients with AF, in order to highlight opportunities that can be adopted to improve OAC management and patient care.
Atrial Fibrillation
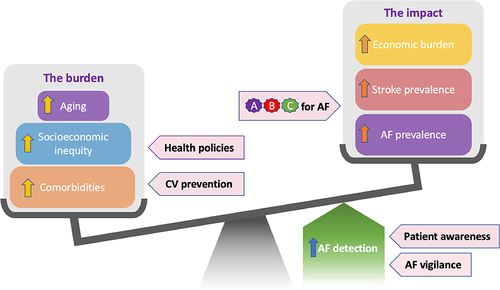
Atrial Fibrillation (AF) is the most commonly diagnosed cardiac dysrhythmiaCitation55 and with the highest risk (3- to 5-fold) for the occurrence of severe ischemic stroke (IS).Citation56–58 Advancing age is the most notable, non-modifiable risk factor for the development of AF with the incidence of AF, doubling with each decade, after the age of 60.Citation57 AF has a prevalence of >10% (versus 2.5% in the overall population) reported for those over 80 years of age,Citation57,Citation59–62 increasing to as high as 38%, for those residents of care and nursing homes, where advanced age residents suffering from multiple LTCs, are concentrated.Citation63–67 This disproportional increase in AF prevalence in older aged adults is aligned with a concomitant increase in the incidence of IS (23.5% versus 1.8%).Citation57,Citation58,Citation62,Citation65 With increasing global life expectancy and a concurrent increase in LTCs, the prevalence and incidence of AF will continue to rise, with the global burden of AF predicted to reach 62.5 million by 2050Citation68 and with that an elevated risk of life-threatening strokes and increased healthcare resource burden ().
Figure 3 Factors promoting adherence to OAC therapy in AF patients.
Oral Anticoagulants
Since their approval in the 1950’s, although not routinely prescribed until the 1990’s, oral anticoagulants (OACs) are considered one of the most effective and safe therapeutic strategies for reducing and preventing stroke in patients diagnosed with AF.Citation70,Citation71 The superior effectiveness of vitamin K antagonists (VKAs), such as warfarin, to reduce stroke and mortality, when compared to then commonly prescribed anti-platelets, such as aspirin, were demonstrated in a series of randomised trials in the 1980’s and 1990’s.Citation72–74 Despite the obvious benefits of VKA therapy, these are also associated with some considerable risk of serious adverse events and therapeutic failures when not used properly, including a higher bleeding risk.Citation73 VKAs have a slow onset of action and narrow therapeutic index that requires careful monitoring to ensure that anticoagulation levels neither fall under or over the required therapeutic range, which can either increase the risk of IS or of an intracranial bleed, respectively.Citation73,Citation75 The risk of bleeding whilst using anticoagulants is particularly notable in the elderly who are prone to syncope and falls, both as a consequence of older age and also of AF, which can increase the possibility of intracranial hematomas occurring.Citation76–78 Risk of falls in the elderly is one of the main reasons that OACs are not often prescribed by physicians or if prescribed, under-dosed to the extent that they are subtherapeutic.Citation79–82 Elderly people suffering with AF and a history of falls were found to be 17% less likely to be prescribed OACs than those with no prior falls.Citation83 Yet the current guidelines from the National Institute for Health Care Excellence (NICE) and the European Society of Cardiology (ESC) clearly state that OACs should not be withheld because of age or a risk of falls, as the benefit of stroke reduction, far outweighs the risk of intracranial haemorrhage.Citation39,Citation71,Citation83 It has been estimated that for the risk of bleeding to outweigh the benefit of taking OACs to prevent stroke, an elderly person would need to fall around 295 times a year, when the average an elderly person will fall is 1.81 times per year.Citation84–86 However, even for those correctly prescribed patients, the narrow therapeutic index of VKAs makes it difficult to maintain patients within the recommended international normalized ratio (INR) anticoagulation range, of 2.0–3.0. One large study found that in a large population of AF diagnosed individuals (6454) who were taking warfarin, their INR was outside of the desired 2.0–3.0 range, 50% of the time.Citation87 As well as requiring frequent monitoring and dose adjustments, to ensure appropriate INRs, VKAs also interact with other drugs and certain foods, which can increase bleeding risk and effectiveness, making for complicated therapeutic and dietary regimes for elderly patients, who are often taking many other medications.Citation88,Citation89 These factors have been shown to be linked to reduced patient satisfaction with their OAC treatmentCitation90 which in turn leads to inadequate adherence to VKAs and subsequently poorly anticoagulated patients.Citation91 In one study, adherence, defined as being greater than 80% was recorded in 51.2% of patients after one year of being prescribed VKAs, following an AF diagnosis.Citation92 Whilst persistence, defined as continuing to take medication after one year (whether adherent, >80% or non-adherent, <80%), was 63.4%.Citation92
Direct-Acting Oral Anticoagulants
The introduction of direct-acting oral anticoagulants (DOACs) following approval by the Food and Drug Administration (FDA) in 2010Citation93 and the European Medicine Agency (EMA) in 2012 heralded the possibility for improved adherence among nonvalvular AF patients.Citation79 Unlike VKAs, DOACs do not require continual monitoring of the INR, have less drug and dietary interactions, a 50% lower risk of intracranial haemorrhage and significant reduction in all-cause mortality.Citation94–96 Current guidelines recommend DOACs as first-line therapeutics for the prevention of stroke in nonvalvular AF patients.Citation97–100 Worldwide the number of AF patients reported as using OACs has doubled due to the increased prescribing of DOACs.Citation50,Citation101 However, the incidence of OAC prescribing still remains lower for the elderly than for younger patients due to a risk of falls, dementia, and/or previous bleeds.Citation102,Citation103 This is despite studies showing that many patients individually value the benefit of stroke prevention over any inherent bleeding risk.Citation104,Citation105 Furthermore, suboptimal DOAC dosing remains a significant problem in the elderly population, with one large study identifying almost a quarter of patients being either overdosed (15%) or underdosed (5%).Citation106 Persistence to DOACs in the long term (>1 year) is found to be higher than for VKAs in a number of studies.Citation39,Citation50,Citation107 One of the main reasons for non-persistence with DOACs, is cost, followed closely by patient preference, stopping their medication due to a fear of bleeding and/or the absence of any AF-associated symptoms,Citation50 which can lead to medication being considered as “optional” both by patient and physician. A notable example where “optional medication” is commonplace is in hypertension, where adherence rates can range from 9–37%Citation40 and poor blood pressure control as a consequence is estimated to cause 10 million deaths, each year.Citation41 Similarly, once a patient LTC is under control, they may also feel they require no further need for any medication.
