Abstract
Cancer-induced immunosuppression significantly impacts tumors, rendering them the ability to acquire aggressive and treatment-resistant phenotypes. The recent clinical success of drugs targeting the immunosuppressive machinery of tumors highlights the importance of identifying novel drugs that effectively augment antitumor immunity and elicit clinical remission in advanced tumors. T cell immunoglobulin domain and mucin domain-3 (TIM-3) is a critical immunoregulatory molecule that links pattern recognition-mediated innate sensing with antigen-specific immune responses. Recent evidence has elucidated the potential utility of drugs targeting TIM-3 in inducing antitumor responses, particularly in synergy with conventional anticancer regimens. Herein, we provide a comprehensive overview, as well as future perspectives, regarding the role of TIM-3 as an emerging target that may improve clinical responses for cancer patients.
Introduction
Cancer immunoevasion
Emerging evidence has unveiled the indispensable role of the host immune system in defending itself from arising tumors. The host immune system acts to detect and eliminate tumors in three ways: 1) immune cells protect the host against viral infection and suppress virus-induced tumors, 2) innate immune cells eliminate cancer cells through activation of pattern recognition-mediated sensing systems, and 3) antigen-specific T cells recognize tumor rejection antigens on cancer cells. These immune system processes, which suppress tumor initiation and progression, are collectively termed “immunosurveillance.”
However, rare cancer cells escape immunosurveillance and emerge as progressively growing tumors. This ability to escape recognition by the host immune system arises via various mechanisms. For example, genetic and epigenetic alterations can render tumor cells capable of reducing immune recognition (for example, by a loss of antigens). Alternatively, cancer cells may create immunosuppressive states by producing immunoregulatory cytokines such as vesicular endothelial growth factor (VEGF), transforming growth factor-beta, galectin-1, indoleamine 2,3-dioxygenase or by recruiting regulatory immune cells (forkhead box P3 [Foxp3]+ regulatory T cells and myeloid-derived suppressor cells) that function as effectors of immunosuppression.Citation1–Citation8 In addition, host immune systems can also promote tumor progression by the selection of cancer cells that thrive, thus supporting tumor progression and anticancer drug resistance in privileged tumor microenvironments (TMEs). As a result, cancer cells are able to manipulate host immune systems to further enhance tumorigenicity.Citation9,Citation10
Cancer-induced immunoevasion is a major player in the suppression of the antitumor efficacy of immunotherapy.Citation11,Citation12 Moreover, cancer-mediated immunomodulation has a profound impact on many antitumor therapies including chemotherapy and molecular targeting strategies.Citation13,Citation14 Therefore, a deeper understanding of the molecular machineries by which cancer-induced immunomodulation influences the therapeutic responses to anticancer regimens is necessary in order to devise new strategies to improve the clinical prognosis of cancer patients.
Recent evidence has revealed that T cell immunoglobulin (Ig) domain and mucin domain (TIM)-3 functions as a critical checkpoint, regulating numerous aspects of tumor immunomodulation.Citation15 This review provides an overview of the immunoregulatory functions of TIM-3 and perspectives regarding the potential of a TIM-3-targeted strategy as a new option in treating cancer patients.
