Abstract
The treatment of psoriasis has been revolutionized since the introduction of biologic therapies. Prior to their introduction, it was unclear if psoriasis was primarily a keratinocyte signaling dysfunction or an autoimmune T-cell mediated pathway. Nonspecific T-cell targeting treatments had been used with some success, but they were limited by a narrow therapeutic index. The nonspecific nature of these agents was fraught with side effects, and the efficacy of these treatments pales in comparison to current treatments. The initial biologic molecules, alefacept and efalizumab, were not specific for any T-cell driven pathway, and neither are currently available in the USA. The successors to these early therapies have shown high efficacy and low side effects in psoriasis and other autoimmune diseases through the specific targeting of tumor necrosis factor-alpha (TNF-α). Since the initial use of antitumor necrosis factor agents, a renaissance in our understanding of psoriasis has been underway, leading to the elucidation of the T-helper 17 (Th17) from the Th1 pathway. With each new treatment, the pathogenesis for psoriasis continues to be more defined, allowing for improved targeted therapies and the ability to achieve new milestones in efficacy.
Introduction
Emerging role of immunotargeting for psoriasis management
Psoriasis vulgaris is a common skin disease that occurs in up to 1.5%–2% of patients of European descent.Citation1,Citation2 It typically presents with thick, erythematous scaly plaques in characteristic locations, including the elbows, knees, umbilicus, genitals, and scalp. Histopathologically, it consists of epidermal regular acanthosis, increased vasculature in the dermal papilla, and thick hyperkeratosis in the stratum corneum with neutrophil-laden deposits. It can be generalized and can be present in forms other than the chronic plaque psoriasis, including guttate, palmoplantar, pustular, and inverse patterns. Nail involvement is common with onycholysis, oil drops, and pitting as the most characteristic findings. Beyond the skin, it also may cause destructive arthritis and independently increases the risk of cardiovascular mortality.Citation3 The point prevalence of any degree of psoriatic arthritis in a patient survey was around 10%.Citation4 The features of psoriasis are life altering, resulting in a decreased quality of life from itching, arthritis, and disfigurement, which may also result in decreased income potential.Citation5 The decreased quality of life, although resulting from alterations limited to the skin and joints, is similar to other chronic illnesses of internal organs.Citation6 Further effects, including social stigmatization and sexual dysfunction, are significant and have been extensively reviewed.Citation7
Genetics of psoriasis
Psoriasis is a complex multigenic heritable disease. About 30% of patients with psoriasis will have an affected first-degree relative. Twin studies have demonstrated a concordance of 35%–70% among monozygotic twins.Citation8,Citation9 Based on genetics and clinical presentation, two types of inheritance have been described. Type 1 psoriasis includes patients with familial disease and an early age of involvement. It has been linked to the now-known risk factor HLA–Cw6.Citation10,Citation11 Type 2 has low penetrance. Patients are older when they develop it, and the disease burden is typically less severe. Studies in Type 1 psoriasis indicate that the presence of HLA–Cw6, the most highly associated gene among several previously identified as PSORS1, correlated to a 16-fold increased risk of classic young-age psoriasis and a 30-fold increased risk for the development of guttate psoriasis.Citation12 Other associations from the human major histocompatibility complex include HLA–B57, while HLA–B40 has a protective effect.Citation13 Further studies, including genome-wide association studies (GWAS), which have the potential to evaluate single nucleotide polymorphisms (SNPs), confirmed the association with HLA–Cw6, as well as multiple other alleles, including: interleukin (IL)-12; IL-23; and downstream genes associated with tumor necrosis factor-alpha (TNF-α) expression.Citation14 IL-12, IL-23, and TNF-α have all become major targets of biologic therapies in psoriasis. Other genetic associations include increased copies of the human β-defensin geneCitation15 and deletions in the late cornified envelope proteins 3B and 3C.Citation16,Citation17 Recent analyses have implicated SNPs in a human endogenous retrovirus gene that is located near HLA–C but is independent of HLA–Cw6 as another risk factor.Citation18,Citation19
Despite the recent advances in the genetics of psoriasis, there is no simple explanation for the progression from susceptibility to disease. Some environmental risk factors are known, including the development of guttate psoriasis after streptococcal infection,Citation20,Citation21 chronic plaque psoriasis flares after streptococcal infection,Citation22 improvement in psoriasis in patients with recurrent streptococcal tonsillitis undergoing tonsillectomy,Citation23 and psoriasis development after medications that included lithium, beta-adrenergic blockers, and antimalarials.Citation24
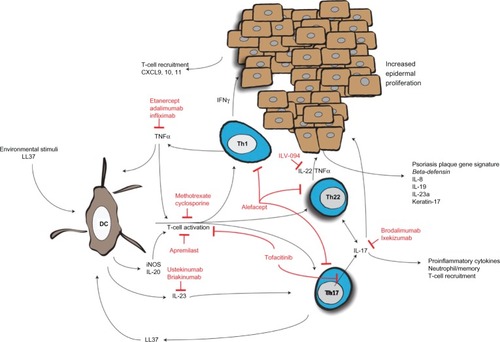
There is no common mechanism that is universal to different variants of psoriasis; however, it is widely accepted that in susceptible individuals, psoriasis can be caused by a single stimulus, cascading down to an increase in the innate immune system activity level, activation of T-cells, subsequent cytokine secretion, and epidermal proliferation ().
Figure 1 Activation of dendritic cells and resulting T-cell cytokine milieu results in epidermal acanthosis and proliferation.