Non-Adherence to DOACs
Surprisingly, despite improved persistence and the many advantages that DOACs bestow over traditional VKAs, under prescribing and poor adherence, still remains an ongoing global challenge. Non-adherence to all DOACs has been reported to occur in up to 40% of AF patients in particular, among the elderly.Citation34,Citation92,Citation108 One large systematic review highlighted that patients were missing on average one dose in four of their DOACs.Citation107 The actual “real-world” level of non-adherence is likely to be much higher, with almost 50% of non-adherent patients in one study, reported not to disclose non-adherence behaviour to their HCPs.Citation34 Compared to VKAs, DOACs have much shorter half-lives, ~12 hours, compared to ~40 hours for VKAs, such that low adherence significantly reduces their efficacy and subsequently, therapeutic benefit to the patient, making DOAC adherence essential.Citation109 There is clear evidence demonstrating a significant risk of stroke and all-cause mortality in patients with AF, when DOAC medication is not adhered to.Citation110,Citation111
Ironically, a lack of INR monitoring for DOACs, and thus the subsequent loss of regular contact with a medical professional has been shown to impact adherence negatively, opposite to what was predicted would happen.Citation34 Other reasons for non-adherence include forgetfulness, having no symptoms, fear of bleeding and not having a reversal agent, and cost of medication for those patients having to pay, all result in occasional doses being missed.Citation34,Citation50 Predictors of greater adherence include older age, increasing co-morbidities, previous history of stroke, hypertension, diabetes, patient knowledge and education, and physician contact (see ).Citation34,Citation93,Citation111,Citation112 Unsurprisingly, reasons for patient non-adherence to OACs, as for most medications for LTCs are multi-factorial, patient specific, and thus not easily resolved with a “one-size-fits-all” approach. It is clear from the evidence, however, that finding practical solutions that ensure that OACS are properly adhered to and optimally used to their full therapeutic benefit, but also identify at-risk patients that are either non-persistent or -adherent, is required. The complexities of adherence to life-long medications, however, have resulted in equally complicated, time and resource consuming interventions that are more often than not, ineffective.Citation46
Role of the Pharmacist in OAC Adherence
Allied HCPs, such as pharmacists, are uniquely positioned to fill this important role in identifying patients who are non-adherent/persistent to their OAC medication, counselling patients on the value of taking their medication, offering written and verbal advice, understanding individual patient needs and burdens, and optimizing patient adherence, safety, and treatment outcomes.Citation113 Physicians report time pressures as a barrier for not tackling adherence issues,Citation114 and subsequently are enlisting pharmacists to act as an intermediary in educating and counselling patients.Citation115,Citation116 In some countries, such as the US, it is already a legal requirement for pharmacists to counsel patients on their medicines.Citation117 Thus, an expansion of the pharmacist role to promote and support adherence, particularly as pharmacists see patients far more frequently than all other primary care,Citation118,Citation119 could be feasible and time/cost effective. Indeed, this role for the pharmacist has already been identifiedCitation120 for the delivery of the Atrial Fibrillation Better Care (ABC: Avoid stroke Better symptom management Cardiovascular and other comorbidities) pathway.Citation121 This framework designed for an individualised, integrated approach to AF management across care settings is recommended in the guidelines of the European Society of Cardiology and Asia Pacific Heart Rhythm Society.Citation71,Citation122 Similarly in the UK, as part of the Community Pharmacy Contractual Framework,Citation123 community pharmacists deliver medicine use reviews (MUR) and the new medicine service (NMS), interventions designed to improve understanding and adherence to medicines in LTCCitation124,Citation125 and comparable services exist globally.Citation126 Whilst in some countries, both hospital and community-based pharmacist are involved in the delivery of anticoagulation management services (AMS) for patients on VKAs, in order to improve INR control and to provide a convenient, local service for patients.Citation127–130 This was extended in 2013, in the US to include DOACs, where a patient can be referred to an AMS service for assessment and ongoing monitoring of their DOAC by a pharmacist.
There have been a number of studies over the past 10-years assessing pharmacist-led interventions for AF screening and OAC management across secondary and primary care (see comprehensive review by Ritchie et al, 2022), which have shown that pharmacist-led interventions can improve adherence to OACs.Citation120 In the next section, I will aim to review some of the most recent studies that have taken place in primary and community care that focus on OAC management and improving adherence.
Pharmacist-Led OAC Management Interventions
Intervention 1
Pharmacist delivering medication optimisation reviews of patients OAC therapy is one way of improving health outcomes for patients with AF. In a recent, retrospective observational study, independent prescribing pharmacists based in GP practices in Bradford delivered a medication optimisation intervention to 616 patients with a confirmed nonvalvular AF diagnosis and on the Quality and Outcomes Framework (QOF) AF register.Citation131 From the medical records, 76% (470/616) were on established OAC treatment pre-intervention, with 37% of these on VKA therapy (172/470), 98% (168/172) of those on warfarin. Post pharmacist intervention, of those patients not previously on any stroke preventative medication but deemed eligible, 25% (37/146) were successfully initiated on DOACs. Of the patients that were on VKA therapy pre-intervention (172/616), 20% (35/172) of them were determined by the pharmacist as eligible to transfer to DOAC therapy, of which 25% (9/35) were successfully converted. The main reasons for non-conversion were patient preference (69%) and cost (19%). Whilst inappropriate dosing of DOACs, both over- and under-dosing, was identified in 24% (73/298) of patients, pre-intervention and was re-optimised in line with recommended guidance and considering individual patient parameters, for 94% (68/73) of them. The most common inappropriately prescribed DOAC was rivaroxaban. Finally, an assessment of patients bleed risk resulted in 73% of the patients having a reduction in their HAS-BLED score by at least one point (3.00 ± 0.65 vs 2.22 ± 0.79). Overall, the study demonstrated that pharmacists based in UK GP practices and accessing patient records could optimise the safe and effective use of OAC therapy for established AF patients, highlighting the importance of targeted medication optimisation reviews for LTCs such as AF.