TIM-3: brief overview of physiological functions
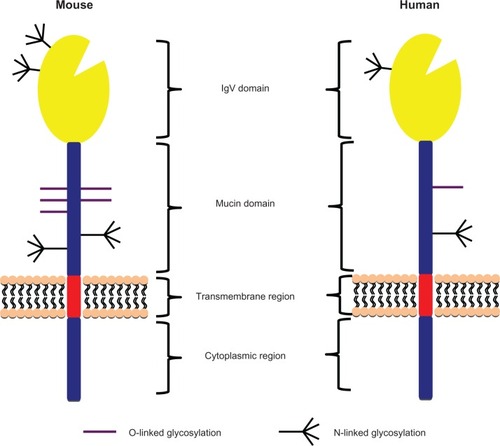
TIM-3 was identified as a molecule expressed on interferon (IFN) γ-producing CD4+ T-helper type 1 (Th1) and cluster of differentiation (CD)8+ T-cytotoxic type 1 (Tc1) cells.Citation15 TIM-3 belongs to the TIM family of molecules that, in mice, contains eight members. Only TIM-1, TIM-3, and TIM-4 are expressed in humans. TIM-3 consists of an N-terminal IgV domain followed by a mucin domain, a transmembrane domain, and a cytoplasmic tail (). Four noncanonical cysteines in the IgV domain are conserved in all TIM-family genes across mice and humans, forming a unique binding cleft not seen in the Ig domain of any other Ig superfamily member.Citation16,Citation17 Interestingly, the cleft structure surrounded by an α-loop in TIM-3 has been shown to be important for binding phosphatidylserine (PS), which mediates uptake of apoptotic cells by macrophages and dendritic cells.Citation18,Citation19
Figure 1 Schema of human and mouse T cell immunoglobulin domain and mucin domain-3 (TIM-3) protein structures. TIM-3 consists of an N-terminal immunoglobulin (Ig) V domain followed by a mucin domain, transmembrane region, and a cytoplasmic region. The IgV and mucin domains contain O- and N-linked glycosylation sites.
TIM-3 interacts with multiple ligands, including galectin-9 (Gal9) and cell-surface PS, leading to various biological consequences. The binding of TIM-3 to Gal9 or high mobility group protein B1 (HMGB1) generates an inhibitory signal that results in the apoptosis of Th1 cells.Citation20,Citation21 Continuous exposure to interleukin 12 induces TIM-3 expression on intra-tumor T cells, triggering functional impairment and exhaustion.Citation22 TIM-3 expressed on other immune cells such as natural killer cells and dendritic cells (DCs) is also involved in immunoregulatory functions. For example, TIM-3 regulates the differentiation and immunogenic activities of natural killer cells.Citation23,Citation24 TIM-3 expressed on DCs promotes the phagocytosis of apoptotic cells through interaction with PS, which enhances antigen presentation and triggers immune tolerance,Citation18,Citation19 whereas it negatively regulates pattern recognition-mediated innate immune systems.Citation25 Further, TIM-3 synergizes with Toll-like receptors to induce inflammation by activating the transcription factor nuclear factor kappa B and enhancing the secretion of pro-inflammatory mediators.Citation26
Together, these findings suggest that TIM-3 controls both the tolerogenic and immunostimulatory properties of various subsets of immune cells. These dual TIM-3 functions may be regulated differentially at various stages of tumorigenicity and/or by distinct TME subtypes ().
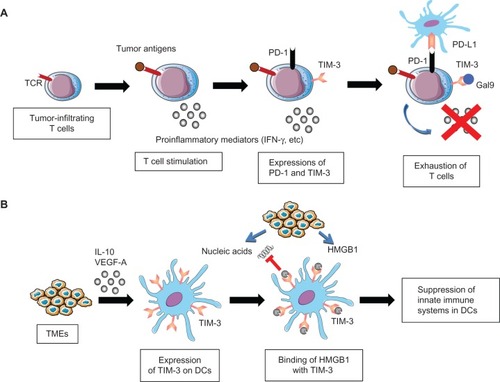
Figure 2 Role of T cell immunoglobulin domain and mucin domain-3 (TIM-3) in cancer immunosuppression.