Review of current and emerging immunotargets, and associated pathophysiology
At one time, it was thought that keratinocytes were the foundation of psoriasis. This has been disproven by genetic studies,Citation10–Citation19 as well as results from stem cell transplantation in humans, which showed that psoriasis can be transferred from donors with psoriasis or cured by stem cell transplantation from a nonafflicted donor.Citation25 It is unclear how streptococcal and other infections cause the initiation of psoriasis, but it is possible that the peptidoglycan in bacterial cell walls can result in Toll-like receptor-2 (TLR-2) activation and subsequent downstream signaling, or that the initial inflammatory milieu produces autoreactive T-cells that react to human keratin 17, which is upregulated in psoriasis and shares homology to a streptococcal M protein.Citation26 TLR-2 is also activated by a gene product of the human endogenous retrovirus K (HerK) in dendritic cells (DCs),Citation19,Citation27 a professional antigen presenting cell, as well as keratinocytes where this receptor appears to be the most concentrated.Citation28 DCs, in this case, produce the cytokines after stimulation with the HerK protein dUTPase, which are typical in psoriasis.Citation19 This indicates that multiple mechanisms activate DCs to initiate the psoriasis cascade.
The DCs is where the different susceptibility genes and the environmental factors converge. Antimicrobial peptides, such as LL-37, among others, are upregulated in psoriatic plaques, and it has been shown that LL-37 is able to complex with self-RNA and DNA to activate endosomal toll-like receptors (TLR) 7, 8, and 9, which induce DCs to produce interferon-α (IFN-α).Citation29,Citation30
Plasmacytoid DCs (PDC), which normally function as early responders in viral infections, have been found to be highly localized in psoriatic lesional skin. They naturally produce IFN-α and may drive the early T-helper 1 (Th1) cytokine expression early in the development of psoriasis,Citation31 and then differentiate into tissue-resident DCs and myeloid DCs (MDC).Citation32 TNF-α also plays a role in psoriasis initiation as seen in a mouse model deficient in interferon receptors.Citation33 There may be a second population of MDC, now called inflammatory myeloid dendritic cells (iMDC) that express CD11c, but not CD1c, like most tissue resident myeloid cells.Citation34 These cells produce inducible nitric oxide synthase (iNOS), IL-20, and IL-23, which are now known to be inhibited early in TNF-α blockage.Citation35–Citation37 The levels of IL-23 correlated well with the development of the psoriatic plaque.Citation38 A combination of IL-6, and transforming growth factor-β1 upregulate the IL-23R on naïve T-cells and allow for differentiation into the Th17 subtype, with IL-23 as a survival factor.Citation39,Citation40 Th17 cells, named for their production of IL-17, and the overall Th17 pathway, now appear to be central to the pathogenesis of psoriasis. IL-17 induces a proinflammatory response, as evidenced by the numerous neutrophils, increased memory T-cells seen in psoriatic lesions.Citation41 TNF-α acts synergistically with IL-17 on the keratinocytes to induce gene expression, specifically elevating human β-defensin 4, IL-8, IL-19, and IL-23A, which all characterize psoriasis plaque gene expression signatures.Citation42 IL-17 inhibition results in a more extensive downregulation of these synergistic genes than TNF-α inhibition, highlighting the central role of the Th17 cell in the pathogenesis of psoriasis.Citation43 IL-17 inhibition blocks upstream genes as well as CD11c dendritic cells, which perhaps is triggered by a positive feedback loop with the LL37/cathelicidin/self-RNA complex that activates the DCs.Citation43 Although IL-17 appears to be central to the inflammatory nature, it does not result in acanthosis that typifies psoriasis. Instead, IL-22 secreted from Th17 and Th22 cells acts as the final messenger for epidermal proliferation and dedifferentiation.Citation44–Citation46 IL-22 upregulates keratin-17, commonly associated with psoriasis, and does this through a Janus kinase (JAK)/signal transducer and activator of transcription (STAT) mechanism, among others.Citation47
Overview of immunotargeted therapies for treatment of psoriasis
Topical therapies, such as vitamin D analogs, corticosteroids, retinoids, coal tar, and anthralin, will not be reviewed in this article as their effects have already been extensively reviewed.Citation48,Citation49
Phototherapy
Phototherapy in the form of photochemotherapy, psoralen ultraviolet A light (PUVA), broadband ultraviolet B (bbUVB), and narrowband UVB (nbUVB) have long been stalwarts in the treatment of psoriasis. Despite their decades of use, the mechanisms have never been fully elucidated. Both nbUVB and PUVA have been found to decrease co-stimulatory expression molecules and antigen expression of human major histocompatibility complex in the dermis in psoriatic plaques.Citation50 Furthermore, light therapy depletes T-cells in the epidermis via apoptosis and has an apoptotic effect on proliferating keratinocytes.Citation51,Citation52 Further studies have shown a strong correlation of nbUVB reduction in dermal dendritic cells CD11c+, CD1c−, and resolution of psoriasis plaques.Citation53 The same study found a significant reduction in downstream cytokines in normalized psoriasis after nbUVB, including IFN-γ, IL-17, and IL-22.Citation53 PUVA may act by contributing to an increase in the number of regulatory T-cells (Tregs), functionality of Tregs, and decreasing circulating Th17 cells in some patients.Citation54 Clinically, both PUVA and nbUVB, which are more effective than bbUVB in most types of psoriasis, are highly effective, with about 80% and 70%, respectively, of treated patients obtaining clearance or marked improvement.Citation55
Phototherapy is a highly effective treatment modality utilizing multiple mechanisms to control psoriasis, but it has the disadvantage of being inconvenient for patients and ineffective for psoriatic arthritis, and in the case of PUVA, it may increase the long-term risk of cutaneous carcinogenesis.
