Abstract
Eosinophilic esophagitis (EoE) is a chronic type 2 inflammation-mediated disease characterized by an eosinophil-predominant inflammation of the esophagus and symptoms of esophageal dysfunction. Relevant treatment outcomes in the setting of EoE include the improvement of histology, symptoms, and endoscopy findings, quality of life (QoL), and the psychological burden of the disease. Established validated tools for the assessment of EoE include questionnaires on dysphagia and QoL (ie, DSQ, EEsAI, and EoE-IQ). More recently, esophageal symptom-specific anxiety and hypervigilance, assessed using the esophageal hypervigilance and anxiety scale (EHAS), have emerged as contributors to disease burden, confirming the importance of psychological aspects in EoE patients. The EoE endoscopic reference score (EREFS) is the only validated endoscopy score in EoE and can quantify mucosal disease burden. However, esophageal panometry using the functional lumen imaging probe (FLIP) and high-resolution manometry (HRM) have shown potential to optimize the assessment of fibrostenotic features of EoE, providing novel insights into the pathophysiology of symptoms. There is a growing number of licenced and off-label therapeutic options in EoE, with various randomized controlled trials demonstrating the efficacy of proton pump inhibitors, topical steroids, food elimination diets, biological drugs, and esophageal dilatation. However, standardized optimal management strategies of EoE are currently lacking. In this review, we provide an overview of established and novel assessment tools in EoE including patient reported outcomes, FLIP panometry, HRM, endoscopy, and histology outcome measures to improve the outcomes of EoE patients. In addition, we summarize available therapeutic options for EoE based on the most recent evidence.
Introduction
Eosinophilic esophagitis (EoE) is a chronic type 2 inflammation-mediated disease characterized by an eosinophil-predominant inflammation of the esophagus in combination with symptoms of esophageal dysfunction.Citation1 The disease is diagnosed and considered histologically active when at least one esophageal biopsy, obtained during an esophagogastroduodenoscopy (EGD), shows 15 or more eosinophils per high-power field (eos/HPF), while <15 eos/HPF on multiple esophageal biopsies designates histological remission.Citation2
Several therapeutic options are currently available to induce and maintain remission in EoE based on randomized controlled trials (RCTs) demonstrating efficacy of proton pump inhibitors (PPIs), topical steroids, food elimination diets, and biological drugs.Citation3–5 In this regard, it must be noted that EoE is a lifelong disease that rapidly recurs when an effective treatment is withdrawnCitation6 and that a proportion of patients may lose response during maintenance therapy.Citation7,Citation8
Despite growing research on EoE over the past three decades,Citation9 international expert recommendations on the monitoring of EoE,Citation10 and the first clinical guidelines addressing the issue of the follow-up of the disease have been published only recently.Citation11
In the setting of EoE, treatment endpoints include the improvement of classical markers of disease activity, namely histology, symptoms, and endoscopy findings, as well as quality of life.Citation12,Citation13 However, an accurate assessment and monitoring of EoE can be complex due to the discrepancies between histological disease activity and reported symptoms,Citation14,Citation15 especially following dilatations of esophageal strictures.Citation16 In addition, endoscopy findings correlate modestly with histological disease activity and response to treatment.Citation17,Citation18 In this regard, recent evidence has suggested a role of esophageal symptom-specific anxiety, hypervigilance, and esophageal dysmotility on symptom severity,Citation19,Citation20 which may affect an accurate assessment of the global disease activity if neglected.Citation21,Citation22 In addition, novel insights into esophageal distensibility and compliance, based on esophageal panometry measured using the functional luminal imaging probe (FLIP), are improving the understanding of the mechanisms underlying symptoms in EoE.Citation23 In this comprehensive review, we summarized updated evidence on the optimal management and clinical assessment of patients with EoE to help clinicians disentangling clinical conundrums and improve the outcomes of EoE patients.
Pathogenesis, Development and Progression of EoE
EoE has been documented across all age groups, from infancy to nearly 100 years old. A retrospective analysis of a large US database, which included both children and adults, revealed that EoE prevalence rises with age, peaking in both males and females between 35 and 39 years, before declining after 45 years of age.Citation24,Citation25 Moreover, patients with EoE are more commonly males, with a 3:1 male to female ratio at all age groups.Citation26 Of note, EoE patients frequently have allergic comorbidities, with most patients showing at least one concomitant allergic disorder.Citation27 The most prevalent disorders among EoE patients are allergic rhinitis, bronchial asthma, and atopic dermatitis.Citation28
The development of EoE involves the interplay between genetic factors and environmental triggers, leading to an immune reaction against food and inhaled allergens that penetrate through a defective esophageal mucosal barrier.Citation17,Citation29 In individuals susceptible to EoE, environmental allergens provoke a chronic inflammatory response in the esophagus. This response is mediated by both the innate and adaptive immune systems and involves eosinophils, mast cells, dendritic cells, basophils, T and B lymphocytes, immunoglobulins (Ig), and cytokines such as interleukin (IL)-4, IL-5, and IL-13, collectively resulting in progressive organ dysfunction. Accordingly, it is believed that EoE progresses over time from a predominantly inflammatory phenotype to a fibro-stenotic disease.Citation30–32
Genetic factors are estimated to contribute around 14.5% to the pathogenesis of EoE, with high concordance rates among probands—58% in monozygotic twins and 36% in dizygotic twins.Citation33 Key genes implicated in EoE pathogenesis impact Th2 lymphocyte responses or epithelial barrier integrity, with significant genes including thymic stromal lymphopoietin (encoded on locus 5q22), CAPN14 (encoded on locus 2p23), and the epidermal differentiation complex (encoded on locus 1q21).Citation29
Role of Symptom Questionnaires in the Assessment of EoE
The hallmark symptom of EoE is dysphagia,Citation25 and this should be investigated querying patients about “troubles swallowing” or “sensation of a slow or delayed passage of food”. Currently recommended patient-reported outcome (PRO) measures for the assessment of EoE symptoms in the setting of RCTs include the dysphagia symptoms questionnaire (DSQ) and the EoE activity index (EEsAI), which investigate dysphagia and dysphagia-related behavioural modifications.Citation13 The latest version of the DSQ (DSQ v4.0) uses a 14-day daily recall period and comprises three questions on the presence and severity of EoE dysphagia. The questionnaire also includes a fourth standalone item on the presence of pain during swallowing, whose score was not part of the psychometric validation of the questionnaire and should therefore not be included in the calculation of the DSQ.Citation34 Higher DSQ scores correspond to increased severity of dysphagia and change in DSQ scores correlate with disease-level changes, with higher DSQ scores corresponding to increased esophageal eosinophilic burden. The EEsAI is a PRO questionnaire based on items investigating dysphagia severity and behavioural adaptation over a 7-day recall period.Citation35 The questionnaire investigates dysphagia caused by eating foods of different consistencies and takes into account behavioural adaptations including avoidance, modification, and slow eating of food. In addition, the EEsAI includes a domain addressing chest pain, heartburn, and acid regurgitation independent of eating or drinking. In the validation study, an increase in the EEsAI score demonstrated association with highly active EoE based on endoscopic and histologic findings.Citation35
With regards to daily practice, although there are no consensus-recommended questionnaires,Citation13 the use of EoE ad-hoc questionnaires has potential to improve the quantification of symptoms burden, increasing the likelihood of obtaining a correct diagnosis and optimizing management. In particular, the DSQ and EEsAI can be administered within a reasonable amount of time and using a short recall period,Citation35,Citation36 and should be thus implemented in routine clinical practice to investigate and monitor EoE patients. In addition, a recent study developed a point-of-care artificial intelligence tool to predict a diagnosis of EoE in patients with dysphagia based on reported symptoms and clinical data, prior to biopsy collection.Citation37 Although integrating the use of questionnaires into clinical practice may be challenging, the spread of telemedicine in the post-COVID era and the increasing use of informatic applications in daily clinical settings could be of help. Free applications (eg, Google Forms, Microsoft Forms), allow for the rapid creation of online questionnaires that patients can easily access through their smartphones. This approach enables the collection of questionnaire data during follow-up periods without the need for direct phone call to patients or prolonged time during outpatient clinics.
Importantly, the correlation between dysphagia and histological disease activity is weak in EoE.Citation15,Citation16,Citation34,Citation35 In this regard, recent studies have shown that esophageal symptom-specific anxiety assessed using the validated esophageal hypervigilance and anxiety scale (EHAS),Citation38 esophageal dysmotility, and adaptive behaviours may have an impact on perceived dysphagia severity,Citation19,Citation21,Citation39 and these should therefore be considered as possible confounders when clinical and histological disease activities are discordant. Moreover, other upper gastrointestinal symptoms, especially gastroesophageal reflux disease (GERD)-like symptoms, are not infrequent in EoE patients due to the overlap with GERD or esophageal motility disorders.Citation29,Citation40–43 Although there are no validated questionnaires that uniquely address non-dysphagia symptoms in EoE, such symptoms should be queried, recorded, and actioned when present.
