Abstract
Inflammatory lipids receive much attention due to their important biological activities. Knowledge of the chemokine system has also reached a level that makes it interesting in clinics, which prompted clinical trials into compounds manipulating chemokines or their receptors. However, little attention has been devoted to understand the relations between these two systems. Here, we will review the role of inflammatory lipids and chemokines in innate and adaptive immunity with an attempt to link the two systems and with emphasis on their importance in cancer development.
Introduction
A fundamental aspect of leukocyte function is their migration. All leukocytes migrate from the bone marrow via the blood to reach their sites of action. This tightly regulated process involves multiple steps, of which chemokines only account for a few. After selectin-dependent rolling and integrin-dependent adhesion, chemokines induce polarization of the leukocytes as they change their shape and start crawling towards an extracellular gradient of the chemokines.Citation1 Chemokine receptor signaling evokes cytoskeleton remodeling resulting in expansion of a lamellipodium in front and contraction in a myosin-dependent manner of the uropod at the back. Thus, by releasing molecules binding to the substrate below, cells move through tissues. As they navigate through the tissues, different chemokines and adhesion molecules in the microenvironment help in localizing various subsets of cells depending on their expression patterns of chemokine receptors.
Chemokines and chemokine receptors
Chemokines are important in health and disease as they orchestrate the infiltration of leukocytes.Citation2 These molecules are divided into four subfamilies based on the position of the cysteine residue in the amino terminal end of the molecules; these are known as CXC or α, CC or β, C or γ, and CX3C or δ chemokines. Chemokines and their receptors are also classified based on their functions as inflammatory chemokines or inflammatory chemokine receptors, or they are classified based on house-keeping functions that are involved in the circulation and homing of cells under physiological conditions.Citation3 All chemokine receptors activate heterotrimeric G proteins and various intracellular signaling pathways.Citation4
Inflammatory lipids in innate immunity
Lipids are important second messengers and much is yet to be known about their complex biology. We will focus here on the current knowledge of lipids and chemokines in inflammation, with an emphasis on cancer.
Lysophospholipids
Among the lysophospholipids, sphingosine 1-phosphate (S1P) is one of the most extensively studied; it binds heptahelical receptors coupled to heterotrimeric G proteinsCitation5 and constitutes a major part of serum and plasma.Citation6,Citation7 We recently reviewed its impact on cancer microenvironment.Citation8 S1P is a multifunctional lipid present in high concentrations up to the micromolar range in the serum and it regulates many cell responses, such as cell proliferation, apoptosis, cell differentiation and migration, as well as immunological responses.Citation7,Citation9,Citation10 It is generated from sphingolipids, which are essential plasma membrane lipids concentrated in liquid-ordered domains, commonly known as lipid rafts.Citation11 S1P can be rapidly metabolized following stimulation of various plasma membrane receptors through the activation of an enzymatic cascade. This pathway has been denoted the sphingomyelin cycle, due to the fact that, for all the steps, reverse reactions may take place catalyzed by specific enzymes such as S1P phosphatases, ceramide synthase, and sphingomyelin synthase.
S1P is synthesized by most cells, but, due to intracellular degradation by S1P lyase and S1P phosphatase-induced dephosphorylation, its level in tissues is low.Citation12–Citation15 The exception is blood with low micromolar levels of this lipid mainly contributed by erythrocytes. In the lymph, the S1P levels are in the hundred nanomolar range.Citation16,Citation17 Serum protein partners might have a role in determining the uptake and intracellular degradation of S1P as free S1P and S1P bound to serum albumin are more susceptible to degradation than when bound to lipoproteins such as high-density lipoprotein.Citation18 Also, the concentration gradient between blood and tissues may be ablated by inhibition of S1P lyase activity, resulting in increased levels of S1P in tissues.Citation17 There are reasons to believe that the lymph S1P, as well as its plasma level, is regulated by the endothelium. Secretion of the lipid from these cells is increased by the physiological stimulus of shear stress,Citation19 but that is not the case for plateletsCitation16,Citation19 nor mast cells.Citation20
The direct actions of S1P may be exerted via two different mechanisms. Either via the extracellular S1P receptors or via intracellular modes of action.Citation21,Citation22 The membrane bound receptors for S1P have been cloned, and were first linked to differentiation of endothelial cells. They were therefore named endothelial differentiation geneCitation23 but renamed once it was realized that the ligand for the receptor family was S1P.Citation24 Thus, they are now known as S1PR1, S1PR2, S1PR3, S1PR4, and S1PR. The receptors are expressed in different patterns through the immune system.Citation11 Dendritic cells express all five receptorsCitation25,Citation26 and human natural killer (NK) cells express mRNA for all receptors except for S1PR2.Citation27 All five receptors signal through G-protein coupled receptors, but differ in downstream effects.Citation28,Citation29 Initial findings of overexpression of sphingosine kinase 1 (SphK1) in fibroblasts capable of developing into tumorsCitation30 and enhanced SphK1 mRNA expression in solid tumorsCitation31,Citation32 compared to normal tissues made S1P a possible target of research in the cancer field.
The main function of many of the S1P receptors is migration.Citation27,Citation33–Citation35 Murine mature dendritic cells (mDCs), but not immature dendritic cells (iDCs), migrate towards S1P in a pattern correlated with the upregulation of S1PR and S1PR3 during maturation.Citation36 This action is dependent on signaling through Rac/Cdc42 and Rho as blocking of these small GTPases results in a complete failure to migrate. The S1P receptor agonist fingolimod (FTY720) does not trigger migration of DCs,Citation36 leading to the proposition that part of the immune modulation accomplished by FTY720 may be caused by impaired DC migration.