Intervention 2
Another example of pharmacist-led management of OACs is through the anticoagulation management service (AMS). Originally set up to monitor the INR of patients on VKAs in an outpatient setting by pharmacists, it was extended to include DOACs assessment and monitoring in 2013, in the US. The service was available to any medical provider to refer a patient for assessment. This involved the pharmacist having access to a patients’ medical record, to determine drug selection, dosing, and to access any laboratory test results (eg serum creatinine for renal functioning) or to request tests. They would then contact the patient by telephone to discuss their medication, determine any issues, including affordability, and to educate the patient about the medication and importance of adhering to it. The pharmacist would then make recommendations to the medical care provider for any changes (drug type, dosing, etc.) that needed to be made if required. The pharmacist would follow-up with the patients two weeks later to see how the patient was getting on with the medication and to determine adherence and again 3–6 months later, but this time including a renal function assessment. Patient contact would be ongoing (every 3–6 months) for the duration of their treatment. A retrospective observational study in the US, compared 129 patients that had been through the pharmacist-led DOAC service for one year (2013–2014) and compared them with 129 patients that had received normal care for their DOAC, from the physician, to determine appropriateness of therapy and adherence.Citation132 More than half of the participants were indicated for AF in each cohort. The study found that patients that had been seen through the pharmacist-led DOAC service were significantly more likely to be initiated and continued on the appropriate DOAC and at the correct dose for their condition, than the usual care group (93% vs 79.1% - baseline, 93.7% vs 81.1% - 3–6 months). In addition, patients in the pharmacist-led DOAC service, who received ongoing education and support were significantly more likely to be adherent than the standard care group (91.8% vs 79.3%). As was seen for the previous study, this intervention demonstrated that pharmacists were able to ensure patients were on the correct DOAC and dose for their particular indication, but additionally it showed that continued follow-up with patients, facilitated improved patient adherence to their medication and assisted in overcoming barriers, such as medication affordability.
Intervention 3
Similarly, a community pharmacist-led intervention using the UK New Medicines Service (NMS) framework was piloted in Poland to see if adherence to dabigatran, previously reported as being suboptimal in 50% of AF patientsCitation133 was improved.Citation134 The study demonstrated that using a combination of in-person, involving face-to-face (medication reconciliation, patient education, side-effect management); telephone calls (motivational interviewing around adherence challenges and barriers to adherence) and indirect involving pictogram-enhanced information leaflet and medication labels, smartphone daily medication reminders, correlated with better adherence to dabigatran than a control group receiving regular dispensing care. Over 3 months, the study found that patients receiving the intervention were significantly more adherent to their prescribed dose (twice/day) at each time point, than the control group receiving standard care (82.7% vs 71.4% - day 7; 84.4% vs 58% - day 21; 78.4% vs 39.7% - day 90). Furthermore, the proportion of patients fully adherent at the end of the study (90 days) was significantly higher than the control group (26.1% vs 13.2%) demonstrating that interventions that educate and counsel at the start of a patients’ medication journey and then provide ongoing support, improves patient adherence to their drug therapy and mitigates the habitual decline in adherence over time, observed for many chronic conditions.
Intervention 4
Despite the introduction and popularity of DOACs, warfarin is still the most commonly prescribed oral anticoagulant and widely used by patients with AF. The narrow therapeutic index of warfarin, a range which is indication-dependent and the side effects that can occur when a patient is not on the correct INR, such as a risk of bleeding when over-dosed or risk of stroke when under-dosed means for a patient to achieve maximal therapeutic benefit, they must maintain an optimal therapeutic range (TTR), involving regular monitoring. In recent years, this monitoring service has moved out into the community, where it is more accessible and is frequently run by nurses and pharmacists.Citation128–130 A number of retrospective studies have shown a significant positive correlation between pharmacist-led, warfarin management services and stable TTR, INR parameters and fewer adverse events, compared to those receiving normal care (Aidit et al, 2017, Lee et al, 2016, Phelps et al, 2018).Citation135–137 In a combined retrospective and prospective study based in Brazil, 268 patients with AF diagnosis, who had been receiving warfarin therapy managed by a physician for a least one year and had a TTR < 50% (retrospective) were transferred to a prospective intervention.Citation138 The prospective arm of the study involved 12 weeks under the care of a clinical pharmacist and involved in-person one-to-one consultations, which involved at baseline, medication reconciliation, patient education, side-effect management, possible drug and food interactions, motivational interviewing on the benefits of adherence, INR measurements and made any necessary dose adjustments to achieve target therapeutic range (INR 2.0–3.0). This was followed by four further pharmacist consultations, occurring every 7 days, where INR was tested, warfarin dose adjustments made, and adherence evaluated. After the fourth visit, if the INR was stable, the patient’s next appointment was for 30 days’ time or 7 days if unstable, until 12 weeks of follow-up were completed. Calculation of the mean TTR pre-intervention after >1 year of physician management, and following 12 weeks of pharmacist post-intervention, showed a significant improvement in TTR values from 14.4% to 54.3%. Despite the fact that the TTR achieved is still lower than the recommended optimal TTR level of >70%, possibly due to the short intervention time with very poorly controlled patients, this study showed that pharmacist-led warfarin management significantly improved the therapeutic range of these patients and reduced the risk of adverse events.
Figure 4 Factors promoting adherence to OAC therapy in AF patients. Figure created with BioRender.com.
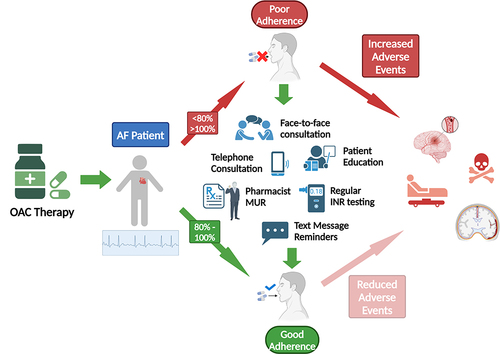
Conclusion
Prescribing of OACs for patients diagnosed with AF will be for the vast majority a life-long commitment. This review has shown that ensuring adherence and persistence with these therapies, for which the benefit of such may not always be perceived by the patient and sometimes the physician, should be at the forefront of clinical practice and interventions. Stroke, along with other noncommunicable diseases such as diabetes, chronic obstructive pulmonary disease, and cancer are responsible for 74% of all deaths globally.Citation8 Non-adherence to life-preserving medications for LTCs is recognised as a global public health issue and is impacting negatively the ability of health services around the globe to achieve population health goals.Citation10 Proper prescribing of medicines and then fully adhering to these medications for LTCs, is considered by policy makers as the most propitious, in terms of positive outcomes for patients and equally overstretched healthcare systems.Citation10 Interventions such as the one’s showcased here, that improve adherence to life-preserving medication such as OACs and target individuals most at risk of non-adherence, such as the elderly, are essential. A pharmacist will see patients of LTCs far more often than any other HCP, due in part to the introduction of the repeat dispensing scheme, which allows patients to receive up to 12 months’ supply of their medicines from the community pharmacist.Citation140 Furthermore, a community pharmacist is often more readily accessible on a day to day basis, than a physician, with a patient twice as likely to visit a pharmacist than a physician.Citation118 In addition, compared to a physician, pharmacists are able to spend more time promoting medication adherence, and counselling the patient on the importance of OAC therapy and the possibility of adverse events. This opportune contact when dispensing medicines, plus a pharmacists’ vast experience of medicines and of delivering reviews of medicines, are activities which the public most closely associate with the role of a pharmacist and trust them to do, compared with some of the more clinical roles now being undertaken.Citation126,Citation141 Despite this rather obvious pathway for medicines management for community pharmacists, however, gaining access to a patient’s clinical records, including having a good working relationship with local physicians and the time available outside of routine dispensing workflows, can limit the success or reach of some interventions, and more research will be needed to address this.Citation142 However, more adherent patients, means more prescriptions dispensed from the pharmacy, which results in increased revenue both from the filled prescriptions and from paid initiatives to improve adherence.Citation143 Here, I have shown some tested examples of pharmacist-led OAC management initiatives that have demonstrated feasibility and improved health outcomes for patients with AF. Combining OAC management together with a pharmacist-led AF screening intervention, for which there have been many reported successes,Citation120 promises wide-reaching benefits to the healthcare system in the future.