Notes: (A) Exhaustion of T cells by TIM-3 and programmed cell death protein 1 (PD-1). Tumor-infiltrating T cells secrete interferon-γ (IFNγ) upon binding of tumor antigens by T cell receptors (TCR). However, TIM-3 and PD-1 are upregulated on tumor-infiltrating T cells upon chronic exposure to antigenic stimuli, and interact with galectin-9 (Gal9) and programmed cell death 1 ligand 1 (PD-L1) expressed on tumors or tumor-infiltrating stromal cells. These interactions impair the effector activities of tumor-infiltrating T cells, leading to cancer immunosuppression. (B) Immunosuppression of dendritic cells (DCs) by TIM-3. TIM-3 expression on DCs is induced via stimulation by interleukin (IL) 10 and vascular endothelial growth factor A (VEGF-A), which are mainly secreted from tumor microenvironments (TMEs). TIM-3 on DCs binds high mobility group box 1 (HMGB1) from inflammatory TMEs, and negatively regulates the HMGB1-mediated recruitment of TMR-derived nucleic acids, thereby suppressing the innate immune systems. As a result, TIM-3 on DCs enables tumors to acquire immunosuppressive capabilities.
Role of TIM-3 in cancer immunosuppression
Accumulating evidence has revealed that TIM-3 plays a critical role in negatively regulating T cell-dependent immunosurveillance. For example, the exhausted phenotype of tumor-infiltrating T cells is manifested by the co-expression of TIM-3 and programmed cell death protein 1 (PD-1), which is closely correlated with dampened tumor antigen-specific responses.Citation27,Citation28 In addition, TIM-3 expression may represent a distinct immunosuppressive feature of Foxp3+ regulatory T cells, although the relative importance of this population compared with other subsets requires clarification.Citation29 These findings collectively suggest that TIM-3 functions as a critical negative regulator of tumor-specific recognition by T cells.
Immune regulation by innate immune cells has emerged as an indispensable defense system against tumor growth and progression.Citation30 Consistent with the role of TIM-3 in regulating antitumor innate immune responses, together with colleagues, we recently showed that TIM-3 functions to suppress the nucleic acid-mediated innate immune responses of tumor-infiltrating DCs.Citation26 TIM-3 is expressed on DCs in tumors at much higher levels than on DCs in normal tissues, and preferentially binds HMGB1, which has a critical role in stimulating nucleic acid-mediated innate immunity.Citation31,Citation32 TIM-3 negatively regulates the HMGB1-mediated recruitment of nucleic acids to the endosomal compartment of DCs, thus shutting down the downstream signaling cascades mediated by Toll-like receptors and cytosolic sensors. These results demonstrate that TIM-3 on DCs enables tumors to evade immunosurveillance by attenuating the sensing of nucleic acids that are potentially released upon anticancer treatment and/or endogenous tumor-associated inflammation.Citation26
More importantly, recent clinical studies have revealed that TIM-3 may serve as a prognostic marker to predict poor responses to anticancer therapies and, therefore, poor survival in patients with non-small cell lung carcinomas and clear-cell renal-cell carcinomas.Citation33,Citation34
Together, TIM-3 serves as a negative regulator of both innate and antigen-specific immune responses, thereby establishing an ideal environment for tumor immunoevasion and tumorigenicity.