Whereas low concentrations of S1P promote chemotaxis in a S1PR1 dependent manner, high concentrations seem to be inhibitory.Citation33,Citation37 An explanation for this inhibition may be downregulation of S1PR1 by high S1P concentrations. Blood concentrations of S1P are high and, therefore, this may be relevant in vivo during transit of cells in blood.Citation11 In certain immune cells this concentration dependence is less evident, and in some cases the chemotactic response is associated with a particular stage of cell differentiation or cell activation, which leads to changes in receptor expression. This is the case for DCs as they mainly express S1PR1 in the immature state but upregulate S1PR3 upon maturation, which then may mediate their chemotactic response towards S1P.Citation36 Interestingly, a similar regulatory capacity may exist for chemokine receptors through S1P receptor agonism as FTY720 at high doses significantly reduced renal expression of CCR (CC chemokine receptor)1, CCR2, and CCR5.Citation38 In this study of partly nephrectomized rats, CCL (CC motif ligand) 2/MCP-1 gene expression as well as plasma concentrations of the proinflammatory cytokines IFN(interferon)-γ, TNF (tumor necrosis factor)-α, IL (interleukin)-6, IL-12 and CCL5/RANTES (regulated on activation, normal T-cell expressed and secreted) were also reduced. The effect may have been mediated by S1PR2 through Gαq and Rac1-dependent signaling pathways.Citation39
The cross talk among chemokines and the S1P system is evident in the study showing that FTY720 stimulated migration towards the lymph nodes is dependent on the CCR7 ligands CCL19 and CCL21.Citation40 For FTY720 enhanced migration in lymphoid compartments, additional chemokine receptors are at work as compared to the homeostatic state.Citation41 In this study, Yopp et alCitation41 showed that FTY720-stimulated migration of T-cells is dependent on CCR2, CCR5, CCR7, and CXCR4 in anatomically restricted compartments. Later, it was shown that overexpression of S1PR1 results in reduced expression of CXCR4 leading to tenfold reduction in migration of Jurkat cells or peripheral blood progenitor cells towards CXCL12/SDF (stromal cell-derived factor)-1α and eightfold reduction in bone marrow homing,Citation42 while S1P receptor agonists may sensitize the cells for CXCR4 signaling via S1PR3.Citation43 This is in line with in vivo and in vitro evidence of increased CXCR4 function in hematopoietic progenitor cells, where activation of S1P receptors by FTY720 modulated the effects of CXCL12/SDF-1α.Citation44 Not only the S1P receptors are important in this respect, as disruption of the S1P gradient towards blood as well as desensitization of its receptors reduced egress of immature progenitors in the steady state due to inhibition of CXCL12/SDF-1α release.Citation45 Functionally, the S1P-CXCR4 cross talk mediates adhesion and transendothelial migration of myeloma cells through upregulation of their adhesion followed by CXCL12/SDF-1α mediated transmigration.Citation46 The FTY720 mediated lymphopenia also relies on chemokine receptors for the initial accumulation of cells in secondary lymphoid organs, as is evident by it being delayed in mice lacking CCR7 and significantly reduced in CXCR5 knockout mice;Citation47 hence, the sequestration of lymphoid cells in the secondary lymphoid organs does not appear to depend on CCR7 and CXCR5.Citation47 Synergism at the second messenger level may explain this. As well as CXCL13/BLC, the ligand for CXCR5 and S1P stimulate the common activation pathway of tyrosine kinases Pyk2 and Rap, which are required for their induction of B-cell migration as well as adhesion to intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion protein 1 (VCAM-1).Citation48 This mechanism may also be important for maturation as splenic T-cell migration towards CCL19/MIP-3β and CXCL12/SDF-1α is enhanced by S1P receptor stimulation, while peripheral lymph node nonactivated and naïve T-cells require both chemokines and S1P receptors stimulation.Citation49
The prostaglandins
Prostaglandin E2 (PGE2) is notable for its many ways of interfering with white blood cell functions. By suppressing acute inflammatory functions of macrophages, granulocytes, naive Th1 and cytotoxic T-cells, as well as NK cells, while promoting Th2 or T regulatory responses, it greatly influences the immune response. By activating its receptor, EP2, PGE2 increases intracellular levels of cAMP (cyclic adenosine monophosphate) in NK cellsCitation50 and limits their cytolytic functionCitation50–Citation52 as well as reducing IFN-γ secretion induced by IL-12 and/or IL-18.Citation53 Accordingly, macrophage phagocytosisCitation54 and bacterial killingCitation55 is inhibited.