Role of Esophageal Panometry in the Assessment of EoE
The FLIP is a novel esophageal function test consisting of a catheter equipped with a compliant cylindrical bag that has 16 1-cm spaced paired impedance planimetry electrodes, as well as a single pressure sensor placed distally. The test provides dynamic information on the cross-sectional area (CSA) of the esophageal lumen and esophageal distensibility during controlled volumetric distension. The current FLIP study protocol involves the positioning the catheter during a sedated endoscopy with 1–2 distal sensors placed inside the stomach. Subsequently, stepwise 10-mL distensions with saline of the cylindrical bag are performed, beginning with 40 mL, and proceeding to the target volume of 70 mL.Citation23,Citation44 The protocol allows to acquire dynamic impedance planimetry topographic plots and quantify esophageal distensibility and contractile responses secondary to distension (ie, secondary peristalsis).Citation45 In the setting of EoE, although FLIP cannot be considered a diagnostic tool and should not be considered prior to performing an EGD, it could be clinically useful by providing a real-time estimate of esophageal diameters, identifying strictures, and assessing the fibrostenotic burden. In addition, FLIP can be leveraged to investigate esophageal biomechanics in response to distension, and identify motility disorders.Citation23
Nicodeme et al investigated the correlation between esophageal distensibility assessed with FLIP and clinically relevant outcomes in EoE.Citation46 In particular, the authors proposed a surrogate measure of esophageal “stiffness” named distensibility plateau (DP), defined as the narrowest, fixed diameter that is observed in response to increasing FLIP volumes and pressures. The study found that a DP <225 mm² (equivalent to a diameter of <17 mm at 70 mL FLIP distension) was associated with an increased risk of food impaction and the need for esophageal dilation over a 4- to 12-month follow-up period. Subsequently, Moosavi et al confirmed that EoE patients have lower values of DP and esophageal compliance compared to asymptomatic controls.Citation47 In addition, the study found that patients with reduced values of both DP and compliance had the highest proportion of severe rings (61%) and strictures (100%) at endoscopy compared to patients with any of the two metrics within normal values. Complementarily, in another study, the severity of esophageal rings identified at endoscopy was associated with lower values of DP, corroborating the reliability of FLIP in the quantification of EoE-related fibrotic features.Citation48
With regards to contractile response patterns of the esophageal body in response to FLIP distension, Carlson et al demonstrated a significant correlation between abnormal contractile response and features of fibrostenotic remodeling in EoE patients.Citation20 Similarly, in another study, reduced esophageal distensibility and endoscopic rings were associated with abnormal contractile responses. In addition, symptom duration and diagnostic delay were negatively correlated with DP, indicating that fibrotic features in EoE are progressive and contribute both to mechanical obstruction and abnormal esophageal motility.Citation30
Carlson et al recently proposed a physio-mechanical classification of esophageal function in EoE based on a combined assessment of esophageal distensibility and motility from FLIP findings.Citation49 The physiomechanical classification is hierarchical: first, the presence of normal (compliance>450mm3/mm and DP>17mm) or reduced (compliance ≤450 mm3/mm or DP ≤17 mm) distensibility should be established. Subsequently, based on contractile response and esophago-gastric junction (EGJ) opening, patients with normal distensibility are sub-classified as normal, weak, or isolated EGJ outflow obstruction, while patients with reduced distensibility are sub-classified as fibrostenosis with normal reactivity, spastic-reactive fibrostenosis, and non-reactive fibrostenosis. Overall, fibrostenotic phenotypes had greater symptom duration, greater diagnostic delay, and higher EoE endoscopic reference (EREFS) scores compared to non-fibrostenotic phenotypes. However, normal and fibrostenotic phenotypes did not differ significantly in terms of dysphagia scores and history previous bolus impaction.Citation49 More recently, esophageal body compliance, contractile response, distensibility plateau, and maximum EGJ diameter were used to develop a composite score named C2D2 score (). In practice, each component of the C2D2 is scored as 0 for normal or 1 to 2 for increasing degree of abnormality, and subsequently summed. In the development study, the C2D2 score showed significant positive correlation with mucosal eosinophil count (rho = 0.24) and total EREFS score (rho= 0.47). In addition, a C2D2 score ≤3 had an odds ratio of 14.5 to predict future PPI response.Citation50 In conclusion, FLIP panometry is emerging as a promising tool for optimizing the assessment of EoE. In particular, FLIP could be of use in patients with persisting symptoms despite optimal therapy, to investigate the presence of providing an increased esophageal stiffness or reduced distensibility because of chronic esophageal fibrosis, which might indicate the need for esophageal dilation or escalation therapy.Citation23
Table 1 C2D2 Score of PhysioMechanical Function in EoE
Role of High-Resolution Manometry in the Assessment of EoE
EoE is a chronic inflammatory disease that causes a transmural inflammation to the esophageal wall and other structural changes that might alter the esophageal wall motility and compliance.Citation23,Citation29,Citation51 High-resolution esophageal manometry (HRM) is considered the gold standard modality for the assessment of esophageal motility and the lower esophageal sphincter functions.Citation52 The practice of HRM systems has permitted a more accurate assessment of esophageal and lower esophageal sphincter functions, with an improved ability to localize the lower esophageal sphincter. Notably, the development of HRM has permitted the creation of the Chicago Classification, currently at its fourth iteration, which is considered as a standardized working algorithm for analyzing and interpreting HRM studies.Citation53 A systematic review of the literature on esophageal motility patterns in EoE reported that, although heterogenous conventional and high-resolution manometry protocols and classifications were used in included studies, motility disorders are not infrequent in patients with EoE.Citation42 In 2009, Bassett et alCitation54 performed the first prospective esophageal motility study in EoE patients using conventional manometry. The authors reported that 23% of patients had non-specific motor disorder whereas 77% had normal esophageal motility. More recently, several groups have studied esophageal motility in EoE and reported inconsistent results; Ghisa et al,Citation40 in a retrospective study, assessed HRM findings of 109 EoE patients and reported that 38% of patients had abnormal findings. Achalasia and other obstructive disorders were found in 15% of cases. Similarly, Savarino et alCitation55 reported that 17% of 35 EoE patients had achalasia or other obstructive disorders. Overall, although various HRM studies on EoE patients have revealed inconsistent findings, achalasia and other esophageal obstructive disorders were not uncommonly reported. Accordingly, provocative measures during HRM, including the rapid drinking challenge, solid swallows, and a test meal could be helpful to disclose EGJ obstructive disorders in patients with EoE.Citation56–58 Finally, some groups have reported that pharmacological treatment of EoE may improve esophageal motility patterns at HRM.Citation59–61
In summary, esophageal motility disorders are not uncommon among EoE patients, especially achalasia and obstructive disorders. Assessment of esophageal motility by means of HRM in EoE might be of paramount significance when evaluating symptomatic refractory cases, especially in patients in histological remission and without esophageal strictures.
Role of Endoscopy in the Assessment of EoE
A diagnostic EGD is required to obtain biopsies and assess the esophageal mucosa of patients with EoE. Inflammatory histological abnormalities of EoE have a patchy nature,Citation62 therefore current guidelines recommend performing at least 6 to 8 biopsies in at least 2 different sites of the esophagus, targeting visible mucosal alterations when present, since they are associated with higher peak eosinophil counts.Citation2,Citation63,Citation64 To optimize biopsy collection, we recommend the “turn-and-suction” technique. When adopting such technique, instead of advancing the biopsy forceps against the esophageal wall and subsequently closing it to collect the tissue sample, the biopsy forceps is drawn back to the endoscope tip in an open position. Subsequently, the endoscope is turned toward the esophageal wall while suctioning and gently advancing the scope, with simultaneous closure of the biopsy forceps to obtain the tissue. Using this technique, biopsy samples are taken in a perpendicular orientation to the esophageal wall, which allows to collect larger tissue samples.Citation65
With regards to mucosal assessment, the EREFS score is the only validated endoscopic scoring system describing endoscopic features of EoE.Citation18 EREFS items include five major findings (Edema, Rings, white Exudates, linear Furrows, and Stricture) that are scored 0 to 9, while the presence of ‘crepe-paper esophagus’ is considered an adjunctive finding ().Citation18 The EREFS score has shown moderate to substantial intra- and inter-observer agreement and should be used in clinical practice to standardize the endoscopic assessment of EoE.Citation66
Figure 1 Examples of the different components of the EoE endoscopic reference score. Edema refers to the loss of normal vascular markings on the esophageal surface. Rings are circumferential ridges that are not modified by esophageal peristalsis. Exudates are white spots on the esophageal mucosa. Furrows are longitudinal red lines with variable depth. Stricture is a discrete luminal narrowing that may be non-negotiable with the endoscope (bottom left a). Crepe-paper esophagus refers to the frail and whitish appearance of the esophageal mucosa, similar to that of crepe-paper (bottom right b).
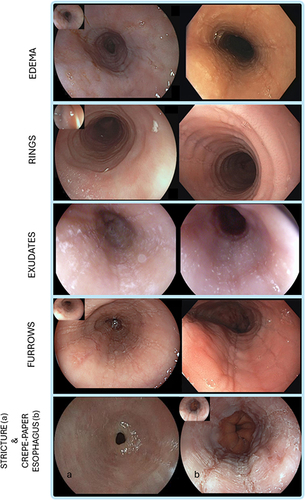
Dellon et al investigated the diagnostic utility of EREFS findings and their change in response to treatment.Citation67 The investigators found that, compared to healthy controls, EoE patients had significantly higher EREFS scores. Accordingly, based on receiver operating characteristic analysis, the EREFS score identified patients with EoE with an area under the curve of 0.94. In addition, another study found that inflammatory features of the EREFS score, including exudates and linear furrows, were associated with the highest concentration of eosinophils, while linear furrows and normal appearing mucosa were not commonly associated with >15 eos/HPF. Interestingly, rings alone, in the absence of linear furrows or white exudates were found in both histologically active and inactive EoE patients.Citation68 It must be noted, however, that approximately 11% of EoE cases present with a normal esophagus.Citation66 Accordingly, a metanalysis on 4678 EoE patients reported a low sensitivity (ranging 15–48%) and sub-optimal specificity (ranging 90–95%) of endoscopic features in the differential diagnosis of EoE.Citation69 Other non-validated endoscopic signs of EoE include the “tug-sign”Citation70 and the “pull sign”,Citation71 that is the feeling of substantial resistance when pulling on the forceps to remove the biopsy sample in EoE compared to non-EoE patients, as well as the Ankylosaurus back sign,Citation72 that is a linear arrangement of pale nodules with the appearance of the back of an Ankylosaurus dinosaur. A prospective cohort study on 83 EoE patients and 121 controls demonstrated that the pull-sign had 98% specificity and 97% positive predictive value for a diagnosis of EoE,Citation71 while a retrospective study showed that the Ankylosaurus back sign, when present, was associated with erosive esophagitis in PPI-responsive EoE.Citation72
More recently, advanced imaging techniques have been used to improve the diagnostic yield of the endoscopic assessment of EoE. In a retrospective study on 189 patients referred for upper endoscopy for dysphagia or food bolus impaction, a high-definition virtual chromoendoscopy system (ie, iSCAN; Pentax EC-3490Fi; Pentax, Tokyo, Japan), could predict a histologically confirmed diagnosis of EoE with sensitivity of 97.6% and specificity of 89.5%.Citation73 In another study, magnifying endoscopy with narrow-band imaging (ME-NBI) was used to assess NBI signs of EoE. The investigators found that the absence of sub-mucosal vascularity (absent cyan vessels), the presence of beige-coloured mucosa and dot-shaped intra-papillary capillary loops was significantly more prevalent in histologically confirmed EoE and lymphocytic esophagitis compared to GERD.Citation74 However, it must be noted that larger prospective studies are needed to validate the utility of advanced endoscopic imaging in the setting of EoE.