Targeting immunoevasion by anticancer drugs: brief overview of current status
Recent advances in clarifying the molecular and cellular mechanisms of immune regulation of tumors have led to breakthroughs for developing novel drugs that specifically target the immunoregulatory functions of lymphocytes and myeloid cells.Citation35,Citation36 In particular, the targeting of immune checkpoint molecules is currently at the forefront of cancer immunotherapy.Citation37 Treatment with an anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) (ipilimumab) has extended the overall survival of patients with advanced melanomas compared with vaccination with gp100 peptide, and the combined regimens of anti-CTLA-4 with dacarbazine significantly improved the overall survival of previously untreated melanoma patients compared with dacarbazine alone.Citation38,Citation39 Moreover, early clinical studies revealed that monoclonal antibody (mAb)-mediated targeting of PD-1 (nivolumab), programmed cell death 1 ligand 1, or the combination of PD-1 and CTLA-4 elicited potent antitumor immune responses and improved clinical responses in patients with metastatic melanomas, non-small cell lung carcinomas or renal-cell carcinomas.Citation40–Citation42 The activation of CD40 by an agonistic mAb (CP-870,893) promoted immunogenic activities of tumor-infiltrating macrophages, which led to the differentiation of tumoricidal stromata and triggered clinical responses in patients with pancreatic cancer.Citation43 These results decisively provide a proof-of-concept that the reversal of tumor-induced immunomodulation by mAb changes the clinical prognosis in patients with treatment-refractory cancers. Additionally, adoptive transfer of T lymphocytes engineered to express chimeric antigen receptor targeting tumor-specific antigens resulted in potent antitumor immune responses and long-term remission in some patients with chronic lymphoid leukemia and solid malignancies.Citation44,Citation45
More importantly, the profound effects of several immune-targeting drugs have prompted researchers and pharmaceutical industries to develop novel immunotherapeutic drugs that target tumors and their immunosuppressive microenvironments. These are mainly comprised of mAbs targeting immune checkpoint and regulatory T cell functions, such as anti-OX40 antibody, anti-CD137 antibody, anti-glucocorticoid-induced tumor necrosis factor receptor-related protein antibody, etc.Citation36,Citation46–Citation48 Together, these advances in the clinical development of immune-targeting drugs in coordination with scientific advances in understanding tumor immune responses should have a profound impact on constructing new strategies to combat malignant diseases in clinical settings.
The potential for targeting TIM-3 as a new anticancer drug
Recent studies have identified TIM-3 as a promising candidate for a therapeutic target that may effectively trigger antitumor immune responses and clinical remission in cancer patients. In a preclinical study utilizing murine tumor models, treatment with an anti-TIM-3 mAb (RMT3-23) resulted in substantial tumor regression by stimulating tumor-specific T cell immunity.Citation49 Further, the anti-TIM-3 mAb synergized with anti-PD-1 mAb to augment antitumor responses by eliminating exhausted T cells in tumor tissues.Citation25,Citation26,Citation50 Therefore, the pharmacological manipulation of TIM-3 functions results in the reversion of immunosuppressive TMEs and thus leads to profound improvements in anticancer responses in coordination with other treatment regimens including immunotherapy, chemotherapy, radiotherapy, and certain subsets of molecular targeting.
In accordance with our assumptions about the role of TIM-3 in the regulation of anticancer drug responses, with colleagues, we demonstrated that treatment with murine or human anti-TIM-3 mAb (RMT3-23 and ATIK2a, respectively) augmented antitumor responses to cytotoxic chemotherapy or immunotherapy by increasing the antitumor immunogenicity of nucleic acids released from TMEs.Citation25,Citation51 Anticancer chemotherapies trigger the release of endogenous inflammatory mediators termed “damage-associated molecular pattern” (DAMP) molecules, such as HMGB1, adenosine triphosphate, and DNA, which serve as enriched sources for innate immune signals.Citation52 Thus, the drugs targeting TIM-3-mediated anti-inflammatory responses enhance the antitumor effects of chemotherapy by sensitizing DAMP molecule-dependent innate immune surveillance. Moreover, recent evidence has revealed the importance of innate immune sensing systems in effectively triggering antigen-specific effector responses and differentiating memory T cells.Citation53 Thus, it is tempting to speculate that TIM-3 stimulates antitumor innate sensing and immunological memory in synergy with inflammatory responses evoked by conventional anticancer regimens, thus opening new perspectives in the field of chemo-immunotherapy.
Despite these promising findings, which may pave the way for developing new and innovative immune targets and therapies for cancer patients, several issues remain unresolved regarding the role of TIM-3 in the regulation of antitumor responses to tumor immunotherapy in the course of tumor immunosurveillance and tumorigenic inflammation. Since TIM-3 serves as a negative regulator of both the innate and adaptive arms of immunity, blockade of TIM-3 should improve the therapeutic efficacy of immunotherapies in the early phase of tumor immunosurveillance. In contrast, the inhibition of TIM-3 may be detrimental in augmenting antitumor responses induced by immunotherapy in the background of pro-tumorigenic inflammation. Thus, our hypothesis highlights the complex and dual features of TIM-3 in the regulation of therapeutic responses to anticancer modalities including immunotherapy.
