Abstract
Signaling pathways mediated by receptor tyrosine kinases (RTKs) and their ligands play important roles in the development and progression of human cancers, which makes RTK-mediated signaling pathways promising therapeutic targets in the treatment of cancer. Compared with small-molecule compounds, antibody-based therapeutics can more specifically recognize and bind to ligands and RTKs. Several antibody inhibitors of RTK-mediated signaling pathways, such as human epidermal growth factor receptor 2, vascular endothelial growth factor, epidermal growth factor receptor or vascular endothelial growth factor receptor 2, have been developed and are widely used to treat cancer patients. However, since the therapeutic options are still limited in terms of therapeutic efficacy and types of cancers that can be treated, efforts are being made to identify and evaluate novel RTK-mediated signaling pathways as targets for more efficacious cancer treatment. The hepatocyte growth factor/c-Met signaling pathway has come into the spotlight as a promising target for development of potent cancer therapeutic agents. Multiple antibody-based therapeutics targeting hepatocyte growth factor or c-Met are currently in preclinical or clinical development. This review focuses on the development of inhibitors of the hepatocyte growth factor/c-Met signaling pathway for cancer treatment, including critical issues in clinical development and future perspectives for antibody-based therapeutics.
Introduction
In living organisms, communication between individual cells and between cells and the environment plays essential roles in various cellular processes including growth, differentiation, migration, and apoptosis.Citation1 These cellular processes are mediated in large part by signaling pathways triggered by interactions between receptor tyrosine kinases (RTKs) and their ligands.Citation1 Upon ligand binding, RTKs on the cell surface activate downstream signaling cascades and regulate target gene expression in a paracrine or autocrine manner.Citation1 These signaling pathways mediated by RTKs and their ligands are critical for both regulation of diverse cellular processes and development and progression of cancers.Citation2–Citation4 RTK-mediated signaling pathways are tightly regulated according to the physiological status of normal cells. In contrast, RTK signaling pathways are dysregulated or hyperactivated in a wide range of cancers via gain-of-function mutations, gene rearrangements, gene amplifications, and overexpression or abnormal stimulation of receptors/ligands.Citation1–Citation4 Furthermore, it has been suggested that loss-of-function by deletion or mutation of RTKs, including fibroblast growth factor receptor 1, EphA, and c-Met, is associated with several diseases, including cancers.Citation5–Citation7 Thus, RTK-mediated signaling pathways have become promising therapeutic targets for treating cancer.Citation2–Citation4
Individual RTK-mediated signaling cascades can be targeted at several levels to develop anti-cancer therapeutic agents that inhibit the signaling pathways via disruption of interactions between RTKs and their ligands, dimerization and phosphorylation of RTKs, and activation of downstream elements.Citation2,Citation4 For monoclonal antibody-based therapeutics, RTK-mediated signaling pathways are mainly inhibited by disrupting interactions between RTKs and their ligands.Citation4 Compared with small-molecule compounds that inhibit the kinase activity and autophosphorylation of a broad spectrum of RTKs, antibody inhibitors have greater target specificity for particular RTKs and/or ligands. Several antibody-based therapeutic agents that inhibit RTK-mediated signaling pathways have already been approved for the treatment of human cancers.Citation4 Trastuzumab (Herceptin™, Genentech/Roche, South San Francisco, CA, USA; approved by the US Food and Drug Administration [FDA] in 1998) binds to human epidermal growth factor receptor 2 (HER2)/neu receptor and is used to treat breast cancer.Citation8 Bevacizumab (Avastin™, Genentech/Roche; approved by the FDA in 2004) binds to vascular endothelial growth factor (VEGF) and is used to treat colorectal cancer, metastatic renal cell carcinoma, recurrent glioblastoma, and non-small cell lung cancer (NSCLC).Citation9–Citation12 Both cetuximab (Erbitux™; developed by Bristol-Myers Squibb, New York, NY, USA/Eli Lilly, Indianapolis, IN, USA and Merck KGaA, Darmstadt, Germany and approved in 2004) and panitumumab (Vectibix™, developed by Amgen (Thousand Oaks, CA, USA) and approved in 2006) bind to epidermal growth factor receptor (EGFR) and are used to treat colorectal and head/neck cancers.Citation13,Citation14 Ramucirumab (Cyramza™, developed by Eli Lilly and approved in 2014) binds to VEGF receptor 2 and is used to treat gastric cancer.Citation15 Although these FDA-approved therapeutic antibodies have contributed to improving clinical outcomes, there are still unmet needs for difficult-to-treat cancers. Identification of additional RTK/ligand targets may enable more effective treatment of these cancers.Citation2–Citation4 Currently, a variety of novel antibody-based inhibitors targeting RTKs such as c-Met, fibroblast growth factor receptor, HER1, HER3, insulin-like growth factor-1 receptor, platelet-derived growth factor receptor, and RON (recepteur d’origine nantais) are in preclinical and clinical development.Citation4
c-Met, the RTK oncogene, was first cloned in 1984.Citation16 The ligand of c-Met, hepatocyte growth factor (HGF; also known as scatter factor) was identified in 1991 as a potent mitogen/morphogen.Citation17,Citation18 The HGF/c-Met signaling pathway has been demonstrated to play important roles in the development and progression of various cancers.Citation19–Citation21 Dysregulation and hyperactivation of HGF or c-Met have been reported in human cancers and linked to poor prognosis.Citation22,Citation23 It has been suggested that the HGF/c-Met signaling pathway may be a promising target for drugs designed to overcome development of resistance to inhibition of other ligand/RTK signaling pathways.Citation19–Citation21 A large number of inhibitors of the HGF/c-Met signaling pathway are under development to treat human cancers.Citation19–Citation21 Similar to other inhibitors of RTK-mediated signaling pathways, agents targeting HGF or c-Met are categorized into two groups, ie, small-molecule drugs and biologics such as monoclonal antibodies.Citation19–Citation21 Currently, multiple therapeutic antibodies targeting the HGF/c-Met signaling pathway are in preclinical or clinical development.Citation24 In this review, we summarize the role of HGF and c-Met in normal tissues and cancers. We then discuss the development of HGF/c-Met signaling pathway inhibitors for cancer treatment, including critical issues in clinical development and future perspectives focusing on monoclonal antibody-based therapeutics.