Nonspecific T-cell therapies
Methotrexate is a competitive antagonist of dihydrofolate reductase, which blocks de novo pyrimidine and purine synthesis, resulting in decreased B- and T-cell proliferation. In addition, methotrexate may have an anti-inflammatory effect as well.Citation56 Recent studies indicate that the effect of low-dose methotrexate may be more related to blocking T-cell adhesion molecule expression than on limiting the cellular proliferation.Citation57 Methotrexate shows varying degrees of effectiveness, typically measured by the Psoriasis Area and Severity Index (PASI), which is used with two common cutoffs, PASI-50 and PASI-75, to describe the percentage of patients that develop a 50% or 75% improvement, respectively. In early studies, PASI-75 with 15 mg weekly was seen in 60% of patients.Citation58 However, this has been inconsistent; a similar study, although with a starting dose of 7.5 mg weekly, demonstrated only a 24% PASI-75.Citation59 Methotrexate has been used as the control in several biologic trials and, typically, is found to have a PASI-75 around 35%–40%.Citation60–Citation62 Dosing is limited, based on side effects and concern for myelosuppression at higher dose; however, methotrexate still represents an affordable option for many patients with psoriasis.
Cyclosporine is a calcineurin inhibitor, originally developed for suppression of rejection of solid organ transplants. Similar to methotrexate, it is not specific for the psoriasis pathways other than for the inhibition of T-cells. It has been found to be slightly more efficacious than methotrexate and typically more rapid,Citation58,Citation59 but its use is limited due to side effects and the development of long-term nephrotoxicity. However, a new small molecule inhibitor, voclosporin or ISA-247, has been found to be effective in psoriasis as well. With improved metabolism compared to cyclosporine, nephrotoxicity will hopefully be less of a concern.Citation63
Leflunomide is an inhibitor of dihydroorotate dehydrogenase, a step in the de novo pyrimidine synthesis pathway. It has shown some efficacy in the treatment of psoriasis, although more so in psoriatic arthritis, with about 17% of patients reaching PASI-75.Citation64,Citation65 Notably, the prospective study included patients with a lower initial degree of involvement than many others focusing on skin psoriasis. In similar medications, other purine and pyrimidine synthesis inhibitors, such as mycophenolate mofetil, have been trialed with some efficacy, although less than methotrexate.Citation66 Chemotherapies, such as hydroxyurea and 6-mercaptopurine, that target the T-cells have been found to be highly effective for the treatment of psoriasis, but as they are only used rarely and their mechanism is not specific, they will not be reviewed.
Phosphodiesterase-4 inhibitors
Apremilast, an oral small molecule phosphodiesterase-4 inhibitor, is in late-stage trials for psoriasis and psoriatic arthritis. Phosphodiesterase-4 is the primary phosphodiesterase of mammalian immune cells and results in increased levels of the cyclic adenosine monophosphate (AMP) molecule and inhibition of active cells, blocking TNF-α, IL-12, IL-23, and increasing inhibitory IL-10 production.Citation67 In early trials, it has shown efficacy in psoriatic arthritis and psoriasis, although PASI-75 scores have ranged from 20%–40% in effective doses from Phase II studies.Citation68–Citation70
JAK inhibitors
Another new class of oral small-molecule inhibitors and topical therapies target the JAK/STAT stimulation pathway. This pathway has been found to be active in cytokine production, common in psoriasis and other autoinflammatory diseases.
Tofacitinib inhibits JAK3/JAK1 signaling for multiple cytokines important in psoriasis, including IL-6 and IL-17.Citation71 Tofacitinib was found to be highly effective and well tolerated in Phase II dose studies, with about 66% of patients achieving PASI-75 improvement at the highest dose.Citation73 Health-related quality of life was also improved rapidly in these patients.Citation74 Tofacitinib is being developed in a topical formulation that also appears effective.Citation75 A topical formulation of a JAK1/JAK2 inhibitor with a similar mechanism is also under development and has shown promising early results.Citation76
Retinoic acid receptor agonists
Acitretin and formerly etretinate (no longer available in the USA) work through the novel retinoid acid receptors. These receptors are part of the steroid hormone receptor family of transcription factors. They result in a decrease in inflammation and an increase in cellular differentiation.Citation77 Acitretin is the only systemic agent that treats psoriasis and is not an immunosuppressant, making it an ideal option in patients with human immunodeficiency virus (HIV) and other immunodeficiencies.Citation78 It has been shown to have no effect on the Th17 or Th22 pathways.Citation79 Its efficacy is frequently limited by side effects; and at doses of 25–50 mg daily, it is not as effective as methotrexate. In fact, it was not even evaluated with PASI-75 in the early trials.Citation80–Citation82 The greatest benefit appears to be in a combination of acitretin and phototherapy. Acitretin, combined with UVB, produced a PASI-75 in 60% of participants;Citation83 while in combination with PUVA, it produced a PASI-90 improvement in 96% of patients.Citation84 Acitretin also shows good activity in psoriatic nail disease.Citation85
Targeted biologics for T-cells
Efalizumab is a humanized monoclonal immunoglobulin (Ig) G1 antibody to lymphocyte functional associated antigen-1 (LFA-1) and, specifically, the CD11, which is a subunit of LFA-1. This targets T-cell activation and migration via interactions with endothelial intercellular adhesion molecule-1. Efalizumab was found to increase circulating levels of T-cells, with a concomitant decrease in tissue-infiltrating T-cells within psoriasis plaques.Citation86 Downregulation of CD11a on T-cells also leads to the development of anergy that is responsive to drug withdrawal.Citation87 It was initially well tolerated in short-term studies and demonstrated PASI-75 rates of 22%–30%.Citation88,Citation89 The 3-year data appeared to show that the medication was relatively safe and well tolerated, with only mild side effects, such as thrombocytopenia, increase in upper respiratory tract infections, and new onset psoriatic arthritis.Citation90 However, this medication was withdrawn from the market in 2009 as multiple cases of progressive multifocal leukoencephalopathy (PML) developed in patients on this medication longer than 3 years. Although both T-cell anergy and inhibition of migration may be the cause of reactivation of the John Cunningham virus,Citation91 migration impairment may be more likely based on a similar humanized monoclonal antibody for multiple sclerosis and Crohn’s disease that inhibits lymphocyte migration using α-4 integrin, natalizumab, in which more than 200 cases of PML have been reported.Citation92
Alefacept is a fusion protein of IgG1, combined with lymphocyte function-associated antigen 3 (LFA3), which binds to the cluster of differentiation 2 (CD2) on activated memory T-cells. LFA3 is typically expressed on antigen-presenting cells to produce a mitogenic signal via CD2 activation. Blockage creates a depletion of peripheral effector memory T-cells, which selectively express more CD2 with a relative preservation of central memory and naïve T-cells.Citation93 Alefacept differs from other biologic therapies in that the dosing is not continuous and typically requires weekly injections for 12 weeks and then 12 weeks off before restarting as needed. Initial trials showed about 30% of patients reaching PASI-75 after the first course of therapy.Citation94,Citation95
Furthermore, selective targeting of memory T-cells did not appear to result in abnormalities in delayed type hypersensitivity or humoral immunity.Citation95,Citation96 This appeared to be effective and safe in up to five courses of treatment, dosed on an as-needed basis.Citation97 Unfortunately, the company stopped promoting and distributing alefacept in 2011, which was unrelated to a safety or product recall.