In DCs, IL-12 production is abrogated while the secretion of IL-10 is increased, leading to IL-4/IL-5 producing Th2 cells. This prompts a Th2-skewness as well as blockage of the development of IL-18-induced CCR7+ NK cells that would otherwise home to the lymph nodes in order to secrete IFN-γ and promote Th1 responses.Citation56 The production of Th1 cytokine IFN-γ, but not Th2 cytokines IL-4 and IL-5, in T-cells is also inhibited by PGE.Citation57 Finally, the Th1-suppressive effects are evident in innate immune cells as IL-12 production is suppressed in monocytesCitation58 and DCs.Citation59
The generally inhibitory impact of PGE2 on innate immunity is especially evident in the case of cancer. The development of DCs is redirected towards myeloid-derived suppressor cells – functionally diverse immature myeloid cells promoting cancer development and suppressing cytotoxic T-lymphocytes responses – when PGE2 is added to the standard regimen of monocyte development.Citation60 The same was observed when DCs were generated in the presence of PGE2 produced by cancer cells as it leads to a tolerogenic M2 phenotype with low expression of costimulatory molecules and altered IL-12/IL-10 balance, which leads to poor capacity to stimulate T-cell proliferation and IFN-γ production.Citation61
PGE2 impact on DCs when already maturated from monocytes is a lot different from the general inhibitory effects on monocytes. For example, PGE2, when added to cultures supplied with IL-β or TNF-α, accelerates the maturation of DCs, elevating their expression of costimulatory moleculesCitation62 and making them superior in inducing IFN-γ release from T-cells. Emphasizing the differential impact of PGE2 on DCs at various differentiation stages, PGE2 enhanced IL-12 secretion from DCs while it reduced the levels secreted from LPS-stimulated DCs.Citation63,Citation64
PGE2 is required for functionally activating monocyte derived DCs upon upregulation of CCR7.Citation65,Citation66 Recently, the mechanism for the upregulation of CCR7 was shown to be due to decreased endogenous secretion of the CCR7 ligand CCL19/MIP-3β by PGE2. This results in the capacity to migrate towards lymph node associated chemokines CCL19/MIP-3β or CCL21/MIP-3α, which is a prerequisite for T-cell priming.Citation66 DCs treated with PGE2 also showed enhanced expression of CCR7 and migration towards its ligands, while they were weak secretors of CCL19/MIP-3β and, hence, unable to attract naïve T-cells.Citation67
PGE2 enhances production of interleukin 8 (CXCL8/IL-8)Citation68 and CCL2/MCP-1Citation69 and is necessary for the migration of human DCs,Citation70 and thus for the recruitment of cells of the innate immune system. However, CCR5 expression on monocytes and macrophages is blocked.Citation71 Similarly, the functions of CCL5 and CXCR3Citation72 are also blocked, thus impeding the potential for NK-DC cross talkCitation73 while at the same time attracting T regulatory cells.Citation74 PGE2 is important as well for the production of CXCL12/SDF-1α, the expression of its receptor, CXCR4, on myeloid-derived suppressor cells,Citation75 and, consequently, for the recruitment of these cells towards ovarian cancer sites. It even increases tumor growth through increased angiogenesis via the induction of CXCL1/GRO-α expression.Citation76 Finally, emphasizing the importance of PGE2 in DC development, replacement of PGE2Citation77,Citation78 and suppression of cyclooxygenase 2Citation79,Citation80 enhances the immunogenic and therapeutic activity of cancer vaccines. On the other hand, a recent knockout study of PGE-1 synthase in mice did not affect maturation or migration of DCs, suggesting that further research is needed in this field.Citation81
Relevance to cancer
The importance of chemokines in cancer was established in early 1980s as MCP-1/CCL2 was identified in cultures of tumor cell lines.Citation82 Knowledge of the important contributions in embryology and physiology has been followed by substantial research on the implications for cancer development and treatment.
Allavena et alCitation83 reviewed the role of chemokines in cancer related inflammation, dividing the connection between chemokines and cancer related inflammation into two pathways: 1) the oncogene-driven intrinsic pathway that triggers the inflammatory cascade; and 2) the leukocyte-driven extrinsic pathway establishing inflammatory conditions, thus increasing cancer risk. Lazennec and RichmondCitation84 provided further insights into the importance of chemokine receptors in this field.
In order to translate the implications of lipids through chemokines and their receptors to the different steps of cancer pathophysiology, we provide a stepwise model (). In this model, seven key steps at which inflammatory lipids and chemokines play important roles are highlighted: 1) the adhesion and rolling of leukocytes as a first step in shaping the inflammatory milieu around cancer cells; 2) the transmigration of cancer cells through the vascular endothelium; 3) the impact of the tumor microenvironment on the leukocytes, implicating functional maturation towards anti- or pro-cancerous phenotypes; 4) the retention of leukocytes, mediated via the mechanisms described in step 3; 5) in malignant transformation. As the first step in cancerogenesis, this is the process by which normal tissue resident cells undergo genetic changes to become cancerous cells – at this point, chemokines do not play any major role, but we will touch upon how S1P and PGE2 do; 6) growth and development of a solid tumor; and 7) dissemination of cancer cells, which is highly dependent on the expression of chemokine receptors.
Figure 1 Development of tumor metastases aided by the chemokine system. The process of tumor dissemination is depicted in seven steps: 1. Adhesion/rolling of leukocytes. 2. Transmigration of leukocytes. 3. Maturation of leukocytes. 4. Retention of leukocytes 5. Malignant transformation. 6. Malignant growth. 7. Metastases.
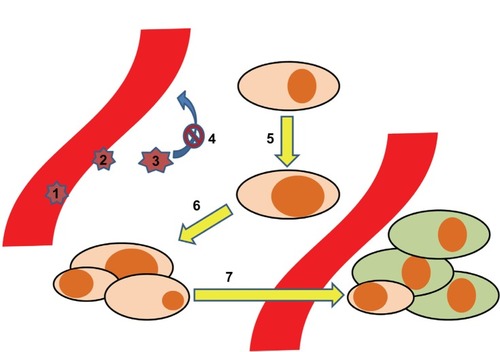
Steps 1–4: Recruitment of leukocytes subsets (steps 1 and 2), followed by maturation of the cells into protumoral phenotypes (step 3), is an important process related to the function of chemokines. In this respect, Negus et al described the involvement of leukocytes in ovarian cancer stroma.Citation85 By immunohistochemical analysis of epithelial ovarian tumor biopsies, they determined the content of leukocyte subsets. CD68+ macrophages accounted for 3,700 cells/mm3, CD8+/CD45RO+ T-cells for 2,200 cells/mm3, and NK cells, B-cells, and mast cells between zero and 200 cells/mm3. There was a correlation between CD8+ T-cells and numbers of cells expressing CCL2/MCP-1 and CCL5/RANTES. Further, a correlation between numbers of macrophages and the numbers of cells expressing CCL2/MCP-1 led to the suggestion that CCL2/MCP-1 may be responsible for leukocyte infiltration into ovarian carcinomas,Citation85 and that epithelial cells are the major source of CCL2/MCP-1.Citation86 Subsequently, in culture with tumor cells, the CD14+CD16- subset of monocytes increased the expression of CCR2,Citation87 trapping them inside the cancer microenvironment. In this milieu, the same monocyte subset also increased its expression of CXCR1, CXCR2, and CXCR4.Citation87 The change was associated with enhanced migration towards CXCL8/IL-8 for the CXCR1 and CXCR2 expressing cells and towards stromal cell-derived factor-1 (CXCL12/SDF-1α) for the CXCR4 expressing ones. This reflects the importance of chemokine receptors in functionally programming the different monocyte subsets, as they express different chemokine receptors.