Disclosure
The author reports no conflicts of interest in this work.
Acknowledgments
I would like to thank Alistair Mathie for his helpful comments on the manuscript.
References
- United Nations Population Division. World Population Prospects; 2022. Available from: https://data.worldbank.org/indicator/SP.DYN.LE00.IN. Accessed January 10, 2024.
- Roser M, Ortiz-Ospina E, Ritchie H Life Expectancy. OurWorldInData.org; 2019. Available from: https://ourworldindata.org/life-expectancy. Accessed January 10, 2024.
- United Nations, Department of Economic and Social Affairs, Population Division (2020). World Population Ageing (ST/ESA/SER.A/444); 2019. Available from: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Report.pdf. Accessed January 10, 2024.
- Department of Health. Long Term Conditions Compendium of Information 3rd edition; 2012. Availabel from: https://assets.publishing.service.gov.uk/media/5a7c638340f0b62aff6c154e/dh_134486.pdf. Accessed January 10, 2024.
- United Nations. In: session S-F, ed, A/RES/75/131 United Nations< Decade of Health Ageing (2021–2030); Resolution/Adopted by the General Assembly on 13 December 2020. New York: UN; 2020. Available from: https://cdn.who.int/media/docs/default-source/decade-of-healthy-ageing/decade-proposal-final-apr2020-en.pdf. Accessed January 10, 2024.
- World Health Organization. United Nation’s Decade of Healthy Ageing (2021–2030). Geneva: World Health Organization; 2020. Available from: https://www.who.int/initiatives/decade-of-healthy-ageing. Accessed January 10, 2024.
- Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43. doi:10.1016/S0140-6736(12)60240-2
- World Health Organization. Noncommunicable diseases; 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases#:~:text=Noncommunicable%20diseases%20(NCDs)%20kill%2041,%2D%20and%20middle%2Dincome%20countries. Accessed January 10, 2024.
- Centers for Disease Control and Prevention. Health and Economic Cost and Chronic Diseases. Available from: https://www.cdc.gov/chronicdisease/about/costs/index.htm. Accessed February 26, 2024.
- Sabate E Adherence to long-term therapies: evidence for action 2003. Geneva, Switzerland: World Health Organization; 2003. Available from: http://www.who.int/chronic_conditions/adherencereport/en/. Accessed July 15, 2024.
- DiMatteo MP, Giordani PJ, Lepper HS, Croghan W. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40(9):794–811. doi:10.1097/00005650-200209000-00009
- Bryson CL, Au DH, Young B, McDonell MB, Fihn SD. A refill adherence algorithm for multiple short intervals to estimate refill compliance (ReComp). Med Care. 2007;45(6):497–504. doi:10.1097/MLR.0b013e3180329368
- Wu JR, Moser DK, de Jong MJ, et al. Defining an evidence-based cutpoint for medication adherence in heart failure. Am Heart J. 2009;157(2):285–291. doi:10.1016/j.ahj.2008.10.001
- Walsh CA, Cahir C, Tecklenborg S, Byrne C, Culbertson MA, Bennett KE. The association between medication non-adherence and adverse health outcomes in ageing populations: a systematic review and meta-analysis. Br J Clin Pharmacol. 2019;85(11):2464–2478. doi:10.1111/bcp.14075
- Strandberg LR. Drugs as a reason for nursing home admissions. J Am Health Care Assoc. 1984;10:2023.
- Luga AO, McGuire MJ. Adherence and health care costs. Risk Manag Health Policy. 2014;7:22–44. doi:10.2147/RMHP.S19801
- Cutler RL, Fernandez-Llimos F, Frommer M, Benrimoj C, Garcia-Cardenas V. Economic impact of medication non-adherence by disease groups: a systematic review. BMJ Open. 2018;8(1):e016982. doi:10.1136/bmjopen-2017-016982
- European Patients Forum. Adherence and concordance EDF position paper; 2015. Available from: https://www.eu-patient.eu/globalassets/policy/adherence-compliance-concordance/adherence-paper-final-rev_external.pdf. Accessed February 26, 2024.
- Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(3):487–497. doi:10.1056/NEJMra050100
- Balkrishnan R. Predictors of medication adherence in the elderly. Clin Ther. 1998;20(4):764–771. doi:10.1016/S0149-2918(98)80139-2
- Office for National Statistics. Prescriptions Dispensed in the Community – statistics for England, 2007–2017. NHS Digital; 2018. Available from: https://digital.nhs.uk/data-and-information/publications/statistical/prescriptions-dispensed-in-The-community/prescriptions-dispensed-in-The-community-england---2007---2017#. Accessed February 26, 2024.
- Barber N, Parsons K, Clifford S, Darracott R, Horne R. Patients’ problems with new medication for chronic conditions. BMJ Qual Saf. 2004;13(3):172–175. doi:10.1136/qshc.2003.005926
- Mukhtar O, Weinman J, Jackson SHD. Intentional non-adherence to medications by older adults. Drugs Ageing. 2014;31(3):149–157. doi:10.1007/s40266-014-0153-9
- Brown MT, Bussell JK. Medication Adherence: WHO Cares? Mayo Clin Proc. 2011;86(4):304–314. doi:10.4065/mcp.2010.0575
- Baker DW, Parker RM, Williams MV, Clark WS, Health Literacy and the risk of hospital admission. J Gen Intern Med. 1998;13(12):791–798. doi:10.1046/j.1525-1497.1998.00242.x
- Ryan AA. Medication compliance and older people: a review of the literature. Int J Nurs Stud. 1999;36(2):153–162. doi:10.1016/S0020-7489(99)00003-6
- Nielsen-Bohlman L, Panzer AM, Kindig D Health Literacy: a Prescription to End Confusion. Washington, DC: Institute of Medicine of the National Academies; 2004. Available from: https://nap.nationalacademies.org/catalog/10883/health-literacy-a-prescription-to-end-confusion. Accessed February 26, 2024.