Physiological role of HGF and c-Met
The HGF/c-Met signaling pathway influences a variety of cellular functions to control diverse biological processes such as embryonic development, epithelial branching morphogenesis, postnatal organ regeneration, and wound healing ().Citation19–Citation21 HGF is a paracrine signaling molecule produced and secreted from mesenchymal cells to affect neighboring epithelial cells expressing c-Met. Protective roles of HGF have been reported in tissue fibrosis, liver cirrhosis, endothelial injury, and lung fibrosis.Citation25–Citation29
Figure 1 HGF/c-Met signaling cascades.
Abbreviations: GRB2, growth factor receptor-bound protein 2; GAB1, GRB2-associated binding protein 1; HGF, hepatocyte growth factor; mTOR, mammalian target of rapamycin; MAPK, mitogen-activated protein kinase; PI3K, phosphatidylinositol-4,5-bisphosphate 3-kinase; STAT3, signal transducer and activator of transcription 3.
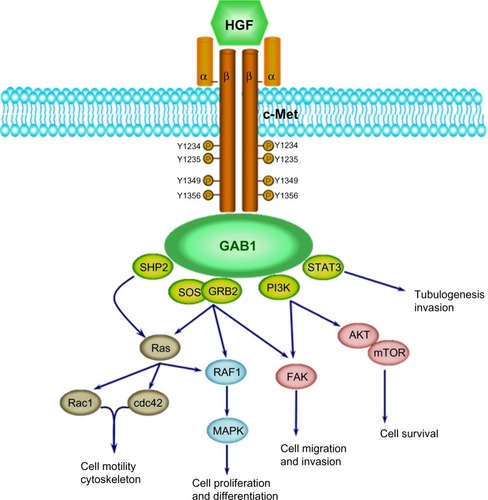
HGF is produced as an inactive single-chain precursor that is processed to yield an active heterodimer of one alpha and one beta chain linked by a disulfide bond.Citation30,Citation31 c-Met is also produced as a single-chain precursor and processed to yield a mature receptor composed of a glycosylated, extracellular alpha subunit disulfide-bonded to a transmembrane beta subunit.Citation32 The extracellular portion of c-Met is composed of a Sema domain (homologous to semaphorins), a cysteine-rich, Met-related-sequence domain, and four immunoglobulin (Ig)-like modules (IgG domains) responsible for binding HGF.Citation32 The intracellular portion of c-Met is composed of a juxtamembrane domain, a tyrosine kinase domain, and a C-terminal regulatory tail responsible for signal transduction.Citation33 Tyrosine residues Tyr 1234 and Tyr 1235 in the tyrosine kinase domain regulate the kinase activity of c-Met, and tyrosine residues Tyr 1349 and Tyr 1356 in the C-terminal regulatory tail (the multisubstrate docking site) are important for recruitment of downstream adapters, including growth factor receptor-bound protein 2 (GRB2) protein and GRB2-associated binding protein 1 (GAB1) ().Citation34,Citation35 HGF/c-Met signaling through these downstream effectors stimulates diverse cellular processes such as cell proliferation, differentiation, migration, invasion, and survival ().Citation19–Citation21
Mice lacking either HGF or c-Met exhibit embryonic lethality due to incomplete liver development.Citation36,Citation37 In addition, loss of HGF signaling in mice decreases proliferation of gastric mucosal cells and delays recovery from mucosal injury.Citation38 Depletion of c-Met in cancer cells results in the inhibition of cell proliferation, invasion, and survival, suggesting an essential role of c-Met in the development, progression, and invasion of cancers.Citation39–Citation42
Role of HGF and c-Met in cancer
In addition to the developmental roles described above, the HGF/c-Met signaling pathway is highly activated in human cancers via overexpression, amplification, or mutation, and promotes development, progression, invasive growth, and metastasis of cancers.Citation43 Abnormal expression of HGF and c-Met has been reported in various solid tumors, including breast, colon, lung, ovary, kidney, and liver cancers ().Citation44,Citation45 HGF secretion from tumor stromal cells has been correlated with overexpression of c-MET. Overexpression of HGF/c-Met has been reported in breast, colon, lung, ovarian, and renal cancer.Citation44,Citation45 HGF/c-Met over-expression has also been correlated with metastasis and poor survival in hepatocellular carcinoma, renal cell carcinoma, and breast cancer.Citation46–Citation50
Table 1 Expression and mutation pattern of HGF/c-Met in human cancers
Both germline and somatic mutations in c-Met have been reported in various cancers. These mutations increase tumorigenic potential via constitutive activation of the c-Met receptor.Citation51 The mutations are found in the tyrosine kinase domain, juxtamembrane domain, and extracellular domain of c-Met. The activating mutations in the tyrosine kinase domain of c-Met induce different downstream cascades and biological processes. D1228H/N and M1250T mutations increase c-Met phosphorylation and Ras activation, while L1195V and Y1230C mutations activate phosphatidylinositol-4, 5-bisphosphate 3-kinase to promote invasive and anchorage-independent growth.Citation52
Amplification of c-Met receptor and overexpression of HGF are associated with resistance to inhibitors targeting other RTKs. Amplification of c-Met activates ERBB3 (HER3)-dependent phosphatidylinositol-4,5-bisphosphate 3-kinase signaling, leading to resistance to gefitinib (a small-molecule inhibitor of EGFR) in lung cancer.Citation53 In addition, HGF is involved in decreased susceptibility to irreversible EGFR tyrosine kinase inhibition in lung cancer with the EGFR T790M mutation.Citation54 Similarly, amplification of c-Met is involved in resistance to the anti-EGFR monoclonal antibodies, cetuximab and panitumumab, in metastatic colorectal cancer.Citation55 Recent studies have demonstrated that the copy number of HGF and MET genes correlates with sensitivity to treatment with trastuzumab in HER2-positive metastatic breast cancer. An increased copy number for the MET gene has been linked to a higher failure rate of trastuzumab treatment and to a shorter time to progression, which means the length of time from the date of diagnosis or the start of treatment for a breast cancer patient until the breast cancer starts to get worse or spreads to other parts of the body. Increased copy number for the HGF gene is also linked to a higher failure rate of trastuzumab treatment.Citation56 These studies on mutation and amplification of HGF and MET genes provide important information for the development of therapeutic agents targeting the HGF/c-Met signaling pathway.Citation57,Citation58 These results indicate that the c-Met receptor, together with other RTK signaling pathways such as ERBB3 (HER3), EGFR, and ERBB2 (HER2), has a synergic role in tumor progression in certain types of cancers. Therefore, combination therapy targeting both c-Met and other RTKs may be more effective for cancer treatment compared with monotherapy.