An example of how differences in the tumor microenvironment affects leukocyte chemokine expression comes from ovarian cancer. While CCR1 and CCR5 are the only CC chemokine receptors that are consistently expressed in ovarian tumors, leukocytes in ascites of advanced ovarian cancer show expression patterns of chemokine receptors comparable to that which is found in peripheral blood. This has been proposed to be related to the microenvironment in which these cells are found, characterized by differential concentration levels of chemokines and cytokines, but also physiological factors such as hypoxia.Citation85,Citation88,Citation89 Importantly, CCR2 is downregulated on tumor cells associated with macrophage phenotypes responding to local TNF-α production, which was suggested to serve as a mechanism to arrest and retain recruited macrophages (step 4).
The tumor chemokine microenvironment is further notable in that it is suppressive of specific anticancer responses. Exposure of macrophages to this milieu leads to their maturation into type-2 macrophages, or tumor-associated macrophages.Citation90 Their release of IL-10 and TGF-β as well as CCL2/MCP-1 polarizes the immune response towards Th2, thus inhibiting macrophage and CD8+ T-cell killing of cancer cells. In ovarian cancer, tumor cell production of CXCL12/SDF-1α reduces immunity by attracting and protecting CXCR4-expressing plasmacytoid DCs but not myeloid DCs, hence weakening immunity.Citation91 Emphasizing the importance of the chemokine system as a pro-cancerous mediator, data from mouse models suggest that the net effect may be the promotion of growth, angiogenesis, apoptosis, and metastasis.Citation92–Citation94
Other observations indicate that it may be a matter of balancing pro- and anti-cancerous effects. This seems to be most important regarding angiogenesis,Citation95 including human non-small-cell lung carcinoma, in which the ratio of glutamic acid-leucine-arginine to non-acid-leucine-arginine CXC chemokine expression is high, and in a severe combined immunodeficiency (SCID) mouse model where neutralization of endogenous tumor-derived CXCL8/IL-8 could inhibit tumor growth and metastasis by about 50% through a decrease in tumor-derived vessel density without directly affecting tumor cell proliferation. An interesting perspective is studies of D6 and the Duffy antigen, which are involved in post inflammatory clearance of chemokines,Citation96 as they are related to general protection from cancer.Citation97,Citation98
Step 5 describes the malignant transformation. The chemokines do not directly play a major role at this point, however S1P and PGE2 do. S1P is regarded a prosurvival lipid due to its involvement in many of the processes implicated in the shaping of a favorable tumor microenvironment.Citation8 Concerning the malignant transformation specifically, overexpression of sphingosine kinase 1 (SK1) – one of the two kinases that catalyze S1P – resulted in tumor formation in 3T3 fibroblastsCitation30,Citation99 while its deletion resulted in reduced head and neck squamous cell carcinogenesis.Citation100 Very recent developments in the field suggest that SK interaction with oncogenes is critical in early development of cancer, as was recently reviewed.Citation101
In the case of colon cancer, S1P and SK1 both independently stimulate the expression of cyclooxygenase 2, leading to increased PGE2 levels.Citation102 In this study, while 75% of the rat colon adenocarcinomas stained strongly positive for SK1, none of the normal epithelium samples did. The same group also linked the expression of the PGE2 receptor EP1 to colon cancer development as its knockdown reduced cancer incidence.Citation103
In step 6, we describe the axis of another chemokine/chemokine receptor in terms of enhancing cell growth.Citation104 CXCR2 inhibits growth; its knockout reduces senescence (inability of division) that is oncogene-induced as well as replication-inducedCitation105 while CXCR2 overexpression leads to premature senescence. CXCL1/GRO-α, the ligand for CXCR1 and CXCR2, also serves to reprogram cancer-associated stromal fibroblasts to a senescent protumorigenic state in ovarian cancer.Citation106 Hence, CXCR2 and its ligands are regarded as gate keepers of tumor growth by increasing senescence.Citation84 CXCR2 also enhances neoangiogenesis and leukocyte infiltration,Citation104 suggesting a function in establishing a sustainable tumor microenvironment rather than uncontrolled growth.
In the last step, we describe the process of dissemination, which is caused by cells traveling through the blood stream to reside in various tissues. These cells are a lot more susceptible to the effects of cytostatic drugs, which is promising in therapeutic terms. The consensus is that the gradients towards which the cancer cells migrate are generated by tissue resident cells,Citation107 but a more autonomous role has been suggested. It implicates an autocrine mechanism by which cancer cells generate their own chemokine gradient, which has been described for CCL19/MIP-3β and CCL21/MIP-3α.Citation108
Some examples of chemokine directed dissemination are the chemokine receptor/chemokine axes CXCL12-CXCR4, CCL19-CCR7 or CCL21-CCR7, and CCL27-CCR10, which are associated with metastasis to bone, lymph nodes, and skin, respectively.Citation107 Recent studies further link CX3CR1 expressed in pancreatic ductal adenocarcinoma cells to migration towards CX3CL1/fraktalkine produced by neurons and nerve fibers.Citation109 Melanoma cells expressing CCR9 metastasize to the small intestineCitation110 and non-melanoma cells expressing CXCR2 spread to the lungs.Citation111 Finally, CCR7 aids cells in migrating into the lymph nodes and CXCR4 into distant organs.Citation104 Based on this, there is a reason to believe that any small effect on the cancer microenvironment that may lead to alteration of the expression levels of these receptors may be of vital importance for clinical end points in cancer.