The EREFS score has proven utility also in the monitoring of response to treatments in EoE. Several RCTs have reported a significant reduction of EREFS scores in treatment responders compared to non-responders.Citation3 Accordingly, a recent post-hoc analysis of a comparative RCT between slurry budesonide and oral fluticasone therapy identified an EREFS score of 2 or less as a threshold to determine endoscopic response in EoE.Citation75
Treatment of Eosinophilic Esophagitis Using Licensed Drugs
EoE is a relatively young disease, and despite a large amount of RCTs investigating EoE-specific drugs,Citation9 budesonide orally disintegrating tablet (BOT) and dupilumab are the only drugs currently approved for the treatment of EoE in Europe,Citation76,Citation77 while budesonide oral suspension (BOS) and dupilumab represent the only approved drugs for EoE in the United States.Citation78,Citation79 In an RCT on 88 patients with EoE, BOT 1 mg twice daily showed efficacy as an induction of remission treatment for active EoE, with 57.6% of patients taking the active drug achieving clinical and histological remission compared to 0% of those in the placebo group (p<0.0001).Citation80 Subsequently, in a maintenance of remission study, both BOT 1 mg and 0.5 mg twice daily maintained persistent remission in 75.0% and 73.5% of patients, respectively, at week 48, compared to 4.4% of patients in the placebo group (P < 0 0.001 for both comparisons).Citation81 More recently, in an RCT on 318 patients with EoE, BOS 2 mg twice daily achieved significantly higher rates of histological and clinical remission compared to placebo (53.1% vs 1.0%; p<0.001 and 52.6% vs 39.1%, p=0.2, respectively) after 12 weeks of treatment.Citation82 In addition, in a randomized withdrawal study where EoE patients in remission were randomized to continue BOS 2 mg daily or placebo, significantly more patients taking active drug maintained clinical and histological remission at week 36 (83.3 vs 50.0%, respectively; p=0.03).Citation83 Similarly, in another RCT on 81 patients with EoE, dupilumab 300 mg once weekly demonstrated superiority compared to placebo, with 60.0% of patients taking the active drug achieving histological remission after 24 weeks of treatment compared to 5% of patients taking placebo (p<0.001). In addition, dupilumab also proved efficacy in the maintenance of remission in EoE, since results observed at week 24 of treatment were maintained or even improved at week 52.Citation84 A recent network meta-analysis confirmed that all approved drugs are more efficacious for the induction of remission and have a comparable safety profile compared to placebo in patients with EoE.Citation3 Among approved drugs, BOT 1mg twice daily, dupilumab 300 mg weekly, and BOS 1mg twice daily or 2mg twice daily were significantly more efficacious than placebo for achieving histological remission defined as <15 eos/HPF. In terms of symptoms improvement, BOT 1mg twice daily and BOS 2mg twice daily were significantly more efficacious than placebo. With regards to improvement of endoscopy findings based on the EREFS score, BOT 1mg twice daily, and BOS 1mg twice daily or 2mg twice daily ranked first and second, while dupilumab could not be included in the analysis because the data reported in the trial were non-extractable.Citation3 Despite these data confirm that all licenced drugs are effective in patients with EoE, it must be noted that the trials included in the network meta-analysis had significant heterogeneity in eligibility criteria and assessment instruments, hampering the establishment of a hierarchy to inform a therapeutic algorithm in active EoE. Recent guidelines have recommended both topical corticosteroids and dupilumab as possible first-line treatments based on treatment efficacy, although topical steroids may be prioritized because of lower costs. However, according to patient-specific clinical scenarios, especially in case of Th-2-mediated comorbidities amenable of dupilumab treatment such as asthma, atopic dermatitis, and chronic rhinosinusitis with nasal polyps, dupilumab may also be considered a first-line treatment.Citation11
Treatment of Eosinophilic Esophagitis Using off-Label Drugs
Historical off-label treatments for EoE include PPIs and aerosolized/swallowed topical steroids originally designed for the treatment of asthma. PPIs can reduce the inflammatory burden of EoE via two main mechanisms: one is the reduction of the acid refluxate, which favors the restoration of the mucosal barrier and limits environmental allergens exposure;Citation17,Citation85,Citation86 the other is the reduction of eotaxin-3 levels, an eosinophil chemoattractant.Citation87 In an early meta-analysis of mostly retrospective observational studies, although there was a significant publication bias in favour of studies reporting histologic responses to PPIs, PPIs were found to be useful for the induction of histological remission and clinical response in up to 50.5% and 60.8% of patients, respectively. Of note, twice daily administration of full-dose PPIs may achieve better outcomes compared to lower doses, and patients with a stricturing phenotype may respond less to PPIs.Citation88 These early findings were recently confirmed in a more recent European multi-centre population-based study conducted on 630 patients with EoE, in which PPIs achieved histological remission in 48.8% and decreased symptom scores in 71% of patients.Citation89 Importantly, in a recent study on 305 patients with EoE, it has been shown that twice-daily PPI dosing can achieve better outcomes compared to once-daily dosing regardless of total daily dose. In particular, omeprazole 20mg twice daily achieved significantly better outcomes compared to omeprazole 40mg once daily, with 52.8% vs 10.0% of patients achieving histological response, respectively.Citation90 In addition, PPIs could also be of use for maintaining histological remission in patients who are intolerant to or develop adverse events under topical steroid treatment.Citation86 Off-label topical steroids include aerosolized and subsequently swallowed budesonide or fluticasone. Administered doses of fluticasone usually vary between 880 and 1760 mcg/day for induction and 440–880 mcg/day for maintenance of remission, while for budesonide a dose of 2 mg/day and 1mg/day is usually employed for induction and maintenance of remission, respectively.Citation1 In a recent multi-centre population-based study conducted on 866 patients, doses ≥800 mcg/day achieved combined clinical and histological remission in 65% of patients.Citation91 Despite having shown utility in patients with EoE, off-label topical steroids are significantly less efficacious than approved EoE treatments based on a recent network meta-analysis.Citation3 Based on available evidence, current guidelines suggest that PPIs may be preferred in patients with concomitant GERD-like symptoms and that the use of off-label topical steroids should be limited to settings where other options are not available.Citation11 However, it must be noted that, due to lower costs, PPIs may still represent a first-line treatment in resource-limited settings, especially in mild disease phenotypes without esophageal stricturing or in cases with overlapping GERD symptoms.
Dietary Treatment of Eosinophilic Esophagitis
Seminal reports have demonstrated that food allergens trigger the inflammatory cascade of EoE, and that food avoidance can lead to histological remission.Citation29,Citation92,Citation93 The most common foods that are responsible for triggering an eosinophil-predominant inflammation in patients with EoE are dairy/milk, wheat/gluten, egg, soy/legumes, and seafood.Citation94 Accordingly, a possible treatment for EoE consists in the elimination of specific foods or groups of foods from the diet. Available dietary regimens include elemental diets and empirical elimination diets, while allergy tests-directed elimination diets are not superior to empiric elimination diets,Citation95 and are currently not recommended by clinical guidelines.Citation11,Citation63 Elemental diets involve feeding with amino-acidic-based formulas while avoiding all kinds of table foods. Although elemental diets can be efficacious in more than 90% of patients, the complete restriction of food and poor palatability hamper their routine use in clinical practice.Citation96 Historical empiric dietary regimens were designed with a top-down approach and started with an initial large food restriction (ie, six-food elimination diet, SFED) followed by sequential reintroductions of foods with endoscopic assessment at each reintroduction. In contrast, the novel step-up strategy involves starting from the least restrictive regimen followed by sequential restrictions based on the histological response.Citation97 The step-up design has proven to be more cost-effective and should be preferred when initiating elimination diets.Citation11,Citation98 An early study on 131 patients undergoing elimination diet showed that a two-, four-, and six-food elimination diet (TFED, FFED, SFED), could achieve histological remission in up to 43%, 60%, and 79% of patients, respectively.Citation98 A recent meta-analysis expanded early results, and showed that a one food elimination diet (OFED), TFED, FFED, and SFED could achieve histological remission in up to 51.4%, 45.7%, 49.4%, and 53.8% of patients, respectively.Citation99 More recently, an RCT on 129 patients with EoE found no difference in a milk-free dietary regimen compared to a SFED in terms of induction of histological remission (34% vs 40%, p=0.58),Citation4 corroborating that minimal restrictions and possible subsequent larger restrictions should be preferred in clinical practice.