In addition, the expression and function of TIM-3 in TME components other than immune cells, such as endothelial cells, fibroblasts, and so on, remains largely, un explored. Recent studies have identified TIM-3 as a functional marker that is specifically expressed on acute myeloid leukemia stem cells, raising the possibility that TIM-3 controls tumorigenicity by creating complex networks formed by tumorigenic cells and tumor-associated non-transformed cells.Citation54–Citation56 Further studies of the origins, inducers, and functional specialization of TIM-3-expressing cells in the tumor environment will help to clarify how TMEs regulate the balance between antitumor immunosurveillance and tumor-promoting inflammation.
It also remains unclear how ligation of TIM-3 on different cell types can mediate the multiple immunoregulatory effects of different immune cell subsets. One key feature of TIM-3-mediated immune regulation is that TIM-3 has many ligands through which to exert multiple aspects of its biological activities. For example, Gal9 interacts with TIM-3 through amino-linked carbohydrates, whereas HMGB1 and PS bind to TIM-3 through a metal ion-dependent ligand-binding site in the FG loop of the Ig variable domain.Citation17,Citation18,Citation26 The binding of each ligand to the extracellular site of its receptor leads to conformational changes in the cytoplasmic tail, which activate alternative signaling pathways and thus exert distinct biological actions. Alternatively, it is tempting to speculate that post-translational modifications such as glycosylation may have a great impact on altering the immunological functions of TIM-3 in TMEs. Further studies should elucidate whether TIM-3 regulates pro- and anti-inflammatory responses by selecting distinct ligands and/or that such regulation is mediated through post-transcriptional modification of TIM-3 itself.
Conclusion
Tumor immunosuppression has emerged as an important hallmark of tumorigenicity.Citation57 Therapeutic intervention to correct immunosuppressive micro-environments may greatly improve the antitumor effects of cancer treatments, which include non-immunotherapeutic regimens such as chemotherapy and molecular target therapy.Citation58,Citation59 Given the recent impressive achievements in developing novel cancer immunotherapies, the therapeutic strategies of combining conventional anticancer modalities with the modification of immunosuppressive TMEs is a profoundly promising strategy for establishing long-term regression of aggressive tumors. Importantly, TIM-3 has been specifically detected on tumorigenic acute myeloid leukemia cells and tumor-associated myeloid cells in contrast to non-tumor counterparts, justifying the targeting of TIM-3 as a new tumor-specific antitumor strategy.Citation26,Citation53,Citation56 Thus, further understanding of the mechanisms for cancer-induced immunoevasion and the additional development of methods to overcome this evasion are critical to improve clinical remission in cancer patients in the future.
Acknowledgments
This study was partially supported by a Grant-in-Aid for Scientific Research and Scientific Research for Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labor and Welfare (MEXT), Japan.
Disclosure
The authors declare no conflicts of interest in this work.