Antibody-based therapeutics targeting HGF and c-Met
Inhibitors of the HGF/c-Met signaling pathway are divided into two groups: while small-molecule compounds block the signaling pathway by inhibiting tyrosine kinase activity and autophosphorylation of c-Met, biologics including truncated HGF, N-terminal Sema domain of HGF, soluble extracellular domain of c-Met (decoy Met), and antibodies against HGF and c-Met suppress the signaling pathway by inhibiting interactions between HGF and c-Met. Compared with small-molecule compounds that often target multiple RTKs, biologics more specifically inhibit the HGF/c-Met signaling pathway. Multiple therapeutic antibodies targeting the HGF/c-Met signaling pathway are currently in preclinical and clinical development ().
Table 2 Monoclonal antibody therapeutics targeting HGF or c-Met under development
Anti-HGF monoclonal antibodies
Rilotumumab (AMG102, Amgen) is a human monoclonal antibody against HGF that blocks interactions between HGF and its receptor c-Met, thereby inhibiting cellular processes driven by the HGF/c-Met signaling pathway.Citation59 In a Phase II clinical trial in gastric and esophagogastric junction cancers, rilotumumab in combination with epirubicin, cisplatin, and capecitabine (ECX) has been shown to improve both progression-free survival and overall survival in patients with tumors expressing high levels of c-Met.Citation60,Citation61 In this study, patients were given placebo or rilotumumab at 15 mg/kg or 7.5 mg/kg on day 1 in addition to ECX (50 mg/m2 epirubicin and 60 mg/m2 cisplatin on day 1, and 625 mg/m2 capecitabine twice a day on days 1–21) every 3 weeks. Median progression-free survival was 5.1 months for patients treated with rilotumumab 15 mg/kg and 6.8 months for patients treated with rilotumumab 7.5 mg/kg, compared with 4.2 months for the placebo group. Objective response rates reported for patients treated with rilotumumab 15 mg/kg, rilotumumab 7.5 mg/kg and placebo were 31%, 48%, and 21%, respectively. Median overall survival times for patients treated with rilotumumab 15 mg/kg, rilotumumab 7.5 mg/kg, and placebo were 9.7, 11.1, and 8.9 months, respectively. Adverse events, including hematologic adverse events, peripheral edema, and venous thromboembolism, were reported for both the placebo and rilotumumab groups, but were more common in the rilotumumab group. Based on the safety profile and results indicating better efficacy of rilotumumab in combination with ECX, a Phase III study is ongoing in c-Met-positive gastric and gastroesophageal junction cancers (RILOMET-1).Citation61 The efficacy of rilotumumab has also been demonstrated in metastatic colorectal cancer with wild-type KRAS. In a Phase II clinical trial of rilotumumab in combination with panitumumab (a fully human anti-EGFR monoclonal antibody), the median progression-free survival was 5.2 months for the combination treatment and 3.7 months for treatment with panitumumab alone.Citation62
Ficlatuzumab (AV-299; SCH 900105, AVEO Pharmaceuticals, Cambridge, MA, USA) is a humanized monoclonal antibody against HGF that inhibits the HGF-induced c-Met signaling pathway by neutralizing HGF/c-Met binding.Citation63 Preclinical studies carried out in the H596 NSCLC xenograft model have demonstrated increased anti-cancer activity of ficlatuzumab in combination with an EGFR inhibitor (erlotinib or cetuximab) compared with single agents.Citation64 In a Phase I study of advanced solid tumors including sarcoma, ovarian cancer, mesothelioma, and glioblastoma multiforme, ficlatuzumab was intravenously administered at 2, 5, 10, or 20 mg/kg once every 2 weeks. At the recommended Phase II dose, ficlatuzumab was administered in combination with erlotinib (150 mg/day). Adverse events including fatigue, peripheral edema, headache, hematologic problems, and pruritus have been reported in the monotherapy group, and common adverse events of rash and diarrhea have been reported in the combination therapy group. From the Phase I study, it has been demonstrated that the selected dose of ficlatuzumab is safe and well tolerated when used in combination with erlotinib at the standard dose.Citation65,Citation66 This was followed by a randomized Phase II study with gefitinib alone or in combination with ficlatuzumab to treat NSCLC patients.Citation67
TAK-701 (Galaxy Biotech) is a humanized monoclonal antibody that binds to HGF with high affinity.Citation68 Combination treatment with TAK-701 and gefitinib (a small-molecule inhibitor of EGFR) inhibits the phosphorylation of both c-Met and EGFR and downstream signaling cascades in HCC827-HGF tumor cells (engineered human NSCLC cells that contain an activating EGFR mutation and stably express HGF).Citation68 Combination treatment with TAK-701 and gefitinib also markedly inhibits tumor growth in HCC827-HGF xenograft models.Citation68 These results suggest that combination treatment with TAK-701 and gefitinib may provide a means of overcoming resistance to EGFR-tyrosine kinase inhibitor therapy in HGF-induced NSCLC. Phase I studies are ongoing for TAK-701 as a single-agent treatment for advanced solid tumors.