The avoidance of trigger foods currently remains the only option targeting the cause, and not the effect, of the disease, and virtually represents a drug-free alternative to treat the disease. Food elimination diets are currently considered one of the possible first-line treatments for EoE, although they require the patient to be strongly motivated to ensure adherence. In this regard, to implement the use of dietary regimens in clinical practice, a rigorous management plan should be defined prior to starting the diet and should involve counselling on possible increased costs for shopping and negative impact on quality of life, as well as a professional dietitian to provide personalized education and tailor a nutritionally balanced and palatable diet.
On a final note, it must be acknowledged that the efficacy of elimination diets can be decreased by the unavoidable inhalation of aeroallergens during the pollen season in sensitized patients,Citation17 and that lack of long-term compliance represents a cause of treatment failure.Citation97,Citation100
Endoscopic Treatment of EoE
Untreated EoE progresses to fibrostenosis and esophageal remodeling with a reduction of the esophageal caliber, increasing the risk of food impaction.Citation25 Fibrostenosis is characterized endoscopically by esophageal rings, luminal narrowing, and strictures.Citation101 In this setting, endoscopy provides critical information to guide treatment and a barium esophagogram may complement endoscopy by increasing the yield for narrow-caliber esophagi and subtle strictures that may be missed at endoscopy.Citation102,Citation103 In a recent Delphi consensus, an esophageal diameter of at least 16 mm was identified as the target threshold for the prevention of food impaction episodes.Citation10 Endoscopic dilatation (ED) is the standard of care in fibrostenotic EoE. ED in EoE can be safely performed using either pneumatic balloons or bougies.Citation104 Although recent meta-analysis reported no significant differences between the two techniques, bougies may be more practical to use in patients with severe and long strictures, while pneumatic balloons may be more viable for short strictures.Citation105 Savary bougies do not necessarily need fluoroscopy and provide a tactile sensation to the endoscopist that helps gauging the caliber and resistance of the stricture. In addition, bougies allow to dilate the entire lumen of the esophagus in patients with a narrow-caliber esophagus. Through-The-Scope (TTS) pneumatic balloon catheters are effective in the management of short strictures and allow to inspect the mucosa trauma in real-time during the procedure (). However, TTS pneumatic balloon catheters can also be used to perform a panesophageal ED using the pull-through technique, that is retrieving the pneumatic balloon slowly and carefully following inflation.Citation106 Recently, a novel dilator device, the BougieCap (Ovesco Endoscopy AG, Tübingen, Germany) has been tested in patients with EoE.Citation107 The BougieCap is a single-use, dome-shaped, transparent hard plastic cap that is attached to the endoscope tip. In a prospective study on 57 patients, the ED with the BougieCap was technically effective in 100% of patients, although in one case the cap detached in the hypopharynx and had to be subsequently retrieved.Citation107 Regardless of the device and technique used for dilating EoE patients, a careful step-up approach with a “start low, go slow” method is advisable, usually with a maximum increase of 3 mm from initial diameter within the same session until a clear mucosal tear is observed.Citation108,Citation109
Figure 2 (a and b) Endoscopic dilatation with through-The-scope pneumatic balloon of a distal esophageal ring; panels (c and d) mucosal trauma after Savary dilatation in a narrow-caliber esophagus. In (a and b), the transparent pneumatic balloon is filled with saline and inflated, which allows to exert a radial pressure and obtain the dilatation of the esophageal stricture. (c and d) show the mucosal tear that results from the dilatation of the stricture. This is the intended outcome when performing stricture dilatations.
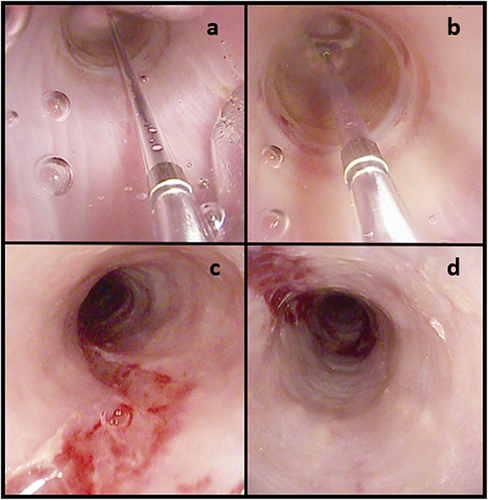
According to a meta-analysis of 27 studies, the efficacy of ED in EoE patients is high, with clinical improvement occurring in up to 95% (95% CI: 90%–98%, I2: 10%, 17 studies) of patients, while the safety profile is good, with perforations, bleeding, and hospitalisation occurring in 0.38% (95% CI: 0.18%–0.85%, I2: 0%, 27 studies), 0.05% (95% CI: 0%–0.3%, I2: 0%, 18 studies), and 0.67% (95% CI: 0.3%–1.1%, I2: 44%, 24 studies) of patients, respectively.Citation110 ED is particularly effective in controlling persistent dysphagia in fibrostenotic EoE patients with strictures or narrowed esophageal lumen but has no anti-inflammatory effect on esophageal eosinophilic infiltrates.Citation103,Citation111 In this regard, esophageal inflammation should be effectively treated before and following ED since this is associated with a decreased need for subsequent dilatations, healthcare costs, and improved quality of life.Citation112 A recent expert consensus agreed on the safety of performing ED to improve dysphagia in histologically active EoE patients.Citation103 However, when clinically viable, it seems reasonable to achieve histological remission before performing ED in patients with EoE.Citation11 On a final note, Safroneeva et al found that ED modifies the association between symptoms and esophageal eosinophilia (eos/HPF)Citation113 and that such effect of ED can last longer than one year,Citation16 making the absence of symptoms less reliable for the assessment of histological disease activity in such patients.
Current Monitoring Strategies in Eosinophilic Esophagitis
The optimal assessment of long-term treatment response in EoE is multifaceted, encompassing the evaluation of endoscopic and histological findings, symptoms, and psychological aspects to accurately determine disease status and consider potential adjustments to therapy.Citation114 Recently, Von Arnim et alCitation10 provided international expert recommendations on long-term monitoring of EoE. The consensus group agreed on the need for a regular clinical follow-up of patients with EoE to ensure treatment compliance and monitor side effects. With regards to timing, the authors proposed that EoE patients with a confirmed clinical and histological remission should receive a clinical follow-up visit 12–24 months after the last endoscopy and that follow-up surveillance endoscopy could be performed 12–24 months after the last endoscopy in patients with a stable disease. Such recommendations were based on retrospective data showing that a gap in care longer than two years was associated with progression to fibrostenosis.Citation32 However, the optimal timing for patients monitoring is yet to be established. Several studies have shown that symptoms have modest accuracy for predicting histological response in EoE,Citation14 possibly because of confounding factors including symptom-specific anxiety and esophageal dysmotility.Citation19,Citation20 Moreover, the EREFS score has proven sub-optimal responsiveness to histologic improvements and should not be used as the sole indicator of disease status in EoE.Citation115,Citation116 In addition, despite increasing research on the topic, there are currently no validated non-invasive or minimally invasive biomarkers to monitor treatment response in EoE.Citation117 Accordingly, endoscopy with biopsy, conducted 8–12 weeks after any treatment modification, is currently considered the benchmark for monitoring EoE, although robust evidence on the optimal timing is still lacking.Citation11,Citation63 Therefore, regardless of endoscopic findings and symptoms, treatment response in EoE should always rely primarily on histological findings.Citation11 In this regard, treatments aim to reduce eosinophil counts below 15/HPF. However, this threshold is somewhat arbitrary, and recent studies suggest that even lower levels of eosinophils may be associated with ongoing disease activity.Citation118 More recently, the EoE histology scoring system (EoEHSS) has been introduced to improve the assessment of typical EoE histology findings beyond peak eosinophil counts per HPF.Citation119 The critical role of a comprehensive histologic analysis is underscored by the association of persistent symptoms with specific histologic features like basal cell hyperplasia despite low eosinophil counts.Citation120 In addition, the EoEHSS has shown excellent agreement between pathologists, can discriminate treated from untreated EoE patients better than the peak eosinophil count, and correlates with symptom scores of EoE.Citation121 Therefore, we feel that a thorough histological examination is pivotal to guide treatment adjustments, with the aim of achieving the minimal eosinophil presence and improvement of adjunctive histological findings to ensure disease remission.