Future perspectives
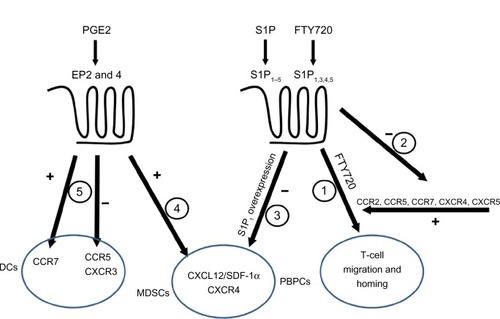
In this review, we attempted to link two complicated systems (ie, chemokines and inflammatory lipids) and to explain their roles in cancer (). We highlighted the importance of chemokine receptor CCR2, which binds CCL2/MCP-1 as responsible for attracting leukocytes that mature into pro-cancerous cells inside the malignant growth site. In the mouse model of pancreatic cancer, CCR2 antagonism decreased metastasis of the cancer cells.Citation112 On the other hand, the antagonists of CXCR2 are beneficial in a combined therapy regiment with oxaliplatin for preclinical colon cancer model.Citation113 This knowledge of the chemokine system can therefore be utilized to prevent dissemination of cancer cells. This is shown to be possible in a mouse model of ovarian cancer where blockade of CXCL12-CXCR4 axis resulted in multiple effects including decreased dissemination of tumor cells corroborated with prolonged survival.Citation114 Further, this has implications with regard to how the knowledge of the chemokine system can be applied to the inflammatory lipids. As indicated above, PGE2 and FTY720 may sensitize CXCR4 signaling, while T-cell migration initiated by FTY720 depends on CXCR4, CCR2, CCR5, and CCR7. This raises a question during the clinical use of FTY720 regarding the implications of systemic alterations in physiologically important systems. The adverse effects of long time treatment are difficult to foresee, but the increased risk of skin cancer upon FTY720 treatment,Citation115 as seen in many in vitro studies, raises serious concerns. Similar problems arise with PGE-2, though the adverse effects for this molecule are well known. Nonsteroidal anti-inflammatory drugs, the inhibitors of the cyclooxygenases important for PGE-2 production, are feared for their gastrointestinal and cardiovascular side effects. Hence, in light of the general inhibitory effects of PGE-2 on innate immunity, preventing normal activation while inducing pro-cancerous phenotypes like MDSC and tumor-associated macrophages, emphasis should be on developing novel pharmacological approaches to limit the effects of PGE-2, other inflammatory lipids, and chemokines in cancer microenvironment.
Figure 2 Interactions among chemokines and inflammatory lipids.
Abbreviations: PGE2, Prostaglandin E2; EP, prostaglandin E receptor; DCs, dendritic cells; CCR, CC chemokine receptor; CXCR, CXC chemokine receptor; SDF, stromal cell-derived factor; MDSCs, myeloid derived supressor cells; PBPCs, peripheral blood progenitor cells; S1P, sphingosine 1-phosphate.
Disclosure
The authors report no conflicts of interest in this work.
References
- MañesSGómez-MoutónCLacalleRAJiménez-BarandaSMiraEMartínez-ACMastering time and space: immune cell polarization and chemotaxisSemin Immunol200517778615582490
- RamanDSobolik-DelmaireTRichmondAChemokines in health and diseaseExp Cell Res201131757558921223965
- MaghazachiAARole of chemokines in the biology of natural killer cellsCurr Top Microbiol Immunol2010341375820369317
- MaghazachiAAInsights into seven and single transmembrane-spanning domain receptors and their signaling pathways in human natural killer cellsPharmacol Rev20055733935716109839
- Oz-ArslanDRüscherWMyrtekDIL-6 and IL-8 release is mediated via multiple signaling pathways after stimulating dendritic cells with lysophospholipidsJ Leukoc Biol20068028729716769764
- PyneSPyneNJSphingosine 1-phosphate signalling in mammalian cellsBiochem J200034938540210880336
- SpiegelSMilstienSSphingosine-1-phosphate: an enigmatic signalling lipidNat Rev Mol Cell Biol2003439740712728273
- RolinJMaghazachiAAEffects of lysophospholipids on tumor microenvironmentCancer Microenviron2011439340321904916
- HuwilerAKolterTPfeilschifterJSandhoffKPhysiology and pathophysiology of sphingolipid metabolism and signalingBiochim Biophys Acta20001485639910832090
- HaitNCOskeritzianCAPaughSWMilstienSSpiegelSSphingosine kinases, sphingosine 1-phosphate, apoptosis and diseasesBiochim Biophys Acta200617582016202616996023
- RiveraJProiaRLOliveraAThe alliance of sphingosine-1-phosphate and its receptors in immunityNat Rev Immunol2008875376318787560
- HannunYAObeidLMPrinciples of bioactive lipid signalling: lessons from sphingolipidsNat Rev Mol Cell Biol2008913915018216770
- MechtcheriakovaDWlachosASobanovJSphingosine 1-phosphate phosphatase 2 is induced during inflammatory responsesCell Signal20071974876017113265
- PeestUSenskenSCAndréaniPHänelPVan VeldhovenPPGrälerMHS1P-lyase independent clearance of extracellular sphingosine 1- phosphate after dephosphorylation and cellular uptakeJ Cell Biochem200810475677218172856
- ZhaoYKalariSKUsatyukPVIntracellular generation of sphingosine 1-phosphate in human lung endothelial cells: role of lipid phosphate phosphatase-1 and sphingosine kinase 1J Biol Chem2007282141651417717379599
- PappuRSchwabSRCornelissenIPromotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphateScience200731629529817363629
- SchwabSRPereiraJPMatloubianMXuYHuangYCysterJGLymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradientsScience20053091735173916151014