Anti-c-Met monoclonal antibodies
Onartuzumab (MetMAb, Genentech) is a humanized, monovalent monoclonal antibody against c-Met. Onartuzumab was developed with the knob-into-hole technology, which allows one-to-one interaction between the antibody and the receptor.Citation69 Onartuzumab potently inhibits binding of HGF, phosphorylation of c-Met, and downstream signaling in the HGF/c-Met pathway with antibody-like pharmacokinetics. Strong anticancer activity has been reported for onartuzumab in preclinical xenograft studies.Citation69 Activated HGF/c-Met signaling has been associated with a poor prognosis and with resistance to EGFR inhibitors in NSCLC. In a Phase II study, prolonged progression-free survival (2.9 months versus 1.5 months) and overall survival (12.6 months versus 3.8 months) have been reported in c-Met-positive patients treated with erlotinib and onartuzumab compared with patients treated with erlotinib alone.Citation70 However, in a randomized Phase III trial, combination therapy with onartuzumab and erlotinib failed to confirm the efficacy demonstrated in the Phase II study, as no improvement in overall survival (6.8 months versus 9.1 months) or progression-free survival (2.7 months versus 2.6 months) was observed in c-Met-positive patients.Citation71 Despite the failure of the Phase III trial, Genentech has continued to develop onartuzumab in two additional Phase III trials in different subgroups of NSCLC, ie, c-Met-positive stage IIIB or IV NSCLC with activating EGFR mutation. Subgroup analyses may provide a means of targeting patients more selectively.Citation72 Another Phase III clinical trial is ongoing in gastric cancer for evaluation of the efficacy and safety of onartuzumab. In this study, onartuzumab is administered in combination with 5-fluorouracil, folinic acid, and oxaliplatin (mFOLFOX6) to treat metastatic HER2-negative and c-Met-positive gastroesophageal cancer.Citation72
Emibetuzumab (LY-2875358, Eli Lilly) is a humanized, bivalent anti-c-Met antibody that inhibits both ligand-dependent and ligand-independent activation of c-Met.Citation73 In the case of HGF-dependent c-Met activation, emibetuzumab inhibits HGF binding to c-Met, c-Met phosphorylation, and tumor growth both in vitro and in vivo, similar to a humanized, one-armed 5D5 anti-c-Met antibody (precursor of onartuzumab). In the case of HGF-independent c-Met activation by MET gene amplification in tumors, emibetuzumab promotes internalization and degradation of c-Met. Decreases in phosphorylated and total c-Met after treatment with emibetuzumab induces inhibition of cell proliferation and tumor growth in the gastric cancer cell lines, MKN-45 and SNU-5, and in the NSCLC cell lines, EBC-1 and H1993. However, the one-armed 5D5 antibody has exhibited no anti-tumor activity in the case of HGF-independent c-Met activation.Citation73 In a Phase I study, treatment with emibetuzumab alone or in combination with erlotinib resulted in a durable partial response in NSCLC and was also shown to be safe and well tolerated. Based on the pharmacokinetic/pharmacodynamic data, the recommended Phase II dose of emibetuzumab for intravenous administration is 750 mg once every 2 weeks as a single agent or in combination with erlotinib.Citation74
ARGX-111 (arGEN-X) is a defucosylated antagonistic anti-c-Met antibody with potent anti-cancer activity based on enhanced antibody-dependent cellular cytotoxicity. A Phase Ib study was initiated in January 2014 to evaluate ARGX-111 in advanced cancers with c-Met overexpression.Citation75 EM1-mAb (Genmab™, Janssen Research and Development, San Diego, CA, USA) is a bispecific anti-EGFR/c-Met antibody that inhibits both EGFR and c-Met signaling pathways. EM1-mAb has exhibited more potent inhibition of downstream signaling cascades compared with the combination of monospecific antibodies.Citation76
Critical analysis for potential of antibody-based HGF/c-Met inhibitors in human cancer
Several antibody-based inhibitors of the HGF/c-Met signaling pathway are under active preclinical/clinical development as novel therapeutic agents to treat cancers. There are important aspects of HGF/c-Met biology that need to be carefully addressed for successful development of these therapeutic antibodies targeting the HGF/c-Met signaling pathway. These include unwanted activation of c-Met by bivalent anti-c-Met antibodies and the recent failure of the Phase III study of onartuzumab in combination with erlotinib in NSCLC.