A significant limitation of esophageal biopsy specimens is the inability to comprehensively capture the esophageal epithelium, as they often contain a minimal amount of the submucosa, including the lamina propria and muscularis.Citation122 In response to this challenge, esophageal panometry with FLIP has been introduced as an innovative tool for assessing esophageal distensibility/compliance and contractile response to distension, offering insights into the structural integrity and mechanical properties of the esophageal wall. FLIP could thus serve as an objective measure of structural changes in the esophagus, and the monitoring of changes in esophageal distensibility before and after treatment can help to evaluate the structural response to therapy, complementing histological and symptomatic assessments.Citation23
While histological improvement is central, the correlation between eosinophil counts and patient symptoms is still imperfect. In fact, many patients report symptom resolution even when histological findings do not fully support this improvement.Citation15 Conversely, some patients continue to experience symptoms despite histological remission.Citation123 This discrepancy highlights the importance of incorporating PRO measures into the treatment response assessment, which could provide a quantitative measure of symptom severity and a subjective response to treatment.Citation124 PRO measures complement histological findings in EoE, and prompt clinicians to tailor therapeutic approaches. Indeed, despite the availability of validated PRO tools for adults with EoE (ie, DSQ, EEsAI), a comprehensive symptomatic management strategy is still missing.Citation125 Recently, Dellon et al developed the Index of Severity for Eosinophilic Esophagitis (I-SEE).Citation126 The I-SEE assesses three domains including symptoms and complications, inflammatory features (at endoscopy and histology), and fibrostenotic features (at endoscopy and histology). The score assesses the severity of EoE using a point scale of 0–6 for mild, 7–14 for moderate, and ≥15 for severe, and has recently been shown to decrease following successful treatment in adult EoE patients.Citation127
EoE affects not only physical health but also significantly impacts psychological well-being, causing symptom-specific anxiety and esophageal hypervigilance, especially related to eating and fear of food impaction.Citation19 Anxiety and hypervigilance in EoE can be assessed using the validated esophageal and hypervigilance anxiety scale (EHAS).Citation38 Psychological factors of EoE patients can worsen symptoms or affect their perceived severity. Research by Carlson et al showed these factors predict dysphagia severity, highlighting the need for psychological evaluations in treatment assessment using tools such as the EHAS.Citation128 Recently, McCann et al validated a novel PRO tool, name EOE impact questionnaire (EoE-IQ), that measures health-related quality of life in patients with EoE.Citation129 The EoE-IQ contains 11 items that evaluate the impact of EoE on emotional functioning, social impact, school or work impact, and sleep disruption. The questionnaire has been shown to correlate with clinical and endoscopy outcome measures in patients with EoE.Citation129 In adult patients, health-related quality of life can be assessed using the EoO-QOL-A,Citation130 a valid and reliable disease-specific tool which includes five main domains: Eating/Diet Impact, Social Impact, Emotional Impact, Disease Anxiety, and Choking Anxiety. This questionnaire has demonstrated excellent internal consistency and test–retest reliability. However, its length and the fact that it has been validated only in English and Spanish have limited its use in clinical practice. For pediatric patients, the Pediatric Eosinophilic Esophagitis Symptom Score (PEESS v2.0) is another validated PRO questionnaire.Citation131 It comprises 20 items that measure four domains of EoE-specific symptoms: dysphagia, GERD, nausea/vomiting, and pain. The score ranges from 0 to 100 and aligns with clinical symptoms and histopathologic severity.
The assessment of esophageal hypervigilance, anxiety, and quality of life in EoE helps to identify conditioned responses that affect the gut-brain interaction, especially in patients with persistent symptoms or those reintroducing foods post-elimination diet.Citation19 Accordingly, an increased awareness of neurogastroenterology and disorders of gut–brain interaction is important in the setting of EoE.Citation132 Integrating psychological support and cognitive-behavioural techniques can enhance treatment success and adherence. In this regard, patients often adopt dietary and lifestyle changes to manage EoE, impacting their nutrition and quality of life,Citation133 emphasizing the importance of comprehensive strategies that include dietary, nutritional, and psychosocial evaluations to fully address the complexity of EoE.
Conclusion
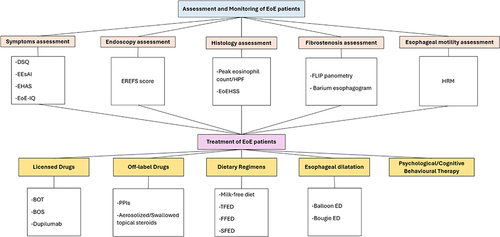
EoE is a complex condition with histological, symptomatic, endoscopic, and psychological aspects that should be addressed by clinicians to optimally manage the condition. In this review, we summarized established and novel assessment tools that should be implemented in clinical practice to optimize care and improve patients’ outcomes. We also provided an overview of available licensed and off-label drug treatments, most recent dietary strategies, and ED strategies. In we provide a summary of available tools for an optimal assessment and management of patients with EoE. Given the increasing incidence of the disease in parallel with the advent of new drugs for its treatment, a standardised and shared approach is highly recommended to improve the clinical management of EoE.
Figure 3 Summary of optimal assessment and management of patients with EoE shows the summary of available options to improve the assessment, monitoring, and treatment of patients with EoE.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. Guarantor of the article: Edoardo V. Savarino. Pierfrancesco Visaggi and Matteo Ghisa joint first authorship.
Disclosure
PV: Has served as speaker for Dr Falk Pharma, Malesci, JB Pharma; MG: Has served as speaker for Sanofi; EV: lecture or consultancy fees from Alfasigma, Dr Falk Pharma, Malesci, Sanofi; AP has served as consultant for Fenix Pharma; EM: Dr Falk and alfaSigma; NdB: Lectures fees from Malesci and Reckitt Benckiser, grant for speech from SANOFI-Genzyme and grants from DrFalk; EVS: has received lecture or consultancy fees from reports personal fees from AbbVie, Abivax, Agave, AGPharma, Alfasigma, CaDiGroup, Celltrion, Dr Falk, EG Stada Group, Fenix Pharma, Galapagos, Johnson&Johnson, JB Pharmaceuticals, Innovamedica/Adacyte, Eli Lilly, Malesci, Mayoly Biohealth, Omega Pharma, Pfizer, Reckitt Benckiser, Sandoz, SILA, Sofar, Takeda, Tillots, Unifarco, grants from Bonollo, Difass, Pfizer, Reckitt Benckiser, SILA, Sofar, Unifarco, Zeta Farmaceutici, personal fees from AbbVie, Agave, Alfasigma, Biogen, Bristol-Myers Squibb, Celltrion, Dr. Falk, Eli Lilly, Fenix Pharma, Johnson&Johnson, JB Pharmaceuticals, Merck & Co, Nestlè, Pfizer, Reckitt Benckiser, Regeneron, Sanofi, SILA, Sofar, Takeda, Unifarco, during the conduct of the study. The authors report no other conflicts of interest in this work.
Data Sharing Statement
No additional data available.
Additional information
Funding
References
- Visaggi P, Savarino E, Sciume G, et al. Eosinophilic esophagitis: clinical, endoscopic, histologic and therapeutic differences and similarities between children and adults. Ther Adv Gastroenterol. 2021;14:1756284820980860. doi:10.1177/1756284820980860
- de Bortoli N, Visaggi P, Penagini R, et al. The 1st EoETALY consensus on the diagnosis and management of eosinophilic esophagitis - definition, clinical presentation and diagnosis. Digestive Liver Dis. 2024;2004:1.
- Visaggi P, Barberio B, Del Corso G, et al. Comparison of drugs for active eosinophilic oesophagitis: systematic review and network meta-analysis. Gut. 2023;72:2019–2030. doi:10.1136/gutjnl-2023-329873
- Kliewer KL, Gonsalves N, Dellon ES, et al. One-food versus six-food elimination diet therapy for the treatment of eosinophilic oesophagitis: a multicentre, randomised, open-label trial. Lancet Gastroenterol Hepatol. 2023;8(5):408–421. doi:10.1016/S2468-1253(23)00012-2
- Marasco G, Visaggi P, Vassallo M, et al. Current and novel therapies for eosinophilic gastrointestinal diseases. Int J Mol Sci. 2023;24(20):15165. doi:10.3390/ijms242015165
- Dellon ES, Woosley JT, Arrington A, et al. Rapid recurrence of eosinophilic esophagitis activity after successful treatment in the observation phase of a randomized, double-blind, double-dummy trial. Clin Gastroenterol Hepatol. 2020;18(7):1483–92.e2. doi:10.1016/j.cgh.2019.08.050
- Molina-Infante J, Rodriguez-Sanchez J, Martinek J, et al. Long-term loss of response in proton pump inhibitor-responsive esophageal eosinophilia is uncommon and influenced by CYP2C19 genotype and rhinoconjunctivitis. Am J Gastroenterol. 2015;110(11):1567–1575. doi:10.1038/ajg.2015.314
- Eluri S, Runge TM, Hansen J, et al. Diminishing effectiveness of long-term maintenance topical steroid therapy in PPI non-responsive eosinophilic esophagitis. Clin Translat Gastroenterol. 2017;8(6):e97. doi:10.1038/ctg.2017.27
- Visaggi P, Ghisa M, Barberio B, et al. Treatment trends for eosinophilic esophagitis and the other eosinophilic gastrointestinal diseases: systematic review of clinical trials. Digestive Liver Dis. 2022;2022:1.
- Arnim UV, Biedermann L, Aceves SS, et al. Monitoring patients with eosinophilic esophagitis in routine clinical practice - international expert recommendations. Clin Gastroenterol Hepatol. 2022;21:2526–2533. doi:10.1016/j.cgh.2022.12.018
- de Bortoli N, Visaggi P, Penagini R, et al. The 1st EoETALY consensus on the diagnosis and management of eosinophilic esophagitis-current treatment and monitoring. Digestive Liver Dis. 2024;2024:1.
- Dellon ES, Gupta SK. A conceptual approach to understanding treatment response in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2019;17(11):2149–2160. doi:10.1016/j.cgh.2019.01.030
- Ma C, Schoepfer AM, Dellon ES, et al. Development of a core outcome set for therapeutic studies in eosinophilic esophagitis (COREOS). J Allergy Clin Immunol. 2022;149(2):659–670. doi:10.1016/j.jaci.2021.07.001
- Chang JW, Yeow RY, Waljee AK, Rubenstein JH. Systematic review and meta-regressions: management of eosinophilic esophagitis requires histologic assessment. Diseas Esophag. 2018;31(8). doi:10.1093/dote/doy049
- Safroneeva E, Straumann A, Coslovsky M, et al. Symptoms have modest accuracy in detecting endoscopic and histologic remission in adults with eosinophilic esophagitis. Gastroenterology. 2016;150(3):581–90.e4. doi:10.1053/j.gastro.2015.11.004
- Safroneeva E, Pan Z, King E, et al. Long-lasting dissociation of esophageal eosinophilia and symptoms after dilation in adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2022;20(4):766–75.e4. doi:10.1016/j.cgh.2021.05.049
- Visaggi P, Savarino E, Del Corso G, et al. Six-food elimination diet is less effective during pollen season in adults with eosinophilic esophagitis sensitized to pollens. Am J Gastroenterol. 2023;118:1957–1962. doi:10.14309/ajg.0000000000002357
- Hirano I, Moy N, Heckman MG, Thomas CS, Gonsalves N, Achem SR. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2013;62(4):489–495. doi:10.1136/gutjnl-2011-301817
- Taft TH, Carlson DA, Simons M, et al. Esophageal hypervigilance and symptom-specific anxiety in patients with eosinophilic esophagitis. Gastroenterology. 2021;161(4):1133–1144. doi:10.1053/j.gastro.2021.06.023
- Carlson DA, Shehata C, Gonsalves N, et al. Esophageal dysmotility is associated with disease severity in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2021;2021:1.