References
- VeselyMDKershawMHSchreiberRDSmythMJNatural innate and adaptive immunity to cancerAnnu Rev Immunol20112923527121219185
- DunnGPOldLJSchreiberRDThe three Es of cancer immunoeditingAnnu Rev Immunol20042232936015032581
- SmythMJDunnGPSchreiberRDCancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicityAdv Immunol20069015016730260
- KhongHTRestifoNPNatural selection of tumor variants in the generation of “tumor escape” phenotypesNat Immunol2002311999100512407407
- FlavellRASanjabiSWrzesinskiSHLicona-LimónPThe polarization of immune cells in the tumour environment by TGFbetaNat Rev Immunol201010855456720616810
- KatzJBMullerAJPrendergastGCIndoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escapeImmunol Rev200822220622118364004
- RabinovichGACrociDORegulatory circuits mediated by lectin-glycan interactions in autoimmunity and cancerImmunity201236332233522444630
- NishikawaHSakaguchiSRegulatory T cells in tumor immunityInt J Cancer2010127475976720518016
- KuraishyAKarinMGrivennikovSITumor promotion via injury- and death-induced inflammationImmunity201135446747722035839
- DeNardoDGJohanssonMCoussensLMImmune cells as mediators of solid tumor metastasisCancer Metastasis Rev2008271111818066650
- DrakeCGJaffeeEPardollDMMechanisms of immune evasion by tumorsAdv Immunol200690518116730261
- RosenbergSAYangJCRestifoNPCancer immunotherapy: moving beyond current vaccinesNat Med200410990991515340416
- GalluzziLSenovillaLZitvogelLKroemerGThe secret ally: immunostimulation by anticancer drugsNat Rev Drug Discov201211321523322301798
- LakeRARobinsonBWImmunotherapy and chemotherapy – a practical partnershipNat Rev Cancer20055539740515864281
- MonneyLSabatosCAGagliaJLTh1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune diseaseNature2002415687153654111823861
- SantiagoCBallesterosATamiCMartínez-MuñozLKaplanGGCasasnovasJMStructures of T Cell immunoglobulin mucin receptors 1 and 2 reveal mechanisms for regulation of immune responses by the TIM receptor familyImmunity200726329931017363299
- CaoEZangXRamagopalUAT cell immunoglobulin mucin-3 crystal structure reveals a galectin-9-independent ligand-binding surfaceImmunity200726331132117363302
- NakayamaMAkibaHTakedaKTim-3 mediates phagocytosis of apoptotic cells and cross-presentationBlood2009113163821383019224762
- DeKruyffRHBuXBallesterosAT cell/transmembrane, Ig, and mucin-3 allelic variants differentially recognize phosphatidylserine and mediate phagocytosis of apoptotic cellsJ Immunol201018441918193020083673
- ZhuCAndersonACSchubartAThe Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunityNat Immunol20056121245125216286920
- RangachariMZhuCSakuishiKBat3 promotes T cell responses and autoimmunity by repressing Tim-3-mediated cell death and exhaustionNat Med20121891394140022863785
- YangZZGroteDMZiesmerSCIL-12 upregulates TIM-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphomaJ Clin Invest201212241271128222426209
- NdhlovuLCLopez-VergèsSBarbourJDTim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicityBlood2012119163734374322383801
- GleasonMKLenvikTRMcCullarVTim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9Blood2012119133064307222323453
- AndersonACAndersonDEBregoliLPromotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cellsScience200731858531141114318006747
- ChibaSBaghdadiMAkibaHTumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1Nat Immunol201213983284222842346
- FourcadeJSunZBenallaouaMUpregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patientsJ Exp Med2010207102175218620819923
- SakuishiKApetohLSullivanJMBlazarBRKuchrooVKAndersonACTargeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunityJ Exp Med2010207102187219420819927
- YanJZhangYZhangJPLiangJLiLZhengLTim-3 expression defines regulatory T cells in human tumorsPLoS One201383e5800623526963
- HayakawaYSmythMJInnate immune recognition and suppression of tumorsAdv Cancer Res20069529332216860661