- YatomiYPlasma sphingosine 1-phosphate metabolism and analysisBiochim Biophys Acta2008178060661117980708
- VenkataramanKLeeYMMichaudJVascular endothelium as a contributor of plasma sphingosine 1-phosphateCirc Res200810266967618258856
- OliveraAMizugishiKTikhonovaAThe sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxisImmunity20072628729717346996
- AlvarezSEHarikumarKBHaitNCSphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2Nature20104651084108820577214
- HaitNCAllegoodJMaceykaMRegulation of histone acetylation in the nucleus by sphingosine-1-phosphateScience20093251254125719729656
- HlaTMaciagTAn abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein-coupled receptorsJ Biol Chem1990265930893132160972
- SpiegelSSphingosine 1-phosphate: a ligand for the EDG-1 family of G-protein-coupled receptorsAnn N Y Acad Sci2000905546010818441
- IdzkoMHammadHvan NimwegenMLocal application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell functionJ Clin Invest20061162935294417080194
- MaedaYMatsuyukiHShimanoKKataokaHSugaharaKChibaKMigration of CD4 T cells and dendritic cells toward sphingosine 1-phosphate (S1P) is mediated by different receptor subtypes: S1P regulates the functions of murine mature dendritic cells via S1P receptor type 3J Immunol20071783437344617339438
- KvebergLBrycesonYInngjerdingenMRolstadBMaghazachiAASphingosine 1 phosphate induces the chemotaxis of human natural killer cells. Role for heterotrimeric G proteins and phosphoinositide 3 kinasesEur J Immunol2002321856186412115604
- SanchezTHlaTStructural and functional characteristics of S1P receptorsJ Cell Biochem20049291392215258915
- SiehlerSManningDRPathways of transduction engaged by sphingosine 1-phosphate through G protein-coupled receptorsBiochim Biophys Acta20021582949912069815
- XiaPGambleJRWangLAn oncogenic role of sphingosine kinaseCurr Biol2000101527153011114522
- FrenchKJSchrecengostRSLeeBDDiscovery and evaluation of inhibitors of human sphingosine kinaseCancer Res2003635962596914522923
- FrenchKJUpsonJJKellerSNZhuangYYunJKSmithCDAntitumor activity of sphingosine kinase inhibitorsJ Pharmacol Exp Ther200631859660316632640
- MatloubianMLoCGCinamonGLymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1Nature200442735536014737169
- GraelerMGoetzlEJActivation-regulated expression and chemotactic function of sphingosine 1-phosphate receptors in mouse splenic T cellsFASEB J2002161874187812468451
- CinamonGMatloubianMLesneskiMJSphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zoneNat Immunol2004571372015184895
- CzelothNBernhardtGHofmannFGenthHForsterRSphingosine-1-phosphate mediates migration of mature dendritic cellsJ Immunol20051752960296716116182
- DorsamGGraelerMHSeroogyCKongYVoiceJKGoetzlEJTransduction of multiple effects of sphingosine 1-phosphate (S1P) on T cell functions by the S1P1 G protein-coupled receptorJ Immunol20031713500350714500646
- SchaierMVorwalderSSommererCRole of FTY720 on M1 and M2 macrophages, lymphocytes, and chemokines in 5/6 nephrectomized ratsAm J Physiol Renal Physiol2009297F769F78019535570
- KawataTIshizukaTTomuraHSphingosine 1-phosphate inhibits migration and RANTES production in human bronchial smooth muscle cellsBiochem Biophys Res Commun200533164064715850807
- HonigSMFuSMaoXFTY720 stimulates multidrug transporter-and cysteinyl leukotriene-dependent T cell chemotaxis to lymph nodesJ Clin Invest200311162763712618517
- YoppACFuSHonigSMFTY720-enhanced T cell homing is dependent on CCR2, CCR5, CCR7, and CXCR4: evidence for distinct chemokine compartmentsJ Immunol200417385586515240672
- RyserMFUgarteFLehmannRBornhauserMBrennerSS1P(1) overexpression stimulates S1P-dependent chemotaxis of human CD34+ hematopoietic progenitor cells but strongly inhibits SDF-1/CXCR4-dependent migration and in vivo homingMol Immunol20084616617118760838
- WalterDHRochwalskyUReinholdJSphingosine-1-phosphate stimulates the functional capacity of progenitor cells by activation of the CXCR4-dependent signaling pathway via the S1P3 receptorArterioscler Thromb Vasc Biol20072727528217158356
- KimuraTBoehmlerAMSeitzGThe sphingosine 1-phosphate receptor agonist FTY720 supports CXCR4-dependent migration and bone marrow homing of human CD34+ progenitor cellsBlood20041034478448614988150
- GolanKVagimaYLudinAS1P promotes murine progenitor cell egress and mobilization via S1P1-mediated ROS signaling and SDF-1 releaseBlood20121192478248822279055
- García-BernalDRedondo-MuñozJDios-EsponeraASphingosine-1-phosphate activates chemokine-promoted myeloma cell adhesion and migration involving α4β1 integrin functionJ Pathol2013229364822711564
- MullerGReitererPHopkenUEGolfierSLippMRole of homeostatic chemokine and sphingosine-1-phosphate receptors in the organization of lymphoid tissueAnn N Y Acad Sci200398710711612727629
- DurandCAWestendorfJTseKWGoldMRThe Rap GTPases mediate CXCL13- and sphingosine1-phosphate-induced chemotaxis, adhesion, and Pyk2 tyrosine phosphorylation in B lymphocytesEur J Immunol2006362235224916821235
- YoppACOchandoJCMaoMLedgerwoodLDingYBrombergJSSphingosine 1-phosphate receptors regulate chemokine-driven transendothelial migration of lymph node but not splenic T cellsJ Immunol20051752913292416116177
