Abstract
Purpose
This study aimed to compare the efficacy and safety of neoadjuvant chemotherapy (NCT) and neoadjuvant immunotherapy combined with chemotherapy (NICT) combined with radical lung cancer resection for the treatment of patients with resectable non-small cell lung cancer (NSCLC). To adjust for confounding factors, we innovatively adopted two matching methods: propensity score (PS) and inverse probability of treatment weighting (IPTW).
Patients and Methods
We conducted a retrospective analysis of the clinicopathological features and prognosis of patients with resectable NSCLC treated with NCT or NICT combined with radical lung cancer resection using propensity score matching (PSM) at a ratio of 1:1 and IPTW to balance potential bias.
Results
After PSM, 116 pairs of patients who had undergone NCT or NICT were selected for the final analysis. The pathological complete remission (pCR) and major pathological remission (MPR) rates were significantly better in the NICT group than in the NCT group (pCR rate of 44.8% vs 2.6%, P< 0.001; MPR rate of 66.4% vs 20.7%, P< 0.001). No significant difference was seen between the NICT and NCT groups in terms of postoperative complications (12.1% vs 9.5%, P=0.182). Patients in the NICT group had significantly better disease-free survival (DFS) and overall survival(OS) than those in the NCT group ([3-year DFS: 75.2% vs 43.3%, P< 0.001] and [3-year OS: 91.5% vs 58.0%, P< 0.001]). Among all patients, those with postoperative pathology of pCR had better DFS (P< 0.001) and OS (P= 0.009). Patients with postoperative pathology of MPR had better DFS (P< 0.001) and OS (P< 0.001). The IPTW method yielded similar pathologic and prognostic results.
Conclusion
Patients with resectable NSCLC treated with NICT had better pathological responses and prognosis, than those treated with NCT, and the safety profiles of NICT and NCT were similar.
Introduction
Globally, lung cancer is the primary cause of cancer-related fatalities, with non-small cell lung cancer (NSCLC) approximately 85% of all lung cancers.Citation1 Radical lung cancer resection is the main therapeutic option for resectable NSCLC; however, the prognosis after surgery is poor, with a considerable number of patients experiencing local regional recurrence (LRR) and distant metastasis (DM) even after complete tumor resection.Citation2,Citation3 DM is thought to result from the progression of minimal residual diseases, which involve metastatic cancer cells that are undetectable on preoperative imaging. Therefore, the early initiation of systemic treatment can maximize the elimination of micrometastases. Preoperative drug administration can inhibit the primary lesion by delivering the drug to the tumor site through the intact vascular system, resulting in a decrease in the tumor stage, further improving the surgical resection rate, reducing the total lung resection rate, and avoiding the possible intraoperative dissemination of tumor cells to distant sites, thereby reducing the incidence of postoperative LRR and DM and prolonging patient survival. However, adding neoadjuvant chemotherapy (NCT) only increased the 5-year survival rate by 5%.Citation4 In the era of immunotherapy, the rise of immune checkpoint inhibitors has provided a new treatment modality for a wide range of tumors.Citation5,Citation6 The efficacy of immunotherapy as a treatment for advanced NSCLC has been demonstrated, with a notable enhancement in the 5-year overall survival (OS) of patients being observed.Citation7,Citation8 In neoadjuvant therapy, immunotherapy offers the opportunity to treat micrometastatic diseases early and enhance the immune response in the presence of large numbers of tumors and tumor antigens.Citation9,Citation10 Preclinical data suggest that neoadjuvant immunotherapy can elicit a more effective systemic antitumor immune response prior to tumor resection by killing tumor cells to release antigens with less impairment of T cell function.Citation11 The efficacy and safety of neoadjuvant immunotherapy have been confirmed in a series of clinical trials.Citation12–24 However, current clinical studies on neoadjuvant immunotherapy in combination with chemotherapy (NICT) show a wide variation in major pathological remission (MPR) rates, from 36.9% in the Checkmate 816 study to 82.9% in the NADIM study.Citation16,Citation23 There is limited evidence comparing NCT and NICT in patients with resectable NSCLC. In this real-world study, we sought to evaluate the pathological response, long-term outcomes, and patterns of failure in patients with resectable NSCLC who underwent NCT and NICT treatments. By utilizing propensity score matching (PSM) and inverse probability of treatment weighting (IPTW), we aimed to balance potential biases, offering insights to assist clinicians in their decision-making and inform future clinical trials.