- Horne R, Chapman SCE, Parham R, Freemantle N, Forbes A, Cooper V. Understanding Patients’ Adherence-Related Beliefs about Medicines Prescribed for Long-Term Conditions: a Meta-Analytic Review of the Necessity-Concerns Framework. PLoS One. 2013;8(12):e80633. doi:10.1371/journal.pone.0080633
- Helier MK, Chapman SC, Horne R. Beliefs about medication predict the misattribution of a common symptom as a medication side effect – evidence from an analogue online study. J Psycho Res. 2015;79(6):519–529. doi:10.1016/j.jpsychores.2015.10.003
- Shelis E, Tillett W, James D, et al. Changing Medication-related Beliefs: a Systematic Review and Meta-analysis of Randomized Controlled Trials. Health Psychol. 2023;43(3):155–170.
- Linn AJ, van Weert JCM, Gebevehu BG, et al. Patients’ online information-seeking behaviour throughout treatment: the impact on medication beliefs and medication adherence. Health Communication. 2019;34(12):1461–1468. doi:10.1080/10410236.2018.1500430
- Al Khaja KAJ, AlKhaja AK, Sequeira RP. Drug information, misinformation, and disinformation on social media: a content analysis study. J Public Health Pol. 2018;39(3):343–357. doi:10.1057/s41271-018-0131-2
- El-Saifi N, Moyle W, Jones C, Tuffaha H. Medication Adherence in Older Patients With Dementia: a Systematic Literature Review. J Pharm Prac. 2018;31(3):322–334. doi:10.1177/08971900177105
- Tarn DM, Shih K, Tseng C-H, Thomas A, Schwartz JB. Reasons for nonadherence to the direct oral anticoagulant Apixaban: a cross-sectional survey of atrial fibrillation patients. JACC Advances. 2023;2(1):100175. doi:10.1016/j.jacadv.2022.100175
- Lau HS, Beuning KS, Postma-Lim E, et al. Non-compliance in elderly people: evaluation of risk factors by longitudinal data analysis. Pharm World Sci. 1996;18(2):63–68. doi:10.1007/BF00579707
- Claxton AJ, Cramer J, Pierce C. A Systematic review of the association between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296–1310. doi:10.1016/S0149-2918(01)80109-0
- Corsonello A, Pedone C, Lattanzio F, et al. Regimen complexity and medication nonadherence in elderly patients. Ther Clin Risk Manag. 2009;5(1):209–216. doi:10.2147/TCRM.S4870
- Liu J, Yu Y, Yan S, et al. Risk factors for self-reported medication adherence in community-dwelling older patients with multimorbidity and polypharmacy: a multicenter cross-sectional study. BMC Geriatr. 2023;23(1):75. doi:10.1186/s12877-023-03768-7
- Mitchell A, Snowball J, Welsh TJ, Watson MC, McGrogan A. Prescribing of direct oral anticoagulants and warfarin to older people with atrial fibrillation in UK general practice: a cohort study. BMC Med. 2021;19(1):189. doi:10.1186/s12916-021-02067-5
- Wetzels GEC, Nelemans P, Schouten JS, Prins MH. Facts and fiction of poor compliance as a cause of inadequate blood pressure control: a systematic review. J Hypertens. 2004;22(10):1849–1855. doi:10.1097/00004872-200410000-00002
- World Health Organization. Global report on hypertension. The race against a silent killer. Licence: CC BY-NC-SA 3.0 IGO. WHO; 2023. Available from: https://www.who.int/publications/i/item/9789240081062. Accessed February 26, 2024.
- DiMatteo MR, Lepper HS, Croghan TW. Depression Is a Risk Factor for Noncompliance with Medical Treatment. Meta-analysis of the Effects of Anxiety and Depression on Patient Adherence. Arch Intern Med. 2000;160(14):2101–2107. doi:10.1001/archinte.160.14.2101
- Sundborn LT, Bingefors K. The influence of symptoms of anxiety and depression on medication nonadherence and its causes: a population based survey of prescription drug users in Sweden. Patient Prefer Adherence. 2013;7:805–811. doi:10.2147/PPA.S50055
- Piette JD, Heisler M, Krein S, Kerr EA. The role of patient-physician trust in moderating medication nonadherence due to cost pressures. Arch Intern Med. 2005;165(15):1749–1755. doi:10.1001/archinte.165.15.1749
- Goff SL, Mazor KM, Meterko V, Dodd K, Sabin J. Patients beliefs and preferences regarding doctors’ medication recommendations. J Gen Intern Med. 2008;23(3):236–241. doi:10.1007/s11606-007-0470-3
- Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;(11):CD000011. doi:10.1002/14651858.CD000011.pub4
- Haskard Zolnierek KB, DiMatteo MR. Physician Communication and Patient Adherence to Treatment: a Meta-analysis. Med Care. 2009;47(8):826–834.36. doi:10.1097/MLR.0b013e31819a5acc
- Maniaci MJ, Heckmann MG, Dawson NL. Functional health literacy and understanding of medications in discharge. Mayo Clinc Proc. 2008;83(5):554–558. doi:10.4065/83.5.554
- Madrid AH, Potpara TS, Dagres N, et al. Differences in attitude, education, and knowledge about oral anticoagulation therapy among patients with atrial fibrillation in Europe: result of a self-assessment patient survey conducted by the European Heart Rhythm Association. EP Europace. 2016;18(3):463–467. doi:10.1093/europace/euv448
- Cao Y, Feng -Y-Y, Du W, et al. Non-persistence to oral anticoagulation therapy in elderly patients with non-valvular atrial fibrillation. Patient Preference Adherence. 2023;17:3185–3194. doi:10.2147/PPA.S435592
- Leventhal H, Cameron L. Behavioural theories and the problem of compliance. Patient Educ Couns. 1987;10(2):117–138. doi:10.1016/0738-3991(87)90093-0
- Horne R, Weinman J, Barber N, Elliot R, Morgan M Concordance, adherence and compliance in medicine taking. Report for the National Co-ordinating Centre for NHS Delivery and Organisation R & D (NCCSDO). NCCSDO; 2005. Available from: https://www.ahpo.net/assets/NCCSDO%20Compliance%202005.pdf. Accessed January, 2024.
- European Medicines Agency. Report of pharmacovigilance tasks from EU Member States and the European Medicines Agency (EMA), 2019–2022. EMA/142695/2023; 2023.