- TianJAvalosAMMaoSYToll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGENat Immunol20078548749617417641
- YanaiHBanTWangZHMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responsesNature200946272699910319890330
- BaiJLiXTongDShiWSongHLiQT-cell immunoglobulin-and mucin-domain-containing molecule 3 gene polymorphisms and prognosis of non-small-cell lung cancerTumour Biol201334280580923359271
- YuanJJiangBZhaoHHuangQPrognostic implication of TIM-3 in clear cell renal cell carcinomaNeoplasia Epub9202013
- MellmanICoukosGDranoffGCancer immunotherapy comes of ageNature2011480737848048922193102
- SharmaPWagnerKWolchokJDAllisonJPNovel cancer immunotherapy agents with survival benefit: recent successes and next stepsNat Rev Cancer2011111180581222020206
- PardollDMThe blockade of immune checkpoints in cancer immunotherapyNat Rev Cancer201212425226422437870
- HodiFSO’DaySJMcDermottDFImproved survival with ipilimumab in patients with metastatic melanomaN Engl J Med2010363871172320525992
- RobertCThomasLBondarenkoIIpilimumab plus dacarbazine for previously untreated metastatic melanomaN Engl J Med2011364262517252621639810
- TopalianSLHodiFSBrahmerJRSafety, activity, and immune correlates of anti-PD-1 antibody in cancerN Engl J Med2012366262443245422658127
- BrahmerJRTykodiSSChowLQSafety and activity of anti-PD-L1 antibody in patients with advanced cancerN Engl J Med2012366262455246522658128
- WolchokJDKlugerHCallahanMKNivolumab plus ipilimumab in advanced melanomaN Engl J Med2013369212213323724867
- BeattyGLChioreanEGFishmanMPCD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humansScience201133160241612161621436454
- PorterDLLevineBLKalosMBaggAJuneCHChimeric antigen receptor-modified T cells in chronic lymphoid leukemiaN Engl J Med2011365872573321830940
- KalosMJuneCHAdoptive T cell transfer for cancer immunotherapy in the era of synthetic biologyImmunity2013391496023890063
- WeinbergADMorrisNPKovacsovics-BankowskiMUrbaWJCurtiBDScience gone translational: the OX40 agonist storyImmunol Rev2011244121823122017441
- LynchDHThe promise of 4-1BB (CD137)-mediated immunomodulation and the immunotherapy of cancerImmunol Rev200822227728618364008
- SchaerDACohenADWolchokJDAnti-GITR antibodies – potential clinical applications for tumor immunotherapyCurr Opin Investig Drugs2010111213781386
- NgiowSFvon ScheidtBAkibaHYagitaHTengMWSmythMJAnti-TIM3 antibody promotes T cell IFN-γ-mediated antitumor immunity and suppresses established tumorsCancer Res201171103540355121430066
- ZhouQMungerMEVeenstraRGCoexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemiaBlood2011117174501451021385853
- BaghdadiMNagaoHYoshiyamaHCombined blockade of TIM-3 and TIM-4 augments cancer vaccine efficacy against established melanomasCancer Immunol Immunother201362462963723143694
- KryskoDVGargADKaczmarekAKryskoOAgostinisPVandenabeelePImmunogenic cell death and DAMPs in cancer therapyNat Rev Cancer2012121286087523151605
- IwasakiAMedzhitovRRegulation of adaptive immunity by the innate immune systemScience2010327596329129520075244
- JanMChaoMPChaACProspective separation of normal and leukemic stem cells based on differential expression of TIM3, a human acute myeloid leukemia stem cell markerProc Natl Acad Sci U S A2011108125009501421383193
- KikushigeYShimaTTakayanagiSTIM-3 is a promising target to selectively kill acute myeloid leukemia stem cellsCell Stem Cell20107670871721112565
- HuangXBaiXCaoYLymphoma endothelium preferentially expresses Tim-3 and facilitates the progression of lymphoma by mediating immune evasionJ Exp Med2010207350552020176801
- HanahanDWeinbergRAHallmarks of cancer: the next generationCell2011144564667421376230
- ZitvogelLGalluzziLSmythMJKroemerGMechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillanceImmunity2013391748823890065
- KnightDANgiowSFLiMHost immunity contributes to the anti-melanoma activity of BRAF inhibitorsJ Clin Invest201312331371138123454771