A monovalent antibody, onartuzumab (MetMAb™), was designed to address issues reported for several bivalent anti-c-Met reagents that induce unwanted stimulation of the c-Met signaling by mimicking c-Met dimerization.Citation69,Citation77 Onartuzumab, the one-armed humanized antibody against c-Met, blocks the interaction between the HGF-α chain and the Sema domain (homologous to semaphorins) of the c-Met receptor, demonstrated by crystallographic, structural, and biochemical analysis.Citation69 However, despite concerns regarding c-Met activation by bivalent anti-c-Met antibodies and the development of the one-armed antibody, onartuzumab, clinical trials are still ongoing for two bivalent antibodies against c-Met (emibetuzumab and ARGX-111).Citation74,Citation75 More important aspects to consider for the development of HGF/c-Met inhibitors include patient stratification and identification of effective combinations of inhibitors targeting the HGF/c-Met pathway along with other interacting signaling pathways such as the EGFR pathway. Since alterations in HGF/c-Met signaling are closely related to tumorigenesis, tumor progression, and metastasis, it is critical to more accurately detect c-Met expression levels and alterations. In the Phase II study of onartuzumab in NSCLC, it has been demonstrated that the intensity of c-Met labeling detected via immunohistochemistry can successfully identify target patient groups for treatment.Citation70 Immunohistochemistry-based detection of c-Met expression levels was also important for patient stratification in the Phase I and II trials of rilotumumab.Citation61,Citation62 However, the same strategy has failed to meet the initial goal in the Phase III study of onartuzumab in combination with erlotinib in NSCLC, possibly resulting from the recruitment of nonspecific patient groups rather than from ineffectiveness of the antibodies.Citation71,Citation72
In addition to immunohistochemistry, different detection methods and other specific targets have also been examined. Elevated c-Met expression levels have been detected via Western blotting of serum proteins from patients with hepatocellular carcinoma and tissues from patients with colorectal cancer and metastasis to the liver.Citation78,Citation79 Phosphorylated c-Met (the active form of c-Met) has been intensively examined in preclinical and clinical studies and via in silico modeling. Antibodies that specifically recognize phosphorylated c-Met can be used to detect this pharmacodynamic biomarker.Citation80–Citation82 In addition to the development of better detection methods and detecting antibodies, well defined scoring systems and minimization of interobserver differences are all required for effective patient diagnosis and stratification.
Although no significant correlation between a high MET gene copy number and patient response to onartuzumab has been reported from clinical trials, results from both preclinical and clinical studies with small-molecule inhibitors have demonstrated a correlation between a high copy number or specific mutation of MET gene and increased clinical response.Citation83–Citation85 Due to the association between MET gene amplification/mutation and poor clinical outcomes, various technologies including next-generation sequencing are under development.Citation86
As reported from studies in NSCLC, inhibitors of the HGF/c-Met pathway may become more potent when combined with antagonists of other signaling pathways. Most clinical trials with anti-c-Met monoclonal antibodies were designed in combination with chemical inhibitors of other signaling pathways. Clinical trials of onartuzumab were conducted in combination with erlotinib, an EGFR inhibitor, to overcome EGFR-mediated resistance.Citation70,Citation71 Synergistic effects of dual inhibition of the HGF/c-Met and VEGF signaling pathways have been reported in preclinical studies, suggesting that combination of HGF/c-Met and VEGF/VEGFR inhibition may allow more effective treatment of human cancers.Citation87–Citation89 The efficacy of onartuzumab and rilotumumab are being evaluated in combination with bevacizumab or panitumumab in the clinical settings of glioblastoma multiforme and metastatic colorectal cancer.Citation86 Successful clinical development of candidates requires a better understanding of the interactions between signaling pathways. The use of two or more therapeutic agents in combination should be carefully designed because this combination treatment may elevate the risk of adverse effects and increase the treatment cost.
Results from clinical studies reflect the highly complex nature of HGF/c-Met signaling in human cancers and suggest that successful development of antibody-based therapeutics targeting the HGF/c-Met signaling pathway requires a better understanding of the pathways involved in the diseases, along with more careful analyses of the clinical data.
Conclusion
Studies carried out over the past two decades have demonstrated that the HGF/c-Met signaling pathway is a promising therapeutic target for treating cancers. Therapeutic agents designed to target HGF/c-Met-mediated signaling cascades have been developed and evaluated in preclinical and clinical studies. In clinical trials in lung, gastric, prostate and renal cancers, inhibitors of the HGF/c-Met signaling pathway have yielded promising results. Like inhibitors of other RTK signaling pathways, inhibitors of the HGF/c-Met signaling pathway are divided into two groups, ie, small-molecule compounds suppressing the tyrosine kinase activity of c-Met and biologics such as monoclonal antibodies that specifically bind to HGF or c-Met.
Among the therapeutic agents targeting HGF or c-Met, a small-molecule inhibitor, cabozantinib (XL184, Exelixis, South San Francisco, CA, USA) became the first agent approved by the FDA for the clinical treatment of progressive metastatic medullary thyroid cancer (in November 2012). In addition, several small-molecule inhibitors of c-Met, including tivantinib (ARQ 197, ArQule, Woburn, MA, USA), golvatinib (E7050, Eisai, Tokyo, Japan), and foretinib (GSK 1363089, GlaxoSmithKline, Brentford, UK) are currently being evaluated in clinical studies for treatment of hepatocellular carcinoma.Citation90
In contrast with the success of cabozantinib, an antibody-based inhibitor of c-Met, onartuzumab failed to improve the clinical benefit in a recent Phase III study in NSCLC. However, the failure in this Phase III trial suggests several key issues to consider for successful clinical development of antibody-based HGF or c-Met inhibitors. Challenges to face for effective clinical application of HGF/c-Met-targeted therapeutics include advances in analytical methods to identify specific patient groups, more careful selection of target populations, development of biomarkers for HGF/c-Met signaling, and identification of effective combination therapies with other RTK inhibitors.