Anti-TNF-α – etanercept, infliximab, adalimumab
Three anti-TNF-α biologic molecules have been US Food and Drug Administration (FDA) approved for the treatment of psoriasis. In addition, others exist that may be used off-label, including golimumab and certolizumab.
Etanercept is a fusion protein, linking TNF-α receptor to IgG1. It was incidentally found to be highly effective in psoriasis and demonstrated very high responses based on PASI-75, up to 40%–50% at 24 weeks in initial studies.Citation98,Citation99 This was the first of the anti-TNF-α agents to be approved for psoriasis.
Infliximab is a chimeric monoclonal antibody that is given intravenously (IV), targeting soluble TNF-α. It has been found to have the highest and most rapid onset of PASI-75 in trials; about 80% of patients develop rapid and extensive improvement in their psoriasis.Citation100,Citation101 As an infusion molecule and the most efficacious, it is noted to have the highest rate of discontinuation due to serious reactions (infusion reactions) of all the anti-TNF molecules.Citation102
Adalimumab is a fully human monoclonal antibody targeting soluble TNF-α. It is administered less frequently and achieves a PASI-75 of up to 70% of patients at the standard dosing of 40 mg every other week.Citation61,Citation103,Citation104
Although there are differences in efficacy and design, the underlying mechanism is the same, and they will be discussed in that fashion. All three are effective in psoriatic arthritis.Citation98,Citation105,Citation106 Inhibition with anti-TNF-α therapies result in a rapid decrease in IL-1, IL-8, IL-23, and iNOS in psoriatic plaques, followed by a decrease in the CD11c DCs, a decrease in messenger RNA for IL-23, and finally a decrease in T-cell infiltration in the psoriatic plaques.Citation107 Further experiments of psoriasis plaques in early involution, 1 month after starting etanercept, noted selective CD11c DCs undergoing apoptosis, although no circulating or dermal T-cells were noted to be affected in such fashion.Citation108 In the follow-up study, it was found that, although etanercept decreases acute response peptides IL-1 and IL-8 in all patients, only the responders developed a decrease in IL-17 to normal levels, indicating that TNF-α is early in the cascade.Citation109 This resulted in further studies to examine more specific inhibition within the pathways that lead to psoriasis.
Anti-IL-12,-23 – ustekinumab, briakinumab
The most recent US FDA-approved biologic is ustekinumab. It is a fully human IgG1 against the p40 subunit of IL-12 and IL-23 that potentiates the survival signal for Th17 cells. IL-12 and IL-23 are linked by a common p40 subunit that exists constitutively on naïve T-cells. Depending on survival signals, and then whether IL-23R or IL-12B2 is produced, the T-cell may differentiate into Th1 or Th17 cells. This has become a highly effective treatment for psoriasis with PASI-75 developing in 60%–70% of patients.Citation110–Citation112 Furthermore, the 3-year data indicates that this medication is safe with few cardiovascular events, no increase in salmonella infections, and no favoring of a Th2 response, such as asthma developing based on inhibition of the Th1 cytokine IL-12.Citation113,Citation114
Briakinumab is another human monoclonal antibody to the p40 subunit of IL-12 and IL-23. While it too was found to be highly effective in Phase III studies,Citation62,Citation115 it has not obtained nor is it currently seeking US FDA approval after seven patients had either a stroke, myocardial infarction, or cardiac death in a separate trial.Citation116
Based on the 3-year safety data, it does appear that ustekinumab is relatively safe, with the most concerning side effects being the major cardiovascular events occurring in 0.3–0.6 per 100 life years, which is within the expected population range of such events.Citation117
Anti-IL-17 – brodalumab, ixekizumab
Recent Phase II trials were just completed for the first two IL-17 inhibitors. Mechanistically, these inhibitors cause upstream and downstream inhibition of genes characteristic of psoriasis.Citation43 Brodalumab is a human anti-IL-17R monoclonal antibody that demonstrates high efficacy, with PASI-75 rates reaching 70%–80% of patients.Citation118 Ixekizumab is a humanized IgG4 monoclonal antibody to IL-17 that also demonstrates efficacy, with about 70%–80% of treated patients reaching PASI-75 in Phase II studies.Citation119 Furthermore, ixekizumab appears to be effective for psoriatic arthritis.Citation119 Further studies will be needed, especially to ensure safety, as neutropenia may be a concerning side effect of inhibition of IL-17.Citation118
Future research
The field of psoriasis therapies has expanded exponentially with numerous small-molecule inhibitors and biologics, some that target novel pathways such as IL-22, and others that are less specific in targeting T-cell activation in the pipeline. Many of these are in early-stage development and have been published in a recent review.Citation120
Implications for enhanced patient care and quality of life
Options have expanded dramatically for patients suffering from psoriasis since the beginning of the biologic era. Psoriasis clearly has a substantial burden on patients throughout the world. Even in developed countries, it continues to have significant treatment dissatisfaction.Citation2,Citation5,Citation121 The era of research and development of small molecule inhibitors, as well as targeted extracellular biologics, has created profound impact on patients throughout the world. It is worth noting that PASI-75 did not become the accepted marker for psoriasis improvement until the biologics evolved, because of the significant improvement in psoriasis treatment efficacy. However, significant limitations still remain: how to increase accessibility to care, despite the significant cost burden;Citation122 how to maximize the time that each biologic is efficaciousCitation102 prior to formation of clearing antibodies; and how to ensure long-term safety of these medications.