- European Innovation Partnership on Active and Healthy Ageing. Adherence to medical plans for older people. European Commission; 2021. Available from: http://chrodis.eu/wp-content/uploads/2015/06/EIP-on-AHA-Infographic-Adherence-to-medical-plans.pdf. Accessed February 26, 2024.
- Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide Epidemiology of Atrial Fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8):837–847. doi:10.1161/CIRCULATIONAHA.113.005119
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–988. doi:10.1161/01.STR.22.8.983
- Schnabel RB, Yin X, Gona P, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386(9989):154–162. doi:10.1016/S0140-6736(14)61774-8
- Ding M, Ebeling M, Ziegler L, Wennberg A, Modig K. Time trends in atrial fibrillation-related stroke during 2001–2020 in Sweden: a nationwide, observational study. The Lancet Regional Health. 2023;28:100596. doi:10.1016/j.lanepe.2023.100596
- Heeringa J, van der Kuip DAM, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27(8):949–953. doi:10.1093/eurheartj/ehi825
- Lane DA, Skjøth F, Lip GYH, Larsen TB, Kotecha D. Temporal trends in incidence, prevalence and mortality of atrial fibrillation in primary care. J Am Heart Assoc. 2017;6(5):e005155. doi:10.1161/JAHA.116.005155
- Lehto M, Haukka J, Aro A, et al. Comprehensive nationwide incidence and prevalence trends of atrial fibrillation in Finland. Open Heart. 2022;9(2):e002140. doi:10.1136/openhrt-2022-002140
- Savickas V, Stewart AJ, Rees-Roberts M, et al. Opportunistic screening for atrial fibrillation by clinical pharmacists in UK general practice during the influenza vaccination season: a cross-sectional feasibility study. PLoS Med. 2020;17(7):e1003197. doi:10.1371/journal.pmed.1003197
- Savickas V, Stewart AJ, Mathie A, et al. Screening for atrial fibrillation in care homes using pulse palpation and the AliveCor Kardia Mobile device: a comparative cross-sectional pilot study. Int J Clin Pharm. 2023;46(2):529–535. doi:10.1007/s11096-023-01672-z
- Ritchie LA, Harrison SL, Penson PE, et al. Prevalence and outcomes of atrial fibrillation in older people living in care homes in Wales: a routine data linkage study 2003–2018. Age Ageing. 2022;51(12):1–10. doi:10.1093/ageing/afac252
- Alcusky M, Lapane KL. Treatment of atrial fibrillation in nursing homes: a place for direct acting oral anticoagulants? J Nurs Home Res Sci. 2018;4:15–19.
- Reardon G, Nelson WW, Patel AA, Philpot T, Neidecker M. Prevalence of Atrial Fibrillation in US Nursing Homes: Results from the National Nursing Home Survey, 1985–2004. J Am Med Dir Assoc. 2012;13(6):529–534. doi:10.1016/j.jamda.2012.03.007
- Kruger K, Sandli M, Geitung J-T, Eide GE, Grimsmo A. Atrial fibrillation and heart failure in seven nursing homes. J Nurs Educ Pract. 2012;2(4):22–32. doi:10.5430/jnep.v2n4p22
- Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation an increasing epidemic and public health challenge. Int J Stroke. 2021;16(2):217–221. doi:10.1177/1747493019897870
- Sagris D, Lip GYH. Atrial fibrillation, a contemporary sign of multimorbidity and irregular social inequity. Lancet Reg Health Eur. 2022;17:100395. doi:10.1016/j.lanepe.2022.100395
- Wardrop D, Keeling D. The story of the discovery of heparin and warfarin. Br J Haematol. 2008;141(6):757–763. doi:10.1111/j.1365-2141.2008.07119.x
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. doi:10.1093/eurheartj/ehaa612
- Aguilar MI, Hart RG. Oral anticoagulants for preventing stroke in patients with non-valvular atrial fibrillation and no previous history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2005;3:CD001927. doi:10.1002/14651858.CD001927.pub2
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–867. doi:10.7326/0003-4819-146-12-200706190-00007
- Katsanos AH, Kamel H, Healey JS, Hart RG. Stroke prevention in Atrial Fibrillation. Circulation. 2020;142(24):2371–2388. doi:10.1161/CIRCULATIONAHA.120.049768
- Hylek EM, Skates SJ, Sheehan MA, Singer DE. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med. 1996;335540:546.
- National institute for Health and care Excellence (NICE)(2013) CG161. Falls in older people assessing risk and prevention; 2013. Available from https://www.nice.org.uk/guidance/cg161. Assessed January 13, 2024.
- Jansen S, Frewen J, Finucane C, de Rooji SE, van der Velde N, Kenny R-A. AF is associated with self-reported syncope and falls in a general population cohort. Age Ageing. 2015;44(4):598–603. doi:10.1093/ageing/afv017
- Montero-Odasso M, van der Velde N, Martin FC, et al. World guidelines for falls prevention and management for older adults: a global initiative. Age Ageing. 2022;51(9):afac205. doi:10.1093/ageing/afad199
- Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GYH. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123(7):638–645. doi:10.1016/j.amjmed.2009.11.025
- Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Int J Stroke. 2011;6(1):42. doi:10.1093/ageing/afr097
- Arbel R, Sergienko R, Hammerman A, et al. Effectiveness and safety of off-label dose-reduced direct oral anticoagulants in atrial fibrillation. Am J Med. 2019;132(7):847–855. doi:10.1016/j.amjmed.2019.01.025
- Steinberg BA, Shrader P, Pieper K, et al. Outcomes registry for better informed treatment of atrial fibrillation (ORBIT-AF) II investigators. Frequency and outcomes of reduced non-vitamin K antagonist anticoagulants: results from ORBIT-AF II (the outcomes registry for better informed treatment of atrial fibrillation II). J Am Heart Assoc. 2018;7(4):e007633. doi:10.1161/JAHA.117.007633
- National Institute for Health and Care Excellence (NICE). Atrial fibrillation: diagnosis and management: NG196; 2021. Available from: https://www.nice.org.uk/guidance/ng196. Accessed January 31, 2024.
- Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med. 1999;159(7):677–685. doi:10.1001/archinte.159.7.677
- Sellers MB, Newby LK. Atrial fibrillation, anticoagulation, fall risk, and outcomes in elderly patients. Am Heart J. 2011;161(2):241–246. doi:10.1016/j.ahj.2010.11.002
- Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319(26):1701–1707. doi:10.1056/NEJM198812293192604
- Boulanger L, Kim J, Friedman M, Hauch O, Foster T, Menzin J. Patterns of use of antithrombotic therapy and quality of anticoagulation among patients with non-valvular atrial fibrillation in clinical practice. Int J Clin Pract. 2006;60(3):258–264. doi:10.1111/j.1368-5031.2006.00790.x
- Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115(21):2689–2696. doi:10.1161/CIRCULATIONAHA.106.653048
- Joint Formulary Committee. British National Formulary (online) London: BMJ Group and Pharmaceutical Press; 2022.