Acknowledgments
The authors thank Sang Hoon Lee and Sang K Park at the Hanwha Chemical Corporation and William B Stallcup at the Sanford-Burnham Medical Research Institute for their valuable advice and discussion during preparation for this review.
Disclosure
The authors report no conflicts of interest in this review.
References
- LemmonMASchlessingerJCell signaling by receptor tyrosine kinasesCell201014171117113420602996
- GschwindAFischerOMUllrichAThe discovery of receptor tyrosine kinases: targets for cancer therapyNat Rev Cancer20044536137015122207
- XuAMHuangPHReceptor tyrosine kinase coactivation networks in cancerCancer Res201070103857386020406984
- FauvelBYasriAAntibodies directed against receptor tyrosine kinases: current and future strategies to fight cancerMAbs20146483885124859229
- DodéCLevilliersJDupontJMLoss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndromeNat Genet200333446346512627230
- FeldheimDANakamotoMOsterfieldMLoss-of-function analysis of EphA receptors in retinotectal mappingJ Neurosci200424102542255015014130
- Kong-BeltranMSeshagiriSZhaJSomatic mutations lead to an oncogenic deletion of met in lung cancerCancer Res200666128328916397241
- ThorAHER2-a discussion of testing approaches in the USAAnn Oncol200112Suppl 1S101S10711521714
- CohenMHGootenbergJKeeganPPazdurRFDA drug approval summary: bevacizumab plus FOLFOX4 as second-line treatment of colorectal cancerOncologist200712335636117405901
- SummersJCohenMHKeeganPPazdurRFDA drug approval summary: bevacizumab plus interferon for advanced renal cell carcinomaOncologist201015110411120061402
- CohenMHShenYLKeeganPPazdurRFDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiformeOncologist200914111131113819897538
- CohenMHGootenbergJKeeganPPazdurRFDA drug approval summary: bevacizumab (Avastin) plus carboplatin and paclitaxel as first-line treatment of advanced/metastatic recurrent nonsquamous non-small cell lung cancerOncologist200712671371817602060
- GarrettCREngCCetuximab in the treatment of patients with colorectal cancerExpert Opin Biol Ther201111793794921557708
- GiustiRMShastriKACohenMHKeeganPPazdurRFDA drug approval summary: panitumumab (Vectibix)Oncologist200712557758317522246
- PooleRMVaidyaARamucirumab: first global approvalDrugs20147491047105824916147
- CooperCSParkMBlairDGMolecular cloning of a new transforming gene from a chemically transformed human cell lineNature1984311598129336590967
- GherardiEStokerMHepatocytes and scatter factorNature199034662812282142751
- NaldiniLVignaENarsimhanRPHepatocyte growth factor (HGF) stimulates the tyrosine kinase activity of the receptor encoded by the proto-oncogene c-METOncogene1991645015041827664
- YouWKMcDonaldDMThe hepatocyte growth factor/c-Met signaling pathway as a therapeutic target to inhibit angiogenesisBMB Rep2008411283383919123972
- CecchiFRabeDCBottaroDPTargeting the HGF/Met signaling pathway in cancer therapyExpert Opin Ther Targets201216655357222530990
- BlumenscheinGRJrMillsGBGonzalez-AnguloAMTargeting the hepatocyte growth factor-cMET axis in cancer therapyJ Clin Oncol201230263287329622869872
- Danilkovitch-MiagkovaAZbarBDysregulation of Met receptor tyrosine kinase activity in invasive tumorsJ Clin Invest2002109786386711927612
- BirchmeierCBirchmeierWGherardiEVande WoudeGFMet, metastasis, motility and moreNat Rev Mol Cell Biol200341291592514685170
- ScagliottiGVNovelloSvon PawelJVThe emerging role of MET/HGF inhibitors in oncologyCancer Treat Rev201339779380123453860
- BiswasPRoyAGongRHepatocyte growth factor induces an endothelin-mediated decline in glomerular filtration rateAm J Physiol Renal Physiol20052881F8F1515583218
- UekiTKanedaYTsutsuiHHepatocyte growth factor gene therapy of liver cirrhosis in ratsNat Med1999522262309930873
- KusumotoKIdoAMoriuchiARepeated intravenous injection of recombinant human hepatocyte growth factor ameliorates liver cirrhosis but causes albuminuria in ratsInt J Mol Med200617350350916465399
- NakamuraYMorishitaRNakamuraSA vascular modulator, hepatocyte growth factor, is associated with systolic pressureHypertension19962834094138794825
- MizunoSMatsumotoKLiMYNakamuraTHGF reduces advancing lung fibrosis in mice: a potential role for MMP-dependent myofibroblast apoptosisFASEB J200519658058215665032
- NakamuraTNishizawaTHagiyaMMolecular cloning and expression of human hepatocyte growth factorNature198934262484404432531289
- GohdaETsubouchiHNakayamaHPurification and partial characterization of hepatocyte growth factor from plasma of a patient with fulminant hepatic failureJ Clin Invest19888124144193276728
- RodriguesGANaujokasMAParkMAlternative splicing generates isoforms of the met receptor tyrosine kinase which undergo differential processingMol Cell Biol1991116296229701710022
- BardelliAPonzettoCComoglioPMIdentification of functional domains in the hepatocyte growth factor and its receptor by molecular engineeringJ Biotechnol19943721091227765452
- LongatiPBardelliAPonzettoCNaldiniLComoglioPMTyrosines 1234–1235 are critical for activation of the tyrosine kinase encoded by the MET proto-oncogene (HGF receptor)Oncogene19949149578302603
- PonzettoCBardelliAZhenZA multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor familyCell19947722612717513258