Conclusion and discussion
The treatment of psoriasis has progressed substantially in the past 10 years. Although many questions remain, especially regarding the initial pathogenesis of psoriasis and the evolutionary advantage of psoriasis, basic details have emerged from targeted inhibition of cytokines. With each new targeted agent, new data becomes available, highlighting new cytokines involved and new cell types. With the significant advances in psoriasis therapy over the past decade, hopefully new targeted therapies will continue to be developed and allow for more safe and efficacious options for patients with psoriasis.
Acknowledgments
All authors had full access to the data in the study, and take responsibility for the integrity of the data and the accuracy of data analysis.
Disclosure
HK Wong reports honoraria from Amgen Inc and is also an investigator for Amgen, Janssen Pharmaceuticals, Abbott Laboratories, and Celgene Corporation. No sponsors participated in this study. The other authors have no conflicts of interest to report.
References
- GelfandJMWeinsteinRPorterSBNeimannALBerlinJAMargolisDJPrevalence and treatment of psoriasis in the United Kingdom: a population-based studyArch Dermatol2005141121537154116365254
- SternRSNijstenTFeldmanSRMargolisDJRolstadTPsoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfactionJ Invest Dermatol Symp Proc200492136139
- GelfandJMNeimannALShinDBWangXMargolisDJTroxelABRisk of myocardial infarction in patients with psoriasisJAMA2006296141735174117032986
- GelfandJMGladmanDDMeasePJEpidemiology of psoriatic arthritis in the population of the United StatesJ Am Acad Dermatol200553457316198775
- GelfandJMFeldmanSRSternRSThomasJRolstadTMargolisDJDeterminants of quality of life in patients with psoriasis: a study from the US populationJ Am Acad Dermatol200451570470815523347
- RappSRFeldmanSRExumMLFleischerABJrReboussinDMPsoriasis causes as much disability as other major medical diseasesJ Am Acad Dermatol1999413 Pt 140140710459113
- ChoiJKooJYQuality of life issues in psoriasisJ Am Acad Dermatol200349Suppl 25761
- BrandrupFHaugeMHenningsenKEriksenBPsoriasis in an unselected series of twinsArch Dermatol19781146874878566529
- DuffyDLSpelmanLSMartinNGPsoriasis in Australian twinsJ Am Acad Dermatol19932934284348349859
- StuartPMalickFNairRPAnalysis of phenotypic variation in psoriasis as a function of age at onset and family historyArch Dermatol Res2002294520721312115023
- NairRPStuartPENistorISequence and haplotype analysis supports HLA–C as the psoriasis susceptibility 1 geneAm J Hum Genet200678582785116642438
- MallonENewsonRBunkerCBHLA–Cw6 and the genetic predisposition to psoriasis: a meta-analysis of published serologic studiesJ Invest Dermatol1999113469369510504461
- FengBJSunLDSoltani-ArabshahiRMultiple Loci within the major histocompatibility complex confer risk of psoriasisPLoS Genet200958e100060619680446
- NairRPDuffinKCHelmsCGenome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathwaysNat Genet200941219920419169254
- HolloxEJHuffmeierUZeeuwenPLPsoriasis is associated with increased beta-defensin genomic copy numberNat Genet2008401232518059266
- Riveira-MunozEHeSMEscaramísGMeta-analysis confirms the LCE3C_LCE3B deletion as a risk factor for psoriasis in several ethnic groups and finds interaction with HLA–Cw6J Invest Dermatol201113151105110921107349
- de CidRRiveira-MunozEZeeuwenPLDeletion of the late cornified envelope LCE3B and LCE3C genes as a susceptibility factor for psoriasisNat Genet200941221121519169253
- LaiOYChenHMichaudHAProtective effect of human endogenous retrovirus K dUTPase variants on psoriasis susceptibilityJ Invest Dermatol201213271833184022437317
- ArizaMEWilliamsMVA human endogenous retrovirus K dUTPase triggers a TH1, TH17 cytokine response: does it have a role in psoriasis?J Invest Dermatol2011131122419242721776007
- WhyteHBaughmanRAcute guttate psoriasis and streptococcal infectionArch Dermatol19648935035614096349
- TelferNRChalmersRJWhaleKColmanGThe role of streptococcal infection in the initiation of guttate psoriasisArch Dermatol1992128139421739285
- GudjonssonJEThorarinssonAMSigurgeirssonBKristinssonKGValdimarssonHStreptococcal throat infections and exacerbation of chronic plaque psoriasis: a prospective studyBr J Dermatol2003149353053414510985
- ThorleifsdottirRHSigurdardottirSLSigurgeirssonBImprovement of psoriasis after tonsillectomy is associated with a decrease in the frequency of circulating T cells that recognize streptococcal determinants and homologous skin determinantsJ Immunol2012188105160516522491250
- AbelEADiCiccoLMOrenbergEKFrakiJEFarberEMDrugs in exacerbation of psoriasisJ Am Acad Dermatol1986155 Pt 1100710222878015
- KaffenbergerBHWongHKJarjourWAndritsosLARemission of psoriasis after allogeneic, but not autologous, hematopoietic stem-cell transplantationJ Am Acad Dermatol201368348949222981608
- GudmundsdottirASSigmundsdottirHSigurgeirssonBGoodMFValdimarssonHJonsdottirIIs an epitope on keratin 17 a major target for autoreactive T lymphocytes in psoriasis?Clin Exper Immunol1999117358058610469066
- ArizaMEWilliamsMVWongHKTargeting IL-17 in psoriasis: from cutaneous immunobiology to clinical applicationClin Immunol2012146213113923314273
- BakerBSOvigneJMPowlesAVCorcoranSFryLNormal keratinocytes express Toll-like receptors (TLRs) 1, 2, and 5: modulation of TLR expression in chronic plaque psoriasisBr J Dermatol200314867067912752123