- GotoTHerbermanRBMaluishAStrongDMCyclic AMP as a mediator of prostaglandin E-induced suppression of human natural killer cell activityJ Immunol1983130135013556185577
- BankhurstADThe modulation of human natural killer cell activity by prostaglandinsJ Clin Lab Immunol1982785916951051
- JoshiPCZhouXCuchensMJonesQProstaglandin E2 suppressed IL-15-mediated human NK cell function through down-regulation of common gamma-chainJ Immunol200116688589111145664
- WalkerWRotondoDProstaglandin E2 is a potent regulator of interleukin-12- and interleukin-18-induced natural killer cell interferon-gamma synthesisImmunology200411129830515009430
- AronoffDMCanettiCPeters-GoldenMProstaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMPJ Immunol200417355956515210817
- CanettiCSerezaniCHAtraszRGWhiteESAronoffDMPeters-GoldenMActivation of phosphatase and tensin homolog on chromosome 10 mediates the inhibition of FcgammaR phagocytosis by prostaglandin E2 in alveolar macrophagesJ Immunol20071798350835618056380
- MailliardRBAlberSMShenHIL-18-induced CD83+CCR7+ NK helper cellsJ Exp Med200520294195316203865
- SnijdewintFGKalinskiPWierengaEABosJDKapsenbergMLProstaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytesJ Immunol1993150532153298390534
- van der Pouw KraanTCBoeijeLCSmeenkRJWijdenesJAardenLAProstaglandin-E2 is a potent inhibitor of human interleukin 12 productionJ Exp Med19951817757797836930
- KalinskiPHilkensCMSnijdersASnijdewintFGKapsenbergMLIL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cellsJ Immunol199715928359200435
- ObermajerNMuthuswamyRLesnockJEdwardsRPKalinskiPPositive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cellsBlood20111185498550521972293
- HeusinkveldMde Vos van SteenwijkPJGoedemansRM2 macrophages induced by prostaglandin E2 and IL-6 from cervical carcinoma are switched to activated M1 macrophages by CD4+ Th1 cellsJ Immunol20111871157116521709158
- JonuleitHKuhnUMullerGPro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditionsEur J Immunol199727313531429464798
- RieserCBockGKlockerHBartschGThurnherMProstaglandin E2 and tumor necrosis factor alpha cooperate to activate human dendritic cells: synergistic activation of interleukin 12 productionJ Exp Med1997186160316089348319
- SharmaSStolinaMYangSCTumor cyclooxygenase 2-dependent suppression of dendritic cell functionClin Cancer Res2003996196812631593
- LuftTJeffordMLuetjensPFunctionally distinct dendritic cell (DC) populations induced by physiologic stimuli: prostaglandin E(2) regulates the migratory capacity of specific DC subsetsBlood20021001362137212149219
- ScandellaEMenYGillessenSForsterRGroettrupMProstaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cellsBlood20021001354136112149218
- MuthuswamyRMueller-BerghausJHaberkornUReinhartTASchadendorfDKalinskiPPGE(2) transiently enhances DC expression of CCR7 but inhibits the ability of DCs to produce CCL19 and attract naive T cellsBlood20101161454145920498301
- YuYChadeeKProstaglandin E2 stimulates IL-8 gene expression in human colonic epithelial cells by a posttranscriptional mechanismJ Immunol1998161374637529759900
- NakayamaTMutsugaNYaoLTosatoGProstaglandin E2 promotes degranulation-independent release of MCP-1 from mast cellsJ Leukoc Biol2006799510416275896
- LeglerDFKrausePScandellaESingerEGroettrupMProstaglandin E2 is generally required for human dendritic cell migration and exerts its effect via EP2 and EP4 receptorsJ Immunol200617696697316393982
- ZeidlerRCsanadyMGiresOLangSSchmittBWollenbergBTumor cell-derived prostaglandin E2 inhibits monocyte function by interfering with CCR5 and Mac-1FASEB J20001466166810744623
- GustafssonKIngelstenMBergqvistLNystromJAnderssonBKarlsson-ParraARecruitment and activation of natural killer cells in vitro by a human dendritic cell vaccineCancer Res2008685965597118632652
- Van ElssenCHVanderlochtJOthTSenden-GijsbersBLGermeraadWTBosGMInflammation-restraining effects of prostaglandin E2 on natural killer-dendritic cell (NK-DC) interaction are imprinted during DC maturationBlood20111182473248221715307
- MuthuswamyRUrbanJLeeJJReinhartTABartlettDKalinskiPAbility of mature dendritic cells to interact with regulatory T cells is imprinted during maturationCancer Res2008685972597818632653
- ObermajerNMuthuswamyROdunsiKEdwardsRPKalinskiPPGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environmentCancer Res2011717463747022025564
- WangDWangHBrownJCXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancerJ Exp Med200620394195116567391
- HokeyDALarreginaATErdosGWatkinsSCFaloLDJrTumor cell loaded type-1 polarized dendritic cells induce Th1-mediated tumor immunityCancer Res200565100591006716267032
- MailliardRBWankowicz-KalinskaACaiQAlpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activityCancer Res2004645934593715342370
- HahnTAlvarezIKobieJJShort-term dietary administration of celecoxib enhances the efficacy of tumor lysate-pulsed dendritic cell vaccines in treating murine breast cancerInt J Cancer20061182220223116331615
- HaasARSunJVachaniACycloxygenase-2 inhibition augments the efficacy of a cancer vaccineClin Cancer Res20061221422216397045