- UeharaYMinowaOMoriCPlacental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factorNature199537365157027057854453
- HuhCGFactorVMSánchezAUchidaKConnerEAThorgeirssonSSHepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repairProc Natl Acad Sci U S A2004101134477448215070743
- NakahiraRMizunoSYoshimineTNakamuraTThe loss of local HGF, an endogenous gastrotrophic factor, leads to mucosal injuries in the stomach of miceBiochem Biophys Res Commun2006341489790316476577
- ZhangSZPanFYXuJFKnockdown of c-Met by adenovirus-delivered small interfering RNA inhibits hepatocellular carcinoma growth in vitro and in vivoMol Cancer Ther20054101577158416227408
- QueWChenJKnockdown of c-Met inhibits cell proliferation and invasion and increases chemosensitivity to doxorubicin in human multiple myeloma U266 cells in vitroMol Med Rep20114234334921468575
- SunBLiuRXiaoZDZhuXc-MET protects breast cancer cells from apoptosis induced by sodium butyratePLoS One201271e3014322253909
- QueWChenJChuangMJiangDKnockdown of c-Met enhances sensitivity to bortezomib in human multiple myeloma U266 cells via inhibiting Akt/mTOR activityAPMIS2012120319520322339676
- GherardiEBirchmeierWBirchmeierCVan de WoudeGTargeting MET in cancer: rationale and progressNat Rev Cancer20121228910322270953
- SmythECSclafaniFCunninghamDEmerging molecular targets in oncology: clinical potential MET/hepatocyte growth-factor inhibitorsOncoTargets Ther2014710011014
- MaPCTretiakovaMSMacKinnonACExpression and mutational analysis of MET in human solid cancersGenes Chromosomes Cancer200847121025103718709663
- MaulikGShrikhandeAKijimaTMaPCMorrisonPTSalgiaRRole of the hepatocyte growth factor receptor, c-Met, in oncogenesis and potential for therapeutic inhibitionCytokine Growth Factor Rev2002131415911750879
- UekiTFujimotoJSuzukiTYamamotoHOkamotoEExpression of hepatocyte growth factor and its receptor c-met proto-oncogene in hepatocellular carcinomaHepatology19972548628669096589
- DaveauMScotteMFrancoisAHepatocyte growth factor, transforming growth factor alpha, and their receptors as combined markers of prognosis in hepatocellular carcinomaMol Carcinog200336313014112619035
- SchutzFAPomerantzMMGrayKPSingle nucleotide polymorphisms and risk of recurrence of renal-cell carcinoma: a cohort studyLancet Oncol2013141818723219378
- LengyelEPrechtelDResauJHC-Met overexpression in node-positive breast cancer identifies patients with poor clinical outcome independent of Her2/neuInt J Cancer2005113467868215455388
- GraveelCRTolbertDVan de WoudeGFMET: a critical player in tumorigenesis and therapeutic targetCold Spring Harb Perspect Biol201357a00920923818496
- GiordanoSMaffeAWilliamsTADifferent point mutations in the met oncogene elicit distinct biological propertiesFASEB J200014239940610657996
- EngelmanJAZejnullahuKMitsudomiTMet amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signalingScience200731658271039104317463250
- YamadaTMatsumotoKWangWHepatocyte growth factor reduces susceptibility to an irreversible epidermal growth factor receptor inhibitor in EGFR-T790M mutant lung cancerClin Cancer Res201016117418320008840
- BardelliACorsoSBertottiAmplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancerCancer Discov20133665867323729478
- MinutiGCappuzzoFDuchnowskaRIncreased MET and HGF gene copy numbers are associated with trastuzumab failure in HER2-positive metastatic breast cancerBr J Cancer2012107579379922850551
- WangWLiQTakeuchiSMet kinase inhibitor E7050 reverses three different mechanisms of hepatocyte growth factor-induced tyrosine kinase inhibitor resistance in EGFR mutant lung cancerClin Cancer Res20121861663167122317763
- NakagawaTTakeuchiSYamadaTCombined therapy with mutant-selective EGFR inhibitor and Met inhibitor for overcoming erotinib resistance in EGFR-mutant lung cancerMol Cancer Ther201211102149215722844075
- GordonMSSweeneyCSMendelsonDSSafety, pharmacokinetics, and pharmacodynamics of AMG-102, a fully human hepatocyte growth factor-neutralizing monoclonal antibody, in a first-in-human study of patients with advanced solid tumorsClin Cancer Res201016269971020068101
- MinZSameerDGisleskogPOPopulation pharmacokinetics of rilotumumab, a fully human monoclonal antibody against hepatocyte growth factor, in cancer patientJ Pharm Sci2014103132833624186235
- IvesonTDonehowerRCDavidenkoIRilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: an open-label, dose de-escalation phase 1b study and a double blind, randomized phase 2 studyLancet Oncol20141591007101824965569
- Van CutsemEEngCNowaraERandomized phase Ib/II trials of rilotumumab or ganitumab with panitumumab versus panitumumab alone in patients with wild-type KRAS metastatic colorectal cancerClin Cancer Res201420164240425024919569
- D’ArcangeloMCappuzzoFFocus on the potential role of ficlatuzumab in the treatment of non-small cell lung cancerBiologics20137616823493885
- MeetzeKABoudrowAConnolyKAnti-tumor activity of SCH 900105 (AV299), an anti-HGF antibody, in non-small cell lung cancer modelsMol Cancer Ther20098Suppl 12 Abstr C173