- GangulyDChamilosGLandeRSelf-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8J Exp Med200920691983199419703986
- LandeRGregorioJFacchinettiVPlasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptideNature2007449716256456917873860
- NestleFOConradCTun-KyiAPlasmacytoid predendritic cells initiate psoriasis through interferon-alpha productionJ Exp Med2005202113514315998792
- BlancoPPaluckaAKGillMPascualVBanchereauJInduction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosusScience200129455461540154311711679
- BoymanOHeftiHPConradCNickoloffBJSuterMNestleFOSpontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-alphaJ Exp Med2004199573173614981113
- ZabaLCKruegerJGLowesMAResident and “inflammatory” dendritic cells in human skinJ Invest Dermatol2009129230230818685620
- ZabaLCCardinaleIGilleaudeauPAmelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responsesJ Exp Med2007204133183319418039949
- LowesMAChamianFAbelloMVIncrease in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a)Pro Natl Acad Sci U S A2005102521905719062
- ChamianFLowesMALinSLAlefacept reduces infiltrating T cells, activated dendritic cells, and inflammatory genes in psoriasis vulgarisPro Natl Acad Sci U S A2005102620752080
- TonelGConradCLaggnerUCutting edge: A critical functional role for IL-23 in psoriasisJ Immunol2010185105688569120956338
- ManganPRHarringtonLEO’QuinnDBTransforming growth factor-beta induces development of the T(H)17 lineageNature2006441709023123416648837
- MorishimaNMizoguchiITakedaKMizuguchiJYoshimotoTTGF-beta is necessary for induction of IL-23R and Th17 differentiation by IL-6 and IL-23Biochem Biophys Res Commun2009386110511019501566
- NogralesKEZabaLCGuttman-YasskyETh17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathwaysBr J Dermatol200815951092110218684158
- ChiricozziAGuttman-YasskyESuárez-FariñasMIntegrative responses to IL-17 and TNF-α in human keratinocytes account for key inflammatory pathogenic circuits in psoriasisJ Invest Dermatol2011131367768721085185
- KruegerJGFretzinSSuárez-FariñasMIL-17 A is essential for cell activation and inflammatory gene circuits in subjects with psoriasisJ Allergy Clin Immunol2012130114515422677045
- BonifaceKGuignouardEPedrettiNA role for T cell-derived interleukin 22 in psoriatic skin inflammationClin Exp Immunol2007150340741517900301
- BonifaceKBernardFXGarciaMGurneyALLecronJCMorelFIL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytesJ Immunol200517463695370215749908
- WilsonNJBonifaceKChanJRDevelopment, cytokine profile and function of human interleukin 17-producing helper T cellsNat Immunol20078995095717676044
- ZhangWDangEShiXThe pro-inflammatory cytokine IL-22 up-regulates keratin 17 expression in keratinocytes via STAT3 and ERK1/2PLoS One201277e4079722808266
- DevauxSCastelaAArchierETopical vitamin D analogues alone or in association with topical steroids for psoriasis: a systematic reviewJ Eur Acad Dermatol Venereol201226Suppl 3526022512681
- CastelaEArchierEDevauxSTopical corticosteroids in plaque psoriasis: a systematic review of efficacy and treatment modalitiesJ Eur Acad Dermatol Venereol201226Suppl 3364622512679
- ErkinGUğurYGürerCKEffect of PUVA, narrow-band UVB and cyclosporin on inflammatory cells of the psoriatic plaqueJ Cutan Pathol200734321321917302604
- KruegerJGWolfeJTNabeyaRTSuccessful ultraviolet B treatment of psoriasis is accompanied by a reversal of keratinocyte pathology and by selective depletion of intraepidermal T cellsJ Exp Med19951826205720687500051
- AufieroBMTalwarHYoungCNarrow-band UVB induces apoptosis in human keratinocytesJ Photochem Photobiol B200682213213916309917
- Johnson-HuangLMSuárez-FariñasMSullivan-WhalenMGilleaudeauPKruegerJGLowesMAEffective narrow-band UVB radiation therapy suppresses the IL-23/IL-17 axis in normalized psoriasis plaquesJ Invest Dermatol2010130112654266320555351
- FuruhashiTSaitoCToriiKNishidaEYamazakiSMoritaAPhoto(chemo)therapy reduces circulating Th17 cells and restores circulating regulatory T cells in psoriasisPLoS One201381e5489523365685
- ArchierEDevauxSCastelaEEfficacy of psoralen UV-A therapy vs narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature reviewJ Eur Acad Dermatol Venereol201226Suppl 3112122512676
- ChanESCronsteinBNMolecular action of methotrexate in inflammatory diseasesArthritis Res20024426627312106498
- JohnstonAGudjonssonJESigmundsdottirHLudvikssonBRValdimarssonHThe anti-inflammatory action of methotrexate is not mediated by lymphocyte apoptosis, but by the suppression of activation and adhesion moleculesClin Immunol2005114215416315639649
- HeydendaelVMSpulsPIOpmeerBCMethotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasisN Engl J Med2003349765866512917302
- FlytströmIStenbergBSvenssonABergbrantIMMethotrexate vs ciclosporin in psoriasis: effectiveness, quality of life, and safety. A randomized controlled trialBr J Dermatol2008158111612117986302
- BarkerJHoffmannMWozelGEfficacy and safety of infliximab vs methotrexate in patients with moderate-to-severe plaque psoriasis: results of an open-label, active-controlled, randomized trial (RESTORE1)Br J Dermatol201116551109111721910713
- SauratJHStinglGDubertretLEfficacy and safety results from the randomized controlled comparative study of adalimumab vs methotrexate vs placebo in patients with psoriasis (CHAMPION)Br J Dermatol2008158355856618047523