- Visaggi P, Ghisa M, Marabotto E, et al. Esophageal dysmotility in patients with eosinophilic esophagitis: pathogenesis, assessment tools, manometric characteristics, and clinical implications. Esophagus. 2022;2022:1.
- Naik RD, Patel DA. Unlocking the mind might be critical in management of eosinophilic esophagitis: expanding beyond drugs, dilation. and Diet Gastroenterol. 2021;161(4):1099–1101. doi:10.1053/j.gastro.2021.07.032
- Savarino E, Di Pietro M, Bredenoord AJ, et al. Use of the functional lumen imaging probe in clinical esophagology. Off J Am Coll Gastroenterol. 2020;115(11):1.
- Prasad GA, Alexander JA, Schleck CD, et al. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol. 2009;7(10):1055–1061. doi:10.1016/j.cgh.2009.06.023
- Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology. 2018;154(2):319–32.e3. doi:10.1053/j.gastro.2017.06.067
- Arias A, Perez-Martinez I, Tenias JM, Lucendo AJ. Systematic review with meta-analysis: the incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther. 2016;43(1):3–15. doi:10.1111/apt.13441
- Mohammad AA, Wu SZ, Ibrahim O, et al. Prevalence of atopic comorbidities in eosinophilic esophagitis: a case-control study of 449 patients. J Am Acad Dermatol. 2017;76(3):559–560. doi:10.1016/j.jaad.2016.08.068
- González-Cervera J, Arias Á, Redondo-González O, Cano-Mollinedo MM, Terreehorst I, Lucendo AJ. Association between atopic manifestations and eosinophilic esophagitis: a systematic review and meta-analysis. Anna Allergy Asthma Immunol. 2017;118(5):582–90.e2. doi:10.1016/j.anai.2017.02.006
- Sciumè GD, Visaggi P, Sostilio A, et al. Eosinophilic esophagitis: novel concepts regarding pathogenesis and clinical manifestations. Miner Gastroent. 2022;68(1):23–39. doi:10.23736/S2724-5985.20.02807-X
- Araujo IK, Shehata C, Hirano I, et al. The severity of reduced esophageal distensibility parallels eosinophilic esophagitis disease duration. Clin Gastroenterol Hepatol. 2023;22:513–522.e1. doi:10.1016/j.cgh.2023.04.027
- Muir AB, Ackerman SJ, Pan Z, et al. Esophageal remodeling in eosinophilic esophagitis: relationships to luminal captured biomarkers of inflammation and periostin. J Allergy Clin Immunol. 2022;150:649–656.e5. doi:10.1016/j.jaci.2022.03.022
- Chang NC, Thakkar KP, Ketchem CJ, Eluri S, Reed CC, Dellon ES. A gap in care leads to progression of fibrosis in eosinophilic esophagitis patients. Clin Gastroenterol Hepatol. 2022;20(8):1701–8.e2. doi:10.1016/j.cgh.2021.10.028
- Alexander ES, Martin LJ, Collins MH, et al. Twin and family studies reveal strong environmental and weaker genetic cues explaining heritability of eosinophilic esophagitis. J Allergy Clin Immunol. 2014;134(5):1084–92.e1. doi:10.1016/j.jaci.2014.07.021
- Hudgens S, Evans C, Phillips E, Hill M. Psychometric validation of the dysphagia symptom questionnaire in patients with eosinophilic esophagitis treated with budesonide oral suspension. J Patient Rep Outcomes. 2017;1(1):3. doi:10.1186/s41687-017-0006-5
- Schoepfer AM, Straumann A, Panczak R, et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology. 2014;147(6):1255–66.e21. doi:10.1053/j.gastro.2014.08.028
- Warners MJ, Hindryckx P, Levesque BG, et al. Systematic review: disease activity indices in eosinophilic esophagitis. Am J Gastroenterol. 2017;112(11):1658–1669. doi:10.1038/ajg.2017.363
- Visaggi P, Del Corso G, Svizzero FB, et al. Artificial intelligence tools for the diagnosis of eosinophilic esophagitis in adults reporting dysphagia: development, external validation, and software creation for point-of-care use. J Aller Clin Immunol Pract. 2023;12:1008–1016.e1. doi:10.1016/j.jaip.2023.12.031
- Taft TH, Triggs JR, Carlson DA, et al. Validation of the oesophageal hypervigilance and anxiety scale for chronic oesophageal disease. Aliment Pharmacol Ther. 2018;47(9):1270–1277. doi:10.1111/apt.14605
- Alexander R, Alexander JA, Ravi K, et al. Measurement of observed eating behaviors in patients with active and inactive eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2019;17(11):2371–2373. doi:10.1016/j.cgh.2018.12.011
- Ghisa M, Laserra G, Marabotto E, et al. Achalasia and obstructive motor disorders are not uncommon in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2020;2020:1.
- Frazzoni M, Penagini R, Frazzoni L, et al. Role of reflux in the pathogenesis of eosinophilic esophagitis: comprehensive appraisal with off- and on PPI impedance-pH monitoring. Am J Gastroenterol. 2019;114(10):1606–1613. doi:10.14309/ajg.0000000000000379
- Visaggi P, Ghisa M, Barberio B, Marabotto E, de Bortoli N, Savarino E. Systematic review: esophageal motility patterns in patients with eosinophilic esophagitis. Digestive Liver Dis. 2022;54:1143–1152. doi:10.1016/j.dld.2022.01.003
- Visaggi P, Mariani L, Svizzero FB, et al. Clinical use of mean nocturnal baseline impedance and post-reflux swallow-induced peristaltic wave index for the diagnosis of gastro-esophageal reflux disease. Esophagus. 2022;19:525–534. doi:10.1007/s10388-022-00933-6
- Carlson DA, Gyawali CP, Khan A, et al. Classifying esophageal motility by FLIP panometry: a study of 722 subjects with manometry. Am J Gastroenterol. 2021;116(12):2357–2366. doi:10.14309/ajg.0000000000001532
- Carlson DA, Kou W, Lin Z, et al. Normal values of esophageal distensibility and distension-induced contractility measured by functional luminal imaging probe panometry. Clin Gastroenterol Hepatol. 2019;17(4):674–81.e1. doi:10.1016/j.cgh.2018.07.042
- Nicodème F, Hirano I, Chen J, et al. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2013;11(9):1101–7.e1. doi:10.1016/j.cgh.2013.03.020
- Moosavi S, Shehata C, Kou W, et al. Measuring esophageal compliance using functional lumen imaging probe to assess remodeling in eosinophilic esophagitis. Neurogastroenterol Motil. 2023;35(4):e14525. doi:10.1111/nmo.14525
- Chen JW, Pandolfino JE, Lin Z, et al. Severity of endoscopically identified esophageal rings correlates with reduced esophageal distensibility in eosinophilic esophagitis. Endoscopy. 2016;48(9):794–801. doi:10.1055/s-0042-107340
- Carlson DA, Hirano I, Gonsalves N, et al. A physiomechanical model of esophageal function in eosinophilic esophagitis. Gastroenterology. 2023;165(3):552–63.e4. doi:10.1053/j.gastro.2023.05.031
- Carlson DA, Hirano I, Gonsalves N, et al. Composite score of physiomechanical esophageal function using functional lumen imaging probe panometry in eosinophilic esophagitis. Gastrointest Endosc. 2024;99(4):499–510.e1. doi:10.1016/j.gie.2023.10.048
- Savarino E, Bhatia S, Roman S, et al. Achalasia. Nat Rev Dis Primers. 2022;8(1):28. doi:10.1038/s41572-022-00356-8
- Mari A, Savarino E. Advances on neurogastroenterology and motility disorders: pathophysiology, diagnostics and management. J Clin Med. 2022;11(10). doi:10.3390/jcm11102911
- Yadlapati R, Kahrilas PJ, Fox MR, et al. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0(©). Neurogastroenterol Motil. 2021;33(1):e14058. doi:10.1111/nmo.14058
- Bassett J, Maydonovitch C, Perry J, Sobin L, Osgard E, Wong R. Prevalence of esophageal dysmotility in a cohort of patients with esophageal biopsies consistent with eosinophilic esophagitis. Diseas Esophag. 2009;22(6):543–548. doi:10.1111/j.1442-2050.2009.00949.x
- Savarino EV, Tolone S, Bartolo O, et al. The GerdQ questionnaire and high resolution manometry support the hypothesis that proton pump inhibitor-responsive oesophageal eosinophilia is a GERD-related phenomenon. Aliment Pharmacol Ther. 2016;44(5):522–530. doi:10.1111/apt.13718
- Visaggi P, Ghisa M, Del Corso G, et al. Chicago classification v4.0 protocol improves specificity and accuracy of diagnosis of oesophagogastric junction outflow obstruction. Aliment Pharmacol Ther. 2022;56:606–613. doi:10.1111/apt.17101
- Sanagapalli S, McGuire J, Leong RW, et al. The clinical relevance of manometric esophagogastric junction outflow obstruction can be determined using rapid drink challenge and solid swallows. Am J Gastroenterol. 2021;116(2):280–288. doi:10.14309/ajg.0000000000000988
- Ang D, Misselwitz B, Hollenstein M, et al. Diagnostic yield of high-resolution manometry with a solid test meal for clinically relevant, symptomatic oesophageal motility disorders: serial diagnostic study. Lancet Gastroenterol Hepatol. 2017;2(9):654–661. doi:10.1016/S2468-1253(17)30148-6