- MonradSUKojimaFKapoorMGenetic deletion of mPGES-1 abolishes PGE2 production in murine dendritic cells and alters the cytokine profile, but does not affect maturation or migrationProstaglandins Leukot Essent Fatty Acids20118411312121190819
- BottazziBPolentaruttiNAceroRRegulation of the macrophage content of neoplasms by chemoattractantsScience19832202102126828888
- AllavenaPGermanoGMarchesiFMantovaniAChemokines in cancer related inflammationExp Cell Res201131766467321134366
- LazennecGRichmondAChemokines and chemokine receptors: new insights into cancer-related inflammationTrends Mol Med20101613314420163989
- NegusRPStampGWHadleyJBalkwillFRQuantitative assessment of the leukocyte infiltrate in ovarian cancer and its relationship to the expression of C-C chemokinesAm J Pathol1997150172317349137096
- NegusRPStampGWRelfMGThe detection and localization of monocyte chemoattractant protein-1 (MCP-1) in human ovarian cancerJ Clin Invest199595239123967738202
- StecMBaranJBaj-KrzyworzekaMChemokine receptors and chemokine production by CD34+ stem cell-derived monocytes in response to cancer cellsAnticancer Res2012324749475323155238
- SicaASaccaniABottazziBDefective expression of the monocyte chemotactic protein-1 receptor CCR2 in macrophages associated with human ovarian carcinomaJ Immunol200016473373810623817
- GrimshawMJBalkwillFRInhibition of monocyte and macrophage chemotaxis by hypoxia and inflammation – a potential mechanismEur J Immunol20013148048911180113
- SolinasGGermanoGMantovaniAAllavenaPTumor- associated macrophages (TAM) as major players of the cancer-related inflammationJ Leukoc Biol2009861065107319741157
- ZouWMachelonVCoulomb-L’HerminAStromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cellsNat Med200171339134611726975
- OrimoAGuptaPBSgroiDCStromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretionCell200512133534815882617
- MantovaniASavinoBLocatiMZammataroLAllavenaPBonecchiRThe chemokine system in cancer biology and therapyCytokine Growth Factor Rev201021273920004131
- MantovaniAAllavenaPSozzaniSVecchiALocatiMSicaAChemokines in the recruitment and shaping of the leukocyte infiltrate of tumorsSemin Cancer Biol20041415516015246050
- StrieterRMBurdickMDMestasJGompertsBKeaneMPBelperioJACancer CXC chemokine networks and tumour angiogenesisEur J Cancer20064276877816510280
- MantovaniABonecchiRLocatiMTuning inflammation and immunity by chemokine sequestration: decoys and moreNat Rev Immunol2006690791817124512
- VetranoSBorroniEMSarukhanAThe lymphatic system controls intestinal inflammation and inflammation-associated colon cancer through the chemokine decoy receptor D6Gut20105919720619846409
- WangJOuZLHouYFEnhanced expression of Duffy antigen receptor for chemokines by breast cancer cells attenuates growth and metastasis potentialOncogene2006257201721116785997
- LeSEPchejetskiDBannoYOverexpression of sphingosine kinase 1 is an oncogenic event in erythroleukemic progressionBlood20051061808181615890687
- ShiraiKKaneshiroTWadaMA role of sphingosine kinase 1 in head and neck carcinogenesisCancer Prev Res (Phila)2011445446221209394
- PyneSPyneNJNew perspectives on the role of sphingosine 1-phosphate in cancerHandb Exp Pharmacol2013557123563651
- KawamoriTOstaWJohnsonKRSphingosine kinase 1 is up-regulated in colon carcinogenesisFASEB J20062038638816319132
- KawamoriTKitamuraTWatanabeKProstaglandin E receptor subtype EP(1) deficiency inhibits colon cancer developmentCarcinogenesis20052635335715564292
- RamanDBaugherPJThuYMRichmondARole of chemokines in tumor growthCancer Lett200725613716517629396
- AcostaJCO’LoghlenABanitoAChemokine signaling via the CXCR2 receptor reinforces senescenceCell20081331006101818555777
- YangGRosenDGZhangZThe chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesisProc Natl Acad Sci U S A2006103164721647717060621
- Ben-BaruchAOrgan selectivity in metastasis: regulation by chemokines and their receptorsClin Exp Metastasis20082534535617891505
- ShieldsJDFleuryMEYongCTomeiAARandolphGJSwartzMAAutologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signalingCancer Cell20071152653817560334
- MarchesiFPiemontiLFedeleGThe chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinomaCancer Res2008689060906918974152
- AmersiFFTerandoAMGotoYActivation of CCR9/CCL25 in cutaneous melanoma mediates preferential metastasis to the small intestineClin Cancer Res20081463864518245522
- SinghSVarneyMSinghRKHost CXCR2-dependent regulation of melanoma growth, angiogenesis, and experimental lung metastasisCancer Res20096941141519147552
- SanfordDEBeltBAPanniRZInflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axisClin Cancer Res2013193404341523653148
- NingYLabonteMJZhangWThe CXCR2 antagonist, SCH-527123, shows antitumor activity and sensitizes cells to oxaliplatin in preclinical colon cancer modelsMol Cancer Ther2012111353136422391039
- RighiEKashiwagiSYuanJCXCL12/CXCR4 blockade induces multimodal antitumor effects that prolong survival in an immunocompetent mouse model of ovarian cancerCancer Res2011715522553421742774
- CohenJABarkhofFComiGOral fingolimod or intramuscular interferon for relapsing multiple sclerosisN Engl J Med201036240241520089954