- PatnaikAWeissGJPapadopoudosKPhase I study of SCH 900105 (SC), an anti-hepatocyte growth factor (HGF) monoclonal antibody (MAb), as a single agent and in combination with erlotinib (E) in patients (pts) with advanced solid tumorsJ Clin Oncol201028Suppl Abstr 2525
- PatnaikAWeissGJPapadopoulosKPPhase I ficlatuzumab monotherapy or with erlotinib for refractory advanced solid tumours and multiple myelomaBr J Cancer2014111227228024901237
- MokTSKParkKGeaterSLA randomized phase (ph) 2 study with exploratory biomarker analysis of ficlatuzumab (F) a humanized hepatocyte growth factor (HGF) inhibitory monoclonal antibody (MAb) in combination with gefitinib (G) versus gefitinib alone in Asian patients with lung adenocarcinoma (LA)Presented at the European Society for Medical Oncology annual meetingSeptember 28 to October 2, 2012Vienna, Austria
- OkamotoWOkamotoITanakaKTAK-701, a humanized monoclonal antibody to hepatocyte growth factor, reverses gefitinib resistance induced by tumor-derived HGF in non-small cell lung cancer with an EGFR mutationMol Cancer Ther20109102785279220716641
- MerchantMMaXMaunHRMonovalent antibody design and mechanism of action of onartuzumab, a MET antagonist with anti-tumor activity as a therapeutic agentProc Natl Acad Sci U S A201311032E2987E299623882082
- SpigelDRErvinTJRamlauRARandomized phase II trial of onartuzumab in combination with erlotinib in patients with advanced non-small cell lung cancerJ Clin Oncol201331324105411424101053
- SpigelDREdelmanMJO’ByrneKOnartuzumab plus erlotinib versus erlotinib in previously treated stage IIIb and IV NSCLC: Results from the pivotal phase III randomized, multicenter, placebo-controlled METLung (OAM4971g) global trialJ Clin Oncol2014325 SupplAbstr 8000
- CormacSGenentech to salvage anti-MET antibody with subgroup analysisNat Biotechnol201432539940024811494
- ZengWPeekVWortingerMLY2875358, a bivalent MET antibody with anti-tumor activity through blocking HGF as well as inducing degradation of MET, differentiates from a one-armed 5D5 MET antibodyCancer Res201373Suppl 85465
- GoldmanJWRosenLSAlgaziAPFirst-in-human dose escalation study of LY2875358 (LY), a bivalent MET antibody, as monotherapy and in combination with erlotinib (E) in patients with advanced cancerJ Clin Oncol201331Suppl 15 Abstr 8093
- HultbergAHuygheLde JongeNARGX-111, a defucosylated antagonistic anti-MET antibody, displays potent anti-tumor activity through enhanced ADCCPresented at the 12th International Congress on Targeted Anticancer TherapiesMarch 5–7, 2014Washington, DC, USA
- MooresSChiuMBusheyBBispecific antibody targeting EGFR and cMet demonstrates superior activity compared to the combination of single pathway inhibitorsMol Cancer Ther20131211 SupplB241
- MartensTSchmidtNOEckerichCA novel one-armed anti-c-Met antibody inhibits glioblastoma growth in vivoClin Cancer Res20061220 Pt 16144615217062691
- OsadaSKanematsuMImaiHClinical significance of serum HGF and c-Met expression in tumor tissue for evaluation of properties and treatment of hepatocellular carcinomaHepatogastroenterology20085582–8354454918613405
- OsadaSMatsuiSKomoriSGoshimaSEffect of hepatocyte growth factor on progression of liver metastasis in colorectal cancerHepatogastroenterology20105797768020422876
- SrivastavaAKHollingsheadMGWeinerJDevelopment and validation of biomarker assays to assess pharmacodynamic modulation of METPresented at the American Society of Clinical Oncology Annual MeetingJune 3–7, 2011Chicago, IL, USA
- YamazakiSViciniPShenZPharmacokinetic/pharmacodynamic modeling of crizotinib for anaplastic lymphoma kinase inhibition and antitumor efficacy in human tumor xenograft mouse modelsJ Pharmacol Exp Ther2010340354955722129595
- ArriolaECañadasIArumí-UríaMMET phosphorylation predicts poor outcome in small cell lung carcinoma and its inhibition blocks HGF-induced effects in MET mutant cell linesBr J Cancer2011105681482321847116
- BellonSFKaplan-LefkoPYangYc-Met inhibitors with novel binding mode show activity against several hereditary papillary renal cell carcinoma-related mutationsJ Biol Chem200828352675268318055465
- BerthouSAebersoldDMSchmidtLSThe Met kinase inhibitor SU11274 exhibits a selective inhibition pattern toward different receptor mutated variantsOncogene200423315387539315064724
- SequistLVvon PawelJGarmeyEGRandomized phase II study of erlotinib plus tivantinib versus erlotinib plus placebo in previously treated non-small-cell lung cancerJ Clin Oncol201129243307331521768463
- MarounCRRowlandsTThe Met receptor tyrosine kinase: a key player in oncogenesis and drug resistancePharmacol Ther2014142331633824384534
- SulpiceEDingSMuscatelli-GrouxBCross-talk between the VEGF-A and HGF signaling pathways in endothelial cellsBiol Cell2009101952553919281453
- YouWKSenninoBWilliamsonCWVEGF and c-Met blockade amplify angiogenesis inhibition in pancreatic islet cancerCancer Res201171144758476821613405
- RoySNarangBKRastogiSKRawalRKA novel multiple tyrosine-kinase targeted agent to explore the future perspectives of anti-angiogenic therapy for the treatment of multiple solid tumors: cabozantinibAnticancer Agents Med Chem2014151374725181996
- GoyalLMuzumdarMDZhuAXTargeting the HGF/c-MET pathway in hepatocellular carcinomaClin Cancer Res20131992310231823388504