- Lucendo AJ. Motor disturbances participate in the pathogenesis of eosinophilic oesophagitis, beyond the fibrous remodelling of the oesophagus. Aliment Pharmacol Ther. 2006;24(8):1264–1267. doi:10.1111/j.1365-2036.2006.03109.x
- Hempel SL, Elliott DE. Chest pain in an aspirin-sensitive asthmatic patient. Eosinophilic esophagitis causing esophageal dysmotility. Chest. 1996;110(4):1117–1120. doi:10.1378/chest.110.4.1117
- Savarino E, Gemignani L, Zentilin P, et al. Achalasia with dense eosinophilic infiltrate responds to steroid therapy. Clin Gastroenterol Hepatol. 2011;9(12):1104–1106. doi:10.1016/j.cgh.2011.08.002
- Hiremath G, Sun L, Collins MH, et al. Esophageal epithelium and lamina propria are unevenly involved in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2023;21(11):2807–16.e3. doi:10.1016/j.cgh.2023.03.014
- Dhar A, Haboubi HN, Attwood SE, et al. British society of gastroenterology (BSG) and British society of paediatric gastroenterology, hepatology and nutrition (BSPGHAN) joint consensus guidelines on the diagnosis and management of eosinophilic oesophagitis in children and adults. Gut. 2022;71(8):1459–1487. doi:10.1136/gutjnl-2022-327326
- Hirano I, Chan ES, Rank MA, et al. AGA institute and the joint task force on allergy-immunology practice parameters clinical guidelines for the management of eosinophilic esophagitis. Gastroenterology. 2020;158(6):1776–1786. doi:10.1053/j.gastro.2020.02.038
- Levine DS, Reid BJ. Endoscopic biopsy technique for acquiring larger mucosal samples. Gastrointest Endosc. 1991;37(3):332–337. doi:10.1016/S0016-5107(91)70726-8
- van Rhijn BD, Warners MJ, Curvers WL, et al. Evaluating the endoscopic reference score for eosinophilic esophagitis: moderate to substantial intra- and interobserver reliability. Endoscopy. 2014;46(12):1049–1055. doi:10.1055/s-0034-1377781
- Dellon ES, Cotton CC, Gebhart JH, et al. Accuracy of the eosinophilic esophagitis endoscopic reference score in diagnosis and determining response to treatment. Clin Gastroenterol Hepatol. 2016;14(1):31–39. doi:10.1016/j.cgh.2015.08.040
- Salek J, Clayton F, Vinson L, et al. Endoscopic appearance and location dictate diagnostic yield of biopsies in eosinophilic oesophagitis. Aliment Pharmacol Ther. 2015;41(12):1288–1295. doi:10.1111/apt.13201
- Kim H, Vance R, Shaheen N, Dellon E. The Prevalence and Diagnostic Utility of Endoscopic Features of Eosinophilic Esophagitis: a Meta-Analysis. Clin Gastroenterol Hepatol. 2012;10:988–96.e5. doi:10.1016/j.cgh.2012.04.019
- Moawad FJ, Robinson CL, Veerappan GR, Summers TA, Maydonovitch CL, Wong R. The tug sign: an endoscopic feature of eosinophilic esophagitis. Am J Gastroenterol. 2013;108(12):1938–1939. doi:10.1038/ajg.2013.252
- Dellon ES, Gebhart JH, Higgins LL, Hathorn KE, Woosley JT, Shaheen NJ. The esophageal biopsy ”pull” sign: a highly specific and treatment-responsive endoscopic finding in eosinophilic esophagitis (with video). Gastrointest Endosc. 2016;83(1):92–100. doi:10.1016/j.gie.2015.05.046
- Ishimura N, Sumi S, Okada M, et al. Ankylosaurus back sign: novel endoscopic finding in esophageal eosinophilia patients indicating proton pump inhibitor response. Endosc Int Open. 2018;6(2):E165–e72. doi:10.1055/s-0043-122882
- Gregory E, Fort Gasia M, Gui X, Ghosh S, Iacucci M. High-definition-iSCAN virtual chromoendoscopy has high sensitivity and specificity for the diagnosis of eosinophilic esophagitis. Endosc Int Open. 2017;5(7):E613–e21. doi:10.1055/s-0043-111591
- Ichiya T, Tanaka K, Rubio CA, Hammar U, Schmidt PT. Evaluation of narrow-band imaging signs in eosinophilic and lymphocytic esophagitis. Endoscopy. 2017;49(5):429–437. doi:10.1055/s-0043-101685
- Cotton CC, Woosley JT, Moist SE, et al. Determination of a treatment response threshold for the eosinophilic esophagitis endoscopic reference score. Endoscopy. 2022;54(7):635–643. doi:10.1055/a-1675-7860
- EMA. Budesonide orally disintegrating tablet EMA approval; 2023.
- EMA. Dupilumab Approval EMA; 2023. https://www.ema.europa.eu/en/documents/product-information/dupixent-epar-product-information_en.pdf. Accessed July 22, 2024.
- FDA. Budesonide oral suspension FDA approval; 2024.
- FDA. Dupilumab FDA Approval; 2023.
- Lucendo AJ, Miehlke S, Schlag C, et al. Efficacy of budesonide orodispersible tablets as induction therapy for eosinophilic esophagitis in a randomized placebo-controlled trial. Gastroenterology. 2019;157(1):74–86.e15. doi:10.1053/j.gastro.2019.03.025
- Straumann A, Lucendo AJ, Miehlke S, et al. Budesonide orodispersible tablets maintain remission in a randomized, placebo-controlled trial of patients with eosinophilic esophagitis. Gastroenterology. 2020;159(5):1672–85.e5. doi:10.1053/j.gastro.2020.07.039
- Hirano I, Collins MH, Katzka DA, et al. Budesonide oral suspension improves outcomes in patients with eosinophilic esophagitis: results from a phase 3 trial. Clin Gastroenterol Hepatol. 2022;20(3):525–34.e10. doi:10.1016/j.cgh.2021.04.022
- Dellon ES, Collins MH, Katzka DA, et al. Long-term treatment of eosinophilic esophagitis with budesonide oral suspension. Clin Gastroenterol Hepatol. 2021;19:699–706.e4. doi:10.1016/j.cgh.2020.03.060
- Rothenberg ME, Dellon ES, Collins MH, et al. Efficacy and safety of dupilumab up to 52 weeks in adults and adolescents with eosinophilic oesophagitis (LIBERTY EoE TREET study): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol. 2023;8(11):990–1004. doi:10.1016/S2468-1253(23)00204-2
- van Rhijn BD, Weijenborg PW, Verheij J, et al. Proton pump inhibitors partially restore mucosal integrity in patients with proton pump inhibitor-responsive esophageal eosinophilia but not eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2014;12(11):1815–23.e2. doi:10.1016/j.cgh.2014.02.037
- Visaggi P, Svizzero FB, Del Corso G, Bellini M, Savarino E, de Bortoli N. Efficacy of Second PPI course following steroid-induced remission in eosinophilic esophagitis refractory to initial PPI therapy. Am J Gastroenterol. 2022;117:1702–1705. doi:10.14309/ajg.0000000000001943
- Cheng E, Zhang X, Huo X, et al. Omeprazole blocks eotaxin-3 expression by oesophageal squamous cells from patients with eosinophilic oesophagitis and GORD. Gut. 2013;62(6):824–832. doi:10.1136/gutjnl-2012-302250
- Lucendo AJ, Arias A, Molina-Infante J. Efficacy of proton pump inhibitor drugs for inducing clinical and histologic remission in patients with symptomatic esophageal eosinophilia: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016;14(1):13–22.e1. doi:10.1016/j.cgh.2015.07.041
- Laserna-Mendieta EJ, Casabona S, Guagnozzi D, et al. Efficacy of proton pump inhibitor therapy for eosinophilic oesophagitis in 630 patients: results from the EoE connect registry. Aliment Pharmacol Ther. 2020;52(5):798–807. doi:10.1111/apt.15957
- Muftah M, Goldin AH, Barshop K, et al. Twice-daily proton pump inhibitor induces higher remission rate in eosinophilic esophagitis than once-daily regimen regardless of total daily dose. Am J Gastroenterol. 2024;119:991–995. doi:10.14309/ajg.0000000000002712
- Laserna-Mendieta EJ, Navarro P, Casabona-Francés S, et al. Swallowed topical corticosteroids for eosinophilic esophagitis: utilization and real-world efficacy from the EoE CONNECT registry. Unit Europ Gastroenterol J. 2024;12:585–595. doi:10.1002/ueg2.12533
- Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology. 1995;109(5):1503–1512. doi:10.1016/0016-5085(95)90637-1
- Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4(9):1097–1102. doi:10.1016/j.cgh.2006.05.026
- Visaggi P, Mariani L, Pardi V, et al. Dietary management of eosinophilic esophagitis: tailoring the approach. Nutrients. 2021;13(5):1630. doi:10.3390/nu13051630
- Arias A, Gonzalez-Cervera J, Tenias JM, Lucendo AJ. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: a systematic review and meta-analysis. Gastroenterology. 2014;146(7):1639–1648. doi:10.1053/j.gastro.2014.02.006
- Rank MA, Sharaf RN, Furuta GT, et al. Technical review on the management of eosinophilic esophagitis: a report from the AGA institute and the joint task force on allergy-immunology practice parameters. Anna Allergy Asthma Immunol. 2020;124(5):424–40.e17. doi:10.1016/j.anai.2020.03.021
- Visaggi P, Baiano Svizzero F, Savarino E. Food elimination diets in eosinophilic esophagitis: practical tips in current management and future directions. Best Pract Res Clin Gastroenterol. 2023;101825. doi:10.1016/j.bpg.2023.101825