- Salmasi S, Adelakun A, Safari A, et al. Satisfaction with oral anticoagulants among patients with atrial fibrillation: a Prospective Observational Study. CJC Open. 2021;3(11):1347–1356. doi:10.1016/j.cjco.2021.06.015
- Neidecker M, Patel AA, Nelson WW, Reardon G. Use of warfarin in long-term care: a systematic review. BMC Geriatr. 2012;12(1):14. doi:10.1186/1471-2318-12-14
- Banerjee A, Benedetto V, Gichuru P, et al. Adherence and persistence to direct oral anticoagulants in atrial fibrillation: a population-based study. Heart. 2020;106(2):119–126. doi:10.1136/heartjnl-2019-315307
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1138–1151. doi:10.1056/NEJMoa0905561
- Steffel J, Collins R, Antz M, et al. External reviewers. 2021 European Heart Rhythm Association practical guide on the use on non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace. 2021;23:1612–1676. doi:10.1093/europace/euab065
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–962. doi:10.1016/S0140-6736(13)62343-0
- Deshpande CG, Kogut S, Laforge R, Willey C. Impact of medication adherence on risk of ischemic stroke, major bleeding and deep vein thrombosis in atrial fibrillation patients using novel oral anticoagulants. Curr Med Res Opin. 2018;34(7):1285–1292. doi:10.1080/03007995.2018.1428543
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18(11):1609–1678. doi:10.1093/europace/euw295
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1–e76. doi:10.1016/j.jacc.2014.03.022
- Camm AJ, Lip GYH, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33(21):2719–2747. doi:10.1093/eurheartj/ehs253
- Steinberg BA, Gao H, Shrader P, et al. International trends in clinical characteristics and oral anticoagulation treatment for patients with atrial fibrillation: results from the GARFIELD-AF, ORBIT-AF I, and ORBIT-AF II registries. Am Heart J. 2017;194:132–140. doi:10.1016/j.ahj.2017.08.011
- Grymonprez M, Simoens C, Steurbaut S, De Backer TL, Lahousse L. Worldwide trends in oral anticoagulant use in patients with atrial fibrillation from 2010 to 2018: a systematic review and meta-analysis. EP Europace. 2022;24(6):887–898. doi:10.1093/europace/euab303
- Mitchell A, Elmasry Y, van Poelgeest E, Welsh TJ. Anticoagulant use in older persons at risk for falls: therapeutic dilemmas-a clinical review. Eur Geriatr Med. 2023;14(4):683–696. doi:10.1007/s41999-023-00811-z
- Hanon O, Vidal J-S, Le Heuzey J-Y, et al. Oral anticoagulant use in the octogenarian European patients with atrial fibrillation: a subanalysis of PREFER in AF. Int J Cardiol. 2017;232:98–104. doi:10.1016/j.ijcard.2017.01.046
- Lahaye S, Regpala S, Lacombe S, et al. Evaluation of patients’ attitudes towards stroke prevention and bleeding risk in atrial fibrillation. Thromb Haemost. 2014;111(3):465–473. doi:10.1160/TH13-05-0424
- Lowen PS, Ji AT, Kapanen A, McClean A. Patient values and preferences for antithrombotic therapy in atrial fibrillation. Thromb Haemost. 2017;117(6):1007–1022. doi:10.1160/TH16-10-0787
- Sanghai S, Wong C, Wang Z, et al. Rates of potentially inappropriate dosing of direct-acting oral anticoagulants and associations with geriatric conditions among older patients with atrial fibrillation: the SAGE-AF Study. J Am Heart Assoc. 2020;9(6):e014108. doi:10.1161/JAHA.119.014108
- Ozaki AF, Choi AS, Le QT, et al. Real-world adherence and persistence to direct oral anticoagulants in patients with atrial fibrillation. A systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2020;13(3):e005969. doi:10.1161/CIRCOUTCOMES.119.005969
- Gorst-Rasmussen A, Skjøth F, Larsen TB, Rasmussen LH, Lip GYH, Lane DA. Dabigatran adherence in atrial fibrillation patients during the first year after diagnosis: a nationwide cohort study. J Thromb Haemost. 2015;13(4):495–504. doi:10.1111/jth.12845
- Yeh CH, Hogg K, Weitz JI. Overview of the new oral anticoagulants opportunities and challenges. Arterioscler Thromb Vasc Biol. 2015;35(5):1056–1065. doi:10.1161/ATVBAHA.115.303397
- Shore S, Carey EP, Turakhia MP, et al. Adherence to dabigatran therapy and longitudinal patient outcomes: insights from the veterans health administration. Am Heart J. 2014;167(6):810–817. doi:10.1016/j.ahj.2014.03.023
- Borne RT, O’Donnell C, Turakhia MP, et al. Adherence and outcomes to direct oral anticoagulants among patients with atrial fibrillation: findings from the veterans health administration. BMC Cardiovasc Disord. 2017;17(1):236. doi:10.1186/s12872-017-0671-6
- Cross AJ, Elliot RA, Petrie K, Kuruvilla L, George J. Interventions for improving medication-taking ability and adherence in older adults prescribed multiple medications. Cochrane Database Syst Rev. 2020;5(5):CD012419. doi:10.1002/14651858.CD012419.pub2
- Livingstone CR, Pugh ALG, Winn S, Williamson VK. Developing community pharmacy services wanted by local people: information and advice about prescription medicines. Int J Pharmacy Pract. 1996;4(2):94–102. doi:10.1111/j.2042-7174.1996.tb00849.x
- Say RE, Thomson R. The importance of patient preferences in treatment decisions-challenges for doctors. BMJ. 2003;327(7414):542–545. doi:10.1136/bmj.327.7414.542
- Kvarnström K, Airaksinen M, Liira H. Barriers and facilitators to medication adherence a qualitative study with general practitioners. BMJ Open. 2018;8(1):e015232. doi:10.1136/bmjopen-2016-015332
- Rathbone AP, Mansoor SM, Krass I, Hamrosi K, Aslani P. Qualitative study to conceptualise a model of interprofessional collaboration between pharmacists and general practitioners to support patients’ adherence to medication. BMJ Open. 2016;16(6):e010488. doi:10.1136/bmjopen-2015-010488
- American Society of Health-System Pharmacists. ASHP guidelines on pharmacist-conducted patient education and counselling. Am J Health-Sys Pharm. 1997;54(4):431–434. doi:10.1093/ajhp/54.4.431
- Valliant SN, Burbage SC, Pathak S, Urick BY. Pharmacists as accessible health care providers: quantifying the opportunity. J Manag Care Spec Pharm. 2022;28(1):85–90. doi:10.18553/jmcp.2022.28.1.85
- Tsuyuki RT, Beahm NP, Okada H, Al Hamarneh YN. Pharmacists as accessible primary health care providers: review of the evidence. Canadian Pharmacists J. 2018;151(1):4–5. doi:10.1177/1715163517745517
- Ritchie LA, Penson PE, Akpan A, Lip GYH, Lane DA. Integrated care for atrial fibrillation management: the role of the pharmacist. Am J Med. 2022;135(12):1410–1426. doi:10.1016/j.amjmed.2022.07.014
- Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol. 2017;14(11):627–628. doi:10.1038/nrcardio.2017.153
- Chao T-F, Joung B, Takahashi Y, et al. Focused update consensus guidelines of the Asia Pacific Heart Rhythm Society on stroke prevention in Atrial Fibrillation: executive summary. Thromb Haemost. 2021;122(1):20–47. doi:10.1055/s-0041-1739411
- Gov.UK. Community Pharmacy Contractual Framework for 2019/2020 to 2023/24: supporting delivery for the NHS Long Term Plan; 2019. Available from: https://www.gov.uk/government/publications/community-pharmacy-contractual-framework-2019-to-2024. Accessed February 8, 2024.