- ReichKLangleyRGPappKAA 52-week trial comparing briakinumab with methotrexate in patients with psoriasisN Engl J Med2011365171586159622029980
- PappKBissonnetteRRosophLEfficacy of ISA247 in plaque psoriasis: a randomised, multicentre, double-blind, placebo-controlled phase III studyLancet200837196211337134218424323
- KaltwasserJPNashPGladmanDEfficacy and safety of leflunomide in the treatment of psoriatic arthritis and psoriasis: a multinational, double-blind, randomized, placebo-controlled clinical trialArthritis Rheum20045061939195015188371
- Tlacuilo-ParraJAGuevara-GutiérrezERodríguez-CastellanosMAOrnelas-AguirreJMBarba-GómezJFSalazar-PáramoMLeflunomide in the treatment of psoriasis: results of a phase II open trialBr J Dermatol2004150597097615149511
- AkhyaniMChams-DavatchiCHemamiMRFatehSEfficacy and safety of mycophenolate mofetil vs methotrexate for the treatment of chronic plaque psoriasisJ Eur Acad Dermatol Venereol201024121447145120384673
- SchaferPHPartonAGandhiAKApremilast, a cAMP phospho-diesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasisBr J Pharmacol2010159484285520050849
- SchettGWollenhauptJPappKOral apremilast in the treatment of active psoriatic arthritis: results of a multicenter, randomized, double-blind, placebo-controlled studyArthritis Rheum201264103156316722806399
- PappKCatherJCRosophLEfficacy of apremilast in the treatment of moderate to severe psoriasis: a randomised controlled trialLancet2012380984373874622748702
- PappKAKaufmannRThaçiDHuCSutherlandDRohanePEfficacy and safety of apremilast in subjects with moderate to severe plaque psoriasis: results from a phase II, multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison studyJ Eur Acad Dermatol Venereol2013273e376e38323030767
- MeyerDMJessonMILiXAnti-inflammatory activity and neutrophil reductions mediated by the JAK1/JAK3 inhibitor, CP-690,550, in rat adjuvant-induced arthritisJ Inflamm (Lond)201074120701804
- O’sheaJJTargeting the Jak/STAT pathway for immunosuppressionAnn Rheum Dis200463Suppl 2ii67ii7115479876
- PappKAMenterAStroberBEfficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a Phase 2b randomized placebo-controlled dose-ranging studyBr J Dermatol2012167366867722924949
- MamoloCHarnessJTanHMenterATofacitinib (CP-690,550), an oral Janus kinase inhibitor, improves patient-reported outcomes in a phase 2b, randomized, double-blind, placebo-controlled study in patients with moderate-to-severe psoriasisJ Eur Acad Dermatol Venereol Epub172013
- PortsWCKhanSLanSA randomised Phase 2a efficacy and safety trial of the topical Janus Kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasisBr J Dermatol Epub262013
- PunwaniNScherlePFloresRPreliminary clinical activity of a topical JAK1/2 inhibitor in the treatment of psoriasisJ Am Acad Dermatol201267465866422281165
- DuvicMNagpalSAsanoATChandraratnaRAMolecular mechanisms of tazarotene action in psoriasisJ Am Acad Dermatol1997372 Pt 3S18S249270552
- BuccheriLKatchenBRKarterAJCohenSRAcitretin therapy is effective for psoriasis associated with human immunodeficiency virus infectionArch Dermatol199713367117159197824
- CaproniMAntigaEMelaniLVolpiWDel BiancoEFabbriPSerum levels of IL-17 and IL-22 are reduced by etanercept, but not by acitretin, in patients with psoriasis: a randomized-controlled trialJ Clin Immunol200929221021418763027
- MurrayHEAnhaltAWLessardRA 12-month treatment of severe psoriasis with acitretin: results of a Canadian open multicenter studyJ Am Acad Dermatol19912445986021827800
- OlsenEAWeedWWMeyerCJCoboLMA double-blind, placebo-controlled trial of acitretin for the treatment of psoriasisJ Am Acad Dermatol1989214 Pt 16816862530251
- GollnickHBauerRBrindleyCAcitretin versus etretinate in psoriasis. Clinical and pharmacokinetic results of a German multicenter studyJ Am Acad Dermatol19881934584682971692
- RuzickaTSommerburgCBraun-FalcoOEfficiency of acitretin in combination with UV-B in the treatment of severe psoriasisArch Dermatol199012644824862138875
- TanewAGuggenbichlerAHönigsmannHGeigerJMFritschPPhotochemotherapy for severe psoriasis without or in combination with acitretin: a randomized, double-blind comparison studyJ Am Acad Dermatol19912546826841838750
- TostiARicottiCRomanelliPCameliNPiracciniBMEvaluation of the efficacy of acitretin therapy for nail psoriasisArch Dermatol2009145326927119289755
- KruegerJGottliebAMillerBDedrickRGarovoyMWalickePAnti-CD11a treatment for psoriasis concurrently increases circulating T-cells and decreases plaque T-cells, consistent with inhibition of cutaneous T-cellJ Invest Dermatol2000115233310951264
- Guttman-YasskyEVugmeysterYLowesMABlockade of CD11a by efalizumab in psoriasis patients induces a unique state of T-cell hyporesponsivenessJ Invest Dermatol200812851182119118239614
- LebwohlMTyringSKHamiltonTKA novel targeted T-cell modulator, efalizumab, for plaque psoriasisN Engl J Med2003349212004201314627785
- GordonKBPappKAHamiltonTKEfalizumab for patients with moderate to severe plaque psoriasis: a randomized controlled trialJAMA2003290233073308014679270
- LeonardiCMenterAHamiltonTCaroIXingBGottliebABEfalizumab: results of a 3-year continuous dosing study for the long-term control of psoriasisBr J Dermatol200815851107111618373710
- SchwabNUlzheimerJCFoxRJFatal PML associated with efalizumab therapy: insights into integrin αLβ2 in JC virus controlNeurology201278745846722302546