- Molina-Infante J, Arias A, Alcedo J, et al. Step-up empiric elimination diet for pediatric and adult eosinophilic esophagitis: the 2-4-6 study. J Allergy Clin Immunol. 2018;141(4):1365–1372. doi:10.1016/j.jaci.2017.08.038
- Mayerhofer C, Kavallar AM, Aldrian D, Lindner AK, Müller T, Vogel GF. Efficacy of elimination diets in eosinophilic esophagitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2023;21(9):2197–210.e3. doi:10.1016/j.cgh.2023.01.019
- Reed CC, Fan C, Koutlas NT, Shaheen NJ, Dellon ES. Food elimination diets are effective for long-term treatment of adults with eosinophilic oesophagitis. Aliment Pharmacol Ther. 2017;46(9):836–844. doi:10.1111/apt.14290
- Warners MJ, Oude Nijhuis RAB, de Wijkerslooth LRH, Smout A, Bredenoord AJ. The natural course of eosinophilic esophagitis and long-term consequences of undiagnosed disease in a large cohort. Am J Gastroenterol. 2018;113(6):836–844. doi:10.1038/s41395-018-0052-5
- Gentile N, Katzka D, Ravi K, et al. Oesophageal narrowing is common and frequently under-appreciated at endoscopy in patients with oesophageal eosinophilia. Aliment Pharmacol Ther. 2014;40(11–12):1333–1340. doi:10.1111/apt.12977
- Aceves SS, Alexander JA, Baron TH, et al. Endoscopic approach to eosinophilic esophagitis: American society for gastrointestinal endoscopy consensus conference. Gastrointest Endosc. 2022;96(4):576–92.e1. doi:10.1016/j.gie.2022.05.013
- Dougherty M, Runge TM, Eluri S, Dellon ES. Esophageal dilation with either bougie or balloon technique as a treatment for eosinophilic esophagitis: a systematic review and meta-analysis. Gastrointest Endosc. 2017;86(4):581–91.e3. doi:10.1016/j.gie.2017.04.028
- Kim JP, Weingart G, Hiramoto B, Gregory DL, Gonsalves N, Hirano I. Clinical outcomes of adults with eosinophilic esophagitis with severe stricture. Gastrointest Endosc. 2020;92(1):44–53. doi:10.1016/j.gie.2020.01.015
- Madanick RD, Shaheen NJ, Dellon ES. A novel balloon pull-through technique for esophageal dilation in eosinophilic esophagitis (with video). Gastrointest Endosc. 2011;73(1):138–142. doi:10.1016/j.gie.2010.09.034
- Schoepfer AM, Henchoz S, Biedermann L, et al. Technical feasibility, clinical effectiveness, and safety of esophageal stricture dilation using a novel endoscopic attachment cap in adults with eosinophilic esophagitis. Gastrointest Endosc. 2021;94(5):912–9.e2. doi:10.1016/j.gie.2021.05.017
- Pasha SF, Acosta RD, Chandrasekhara V, et al. The role of endoscopy in the evaluation and management of dysphagia. Gastrointest Endosc. 2014;79(2):191–201. doi:10.1016/j.gie.2013.07.042
- Richter JE. Eosinophilic esophagitis dilation in the community--try it--you will like it--but start low and go slow. Am J Gastroenterol. 2016;111(2):214–216. doi:10.1038/ajg.2015.433
- Moawad FJ, Molina-Infante J, Lucendo AJ, Cantrell SE, Tmanova L, Douglas KM. Systematic review with meta-analysis: endoscopic dilation is highly effective and safe in children and adults with eosinophilic oesophagitis. Aliment Pharmacol Ther. 2017;46(2):96–105. doi:10.1111/apt.14123
- Schoepfer AM, Gonsalves N, Bussmann C, et al. Esophageal dilation in eosinophilic esophagitis: effectiveness, safety, and impact on the underlying inflammation. Am J Gastroenterol. 2010;105(5):1062–1070. doi:10.1038/ajg.2009.657
- Schupack DA, Ravi K, Geno DM, et al. Effect of maintenance therapy for eosinophilic esophagitis on need for recurrent dilation. Dig Dis Sci. 2021;66(2):503–510. doi:10.1007/s10620-020-06192-8
- Safroneeva E, Cotton CC, Schoepfer AM, Zwahlen M, Woosley JT, Dellon ES. Dilation modifies association between symptoms and esophageal eosinophilia in adult patients with eosinophilic esophagitis. Am J Gastroenterol. 2020;115(12):2098–2102. doi:10.14309/ajg.0000000000000957
- Larsson H, Strobel MJ, Perez-Guagnelli E. Emotional journey of patients with eosinophilic esophagitis. Adv Ther. 2023;40(12):5254–5270. doi:10.1007/s12325-023-02678-9
- Ma C, Bredenoord AJ, Dellon ES, et al. Reliability and responsiveness of endoscopic disease activity assessment in eosinophilic esophagitis. Gastrointest Endosc. 2022;95(6):1126–37.e2. doi:10.1016/j.gie.2022.01.014
- van Rhijn BD, Verheij J, Smout AJPM, Bredenoord AJ. The endoscopic reference score shows modest accuracy to predict histologic remission in adult patients with eosinophilic esophagitis. Neurogastroenterol Motil. 2016;28(11):1714–1722. doi:10.1111/nmo.12872
- Visaggi P, Solinas I, Baiano Svizzero F, et al. Non-invasive and minimally invasive biomarkers for the management of eosinophilic esophagitis beyond peak eosinophil counts: filling the gap in clinical practice. Diagnostics. 2023;13(17):1.
- Eke R, Li T, White A, Tariq T, Markowitz J, Lenov A. Systematic review of histological remission criteria in eosinophilic esophagitis. JGH Open. 2018;2(4):158–165. doi:10.1002/jgh3.12059
- Collins MH, Martin LJ, Alexander ES, et al. Newly developed and validated eosinophilic esophagitis histology scoring system and evidence that it outperforms peak eosinophil count for disease diagnosis and monitoring. Dis Esophagus. 2016;30(3):1–8.
- Whelan KA, Godwin BC, Wilkins B, et al. Persistent basal cell hyperplasia is associated with clinical and endoscopic findings in patients with histologically inactive eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2020;18(7):1475–82.e1. doi:10.1016/j.cgh.2019.08.055
- Collins MH, Martin LJ, Wen T, et al. Eosinophilic esophagitis histology remission score: significant relations to measures of disease activity and symptoms. J Pediatr Gastroenterol Nutr. 2020;70(5):598–603. doi:10.1097/MPG.0000000000002637
- Pouw RE, Barret M, Biermann K, et al. Endoscopic tissue sampling - Part 1: upper gastrointestinal and hepatopancreatobiliary tracts. European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2021;53(11):1174–1188. doi:10.1055/a-1611-5091
- Alexander JA, Jung KW, Arora AS, et al. Swallowed fluticasone improves histologic but not symptomatic response of adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2012;10(7):742–9.e1. doi:10.1016/j.cgh.2012.03.018
- Mercieca-Bebber R, King MT, Calvert MJ, Stockler MR, Friedlander M. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas. 2018;9:353–367. doi:10.2147/PROM.S156279
- Schoepfer A, Straumann A, Safroneeva E. Patient-Reported Outcomes in Eosinophilic Esophagitis and Achalasia. Curr Treat Options Gastroenterol. 2016;14(1):51–60. doi:10.1007/s11938-016-0084-0
- Dellon ES, Khoury P, Muir AB, et al. A clinical severity index for eosinophilic esophagitis: development, consensus, and future directions. Gastroenterology. 2022;163:59–76. doi:10.1053/j.gastro.2022.03.025
- Cotton CC, Moist SE, McGee SJ, Furuta GT, Aceves SS, Dellon ES. A newly proposed severity index for eosinophilic esophagitis is associated with baseline clinical features and successful treatment response. Clin Gastroenterol Hepatol. 2023;21(10):2534–42.e1. doi:10.1016/j.cgh.2023.03.047
- Carlson DA, Gyawali CP, Roman S, et al. Esophageal hypervigilance and visceral anxiety are contributors to symptom severity among patients evaluated with high-resolution esophageal manometry. Am J Gastroenterol. 2020;115(3):367–375. doi:10.14309/ajg.0000000000000536
- McCann E, Chehade M, Spergel JM, et al. Validation of the novel Eosinophilic Esophagitis Impact Questionnaire. J Patient Rep Outcomes. 2023;7(1):120. doi:10.1186/s41687-023-00654-z
- Taft TH, Kern E, Kwiatek MA, Hirano I, Gonsalves N, Keefer L. The adult eosinophilic oesophagitis quality of life questionnaire: a new measure of health-related quality of life. Aliment Pharmacol Ther. 2011;34(7):790–798. doi:10.1111/j.1365-2036.2011.04791.x
- Martin LJ, Franciosi JP, Collins MH, et al. Pediatric Eosinophilic Esophagitis Symptom Scores (PEESS v2.0) identify histologic and molecular correlates of the key clinical features of disease. J Allergy Clin Immunol. 2015;135(6):1519–28.e8. doi:10.1016/j.jaci.2015.03.004
- Butt MF, Visaggi P, Singh R, Vork L. Lack of awareness of neurogastroenterology and motility within medical education: time to fill the gap. Neurogastroenterol Motil. 2023;35(10):e14666. doi:10.1111/nmo.14666
- Molina-Infante J. Nutritional and psychological considerations for dietary therapy in eosinophilic esophagitis. Nutrients. 2022;14(8). doi:10.3390/nu14081588