- National Health Service. Advanced Service Specification – NHS New Medicine Service (NMS); 2021. Available from: https://www.england.nhs.uk/publication/advanced-service-specification-nhs-new-medicine-service-nms/. Accessed October 8, 2024.
- National Health Service. NHS Community Pharmacy New Medicine Service (NMS) Expansion Pilot: inclusion of Depression as a Therapeutic Area and Revised Service Delivery Model. NHS England; 2022. Available from: https://www.nhsbsa.nhs.uk/sites/default/files/2022-09/NMS%20Expansion%20Pilot%20SLA-ServiceSpec%20FINAL%20v1.0.pdf. Accessed February 8, 2024.
- Hindi AMK, Schafheutle EI, Jacobs S. Patient and public perspectives of community pharmacies in the United Kingdom: a Systematic review. Health Expect. 2018;21(2):409–428. doi:10.1111/hex.12639
- Chartrand M, Lalonde L, Cantin A, et al. Anticoagulation management services in community pharmacy: feasibility of implementing a quality improvement programme through a practice-based research network. J Clin Pharm Ther. 2018;43(6):877–887. doi:10.1111/jcpt.12745
- Ingram SJ, Kirkdale CL, Williams S, et al. Moving of anticoagulation initiation and monitoring services into the community: evaluation of the Brighton and Hove community pharmacy service. BMC Health Ser Res. 2018;18(1):91. doi:10.1186/s12913-018-2901-8
- Harper P, McMichael I, Griffiths D, Harper J, Hill C. The community-based anticoagulation management service achieves a consistently high standard of anticoagulant care. N Z Med J. 2015;128(1422):31–41.
- Rossiter J, Soor G, Telner D, Aliarzadeh B, Lake J. A pharmacist-led point-of-care INR clinic: optimizing care in a family health setting. Int J Family Med. 2013;2013:691454. doi:10.1155/2013/691454
- Sharma R, Hasan SS, Gilkar IA, Hussain WF, Conway BR, Ghori MU. Pharmacist-Led interventions in optimising the use of oral anticoagulants in atrial fibrillation patients in the general practice in England: a retrospective observational study. BJGP Open. 2023;0113. doi:10.3399/BJGPO.2023.0113
- Ashjian E, Kurtz B, Renner E, Yeshe R, Barnes GD. Evaluation of a pharmacist-led outpatient direct oral anticoagulant service. AJHP. 2017;74(7):483–489. doi:10.2146/ajhp151026
- Grzesk G, Janiszewska E, Malinowski B, Kubica A, Wicinski M. Adherence in patients with atrial fibrillation treated with dabigatran. Kardiol Pol. 2018;76(11):1562–1563. doi:10.5603/KP.a2018.0194
- Merks P, Cameron JD, Balcerzak M, et al. Evaluation of a pharmacist-led intervention to improve medication adherence in patients initiating dabigatran treatment: a comparison with standard pharmacy practice in Poland. BMC Primary Care. 2022;23(1):210. doi:10.1186/s12875-022-01821-9
- Aidit S, Soh YC, Yap CS, et al. Effect of standardized warfarin treatment protocol on anticoagulant effect: comparison of a warfarin medication therapy adherence clinic with usual medical care. Front Pharmacol. 2017;8:637.10.3389. doi:10.3389/fphar.2017.00637
- Lee T, Davis E, Kelly J. Clinical impact of a pharmacist-led inpatient anticoagulation service: a review of the literature. Integr Pharm Res Prac. 2016;5:53–63. doi:10.2147/IPRP.S93312
- Phelps E, Delate T, Witt DM, Shaw PB, McCool KH, Clark NP. Effect of increased time in therapeutic range on atrial fibrillation outcomes within a centralized anticoagulation service. Thromb Res. 2018;163:54–59. doi:10.1016/j.thromres.2018.01.024
- Marcatto LR, Sacilotto L, Tavares LC, et al. Pharmaceutical care increases time in therapeutic range of patients with poor quality of anticoagulation with warfarin. Front Pharmacol. 2018;9:1052. doi:10.3389/fphar.2018.01052
- Proietti M, Lane DA. The compelling issue of nonvitamin K antagonist oral anticoagulant adherence in atrial fibrillation patients: a systematic need for new strategies. Thromb Haemost. 2020;120(3):369–371. doi:10.1055/s-0039-1697954
- NHS England. Electronic repeat dispensing (eRD); 2023. Available from: https://www.england.nhs.uk/long-read/electronic-repeat-dispensing-erd/. Accessed February 8, 2024.
- Kelly DV, Young S, Philips L, Clark D. Patient attitudes regarding the role of the pharmacist and interest in expanded pharmacist services. Can Pharm J. 2014;147(4):239–247. doi:10.1177/1715163514535731
- Blalock SJ, Roberts AW, Lauffenburger JC, Thompson T, O’Connor SK. The effect of community pharmacy-based interventions on patient health outcomes: a systematic review. Med Care Res Rev. 2013;70(3):235–266. doi:10.1177/10775587124592
- Al-Khatib A, Andreski M, Pudlo A, Doucette WR. An evaluation of community pharmacies’ actions under value-based payment. JAPHA. 2020;60(6):899–905. doi:10.1016/j.japh.2020.06.014