- BloomgrenGRichmanSHotermansCRisk of natalizumab-associated progressive multifocal leukoencephalopathyN Engl J Med2012366201870188022591293
- ChamianFLinSLLeeEAlefacept (anti-CD2) causes a selective reduction in circulating effector memory T cells (Tem) and relative preservation of central memory T cells (Tcm) in psoriasisJ Transl Med200752717555598
- LebwohlMChristophersELangleyRAn international, random-ized, double-blind, placebo-controlled phase 3 trial of intramuscular alefacept in patients with chronic plaque psoriasisArch Dermatol2003139671972712810502
- EllisCNKruegerGGAlefacept Clinical Study GroupTreatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytesN Engl J Med2001345424825511474662
- GottliebABCasaleTBFrankelECD4+ T-cell–directed antibody responses are maintained in patients with psoriasis receiving alefacept: results of a randomized studyJ Amer Acad Dermatol200349581682514576659
- RobertsJLOrtonneJPTanJKJaraczEFrankelEAlefacept Clinical Study GroupThe safety profile and sustained remission associated with response to multiple courses of intramuscular alefacept for treatment of chronic plaque psoriasisJ Am Acad Dermatol201062696897820392521
- MeasePJGoffeBSMetzJVanderStoepAFinckBBurgeDJEtanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trialLancet2000356922738539010972371
- LeonardiCLPowersJLMathesonRTEtanercept as mono-therapy in patients with psoriasisN Engl J Med2003349212014202214627786
- ChaudhariURomanoPMulcahyLDDooleyLTBakerDGGottliebABEfficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trialLancet200135792711842184711410193
- ReichKNestleFOPappKInfliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trialLancet200536694941367137416226614
- YeungHWanJVan VoorheesASPatient-reported reasons for the discontinuation of commonly used treatments for moderate to severe psoriasisJ Am Acad Dermatol2013681647222846688
- MenterATyringSKGordonKAdalimumab therapy for moderate to severe psoriasis: A randomized, controlled phase III trialJ Am Acad Dermatol200858110611517936411
- GordonKBLangleyRGLeonardiCClinical response to adalimumab treatment in patients with moderate to severe psoriasis: double-blind, randomized controlled trial and open-label extension studyJ Am Acad Dermatol200655459860617010738
- KavanaughAKruegerGGBeutlerAInfliximab maintains a high degree of clinical response in patients with active psoriatic arthritis through 1 year of treatment: results from the IMPACT 2 trialAnn Rheum Dis200766449850517114188
- MeasePJGladmanDDRitchlinCTAdalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trialArthritis Rheum200552103279328916200601
- GottliebABChamianFMasudSTNF inhibition rapidly down-regulates multiple proinflammatory pathways in psoriasis plaquesJ Immunol200517542721272916081850
- MalaviyaRSunYTanJKEtanercept induces apoptosis of dermal dendritic cells in psoriatic plaques of responding patientsJ Am Acad Dermatol200655459059717010737
- ZabaLCSuárez-FariñasMFuentes-DuculanJEffective treatment of psoriasis with etanercept is linked to suppression of IL-17 signaling, not immediate response TNF genesAlefacept Clinical Study GroupJ Allergy Clin Immunol200912451022103019895991
- KimballABGordonKBFakharzadehSLong-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis: results from the PHOENIX 1 trial through up to 3 yearsBr J Dermatol2012166486187222356258
- LeonardiCLKimballABPappKAEfficacy and safety of usteki-numab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1)Lancet200837196251665167418486739
- PappKALangleyRGLebwohlMEfficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2)Lancet200837196251675168418486740
- LebwohlMLeonardiCGriffithsCELong-term safety experience of ustekinumab in patients with moderate-to-severe psoriasis (Part I of II): results from analyses of general safety parameters from pooled Phase 2 and 3 clinical trialsJ Am Acad Dermatol201266573174121930328
- GordonKBPappKALangleyRGLong-term safety experience of ustekinumab in patients with moderate to severe psoriasis (Part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trialsJ Am Acad Dermatol201266574275121978572
- StroberBECrowleyJJYamauchiPSOldsMWilliamsDAEfficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasisBr J Dermatol2011165366166821574984
- GordonKBLangleyRGGottliebABA phase III, randomized, controlled trial of the fully human IL-12/23 mAb briakinumab in moderate-to-severe psoriasisJ Invest Dermatol2011132230431422011907
- ReichKLangleyRGLebwohlMCardiovascular safety of ustekinumab in patients with moderate to severe psoriasis: results of integrated analyses of data from phase II and III clinical studiesBr J Dermatol2011164486287221332467
- PappKALeonardiCMenterABrodalumab, an anti–interleukin-17-receptor antibody for psoriasisN Engl J Med2012366131181118922455412
- LeonardiCMathesonRZachariaeCAnti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasisN Engl J Med2012366131190119922455413
- GudjonssonJEJohnstonAEllisCNNovel systemic drugs under investigation for the treatment of psoriasisJ Am Acad Dermatol201267113914722305044
- BickersDRLimHWMargolisDThe burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative DermatologyJ Am Acad Dermatol200655349050016908356
- FerrándizCGarcíaABlascoAJLázaroPCost-efficacy of adalimumab, etanercept, infliximab, and ustekinumab for moderate-to-severe plaque psoriasisJ Eur Acad Dermatol Venereol201226676877722126264