Materials and Methods
Patient Selection
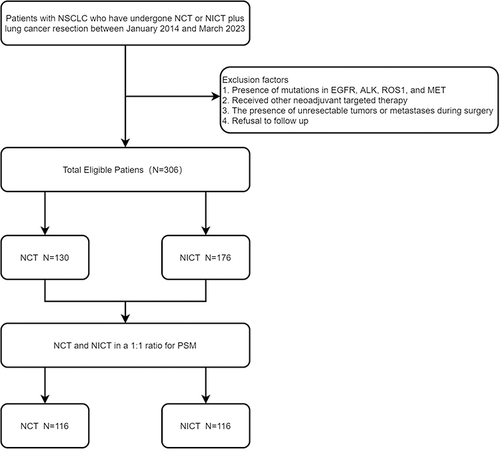
Data from 306 patients with resectable NSCLC treated with NCT or NICT combined with surgery between January 2014 and March 2023 at our institution were retrospectively analyzed. NSCLC (squamous or adenocarcinoma) was pathologically confirmed to be present in all patients, and all patients were genetically tested to exclude mutations in EGFR, ALK, ROS1, and MET, and received NCT with NICT only before surgical treatment. Those patients who had unresectable tumors or metastases during exploratory surgery, those who received other neoadjuvant targeted therapies, and those who refused follow-up were excluded (). This study used the 8th edition of the Union for International Cancer Control/American Joint Committee on Cancer (UICC/AJCC) TNM staging system.Citation25 In this study, we have excluded patients who participated in clinical trials, considering only those treated under routine clinical conditions.The study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of the Shandong Cancer Hospital and Research Institute (No. SDTHEC2023004018).
Neoadjuvant Treatment Regimens
All patients underwent multidisciplinary consultation to assess their condition and determine the appropriate treatment regimen before starting treatment, and computerized tomography (CT) or positron emission tomography-CT (PET-CT) was performed before and after neoadjuvant treatment to assess tumor changes. The main preoperative chemotherapy regimens for patients with NCT are intravenous paclitaxel-like and platinum-based drugs for patients with squamous cell carcinoma and intravenous pemetrexed and platinum-based drugs for adenocarcinoma patients. Platinum-based drugs included carboplatin, with an area under the curve of 5, or cisplatin 25 mg/m2 on days 1–3. Paclitaxel-based drugs include paclitaxel 135–175 mg/m2 or albumin-bound paclitaxel 260 mg/m2. Pemetrexed (500 mg/m2) was then administered. Patients received 1–3 doses of preoperative chemotherapy every three weeks, and the average usage cycle was two in the NICT and NCRT groups. For patients who chose NICT, the preoperative IT regimen consisted of 1–3 cycles of intravenous programmed cell death-1 (PD-1) inhibitor (tislelizumab at a dose of 200 mg, pembrolizumab at a dose of 200 mg, camrelizumab at a dose of 200 mg, toripalimab at a dose of 240 mg, or sintilimab at a dose of 200 mg) every three weeks, and the preoperative chemotherapy regimen was the same as that for NCT.
Surgical Treatment
All enrolled patients were clinically assessed for resectable NSCLC in a multidisciplinary consultation. All patients underwent radical surgical resection of the lung cancer under general anesthesia 4–6 weeks after the completion of the last neoadjuvant treatment. Surgical modalities included video-assisted thoracoscopic surgery (VATS) and thoracotomy. The lungs were excised via lobectomy, sleeve resection, or total unilateral pneumonectomy. All patients returned to the thoracic surgery department postoperatively and were encouraged to cough and spit to promote drainage and lung re-expansion, and were instructed to move early to prevent postoperative complications.
Follow-Up
All selected patients received a regular outpatient review and telephone follow-up since admission, with regular physical examinations, chest-enhanced CT, and, if necessary, PET-CT, ultrasound, tracheoscopy, magnetic resonance imaging, or whole-body bone imaging during the follow-up period. For patients whose last case was recorded in the case system until the cut-off time of this study was greater than 1 month, a telephone follow-up was performed to ask the patients for details of their progress and survival. The follow-up ended on August 1, 2023, with a median follow-up of 20 months for all patients (range 8–117 months). The median follow-up period for patients in the NCT and NICT groups was 24 months (range 8–117 months) and 18 months (range 7–45 months), respectively.
Study Endpoints
The primary study endpoints were disease-free survival (DFS) and OS. Secondary study endpoints included the rates of pathological complete remission (pCR), MPR, R0 resection, failure mode, and surgical complications. Two trained pathologists assessed all postoperative pathological results. The time interval between the radical resection of lung cancer and the first recording of a recurrence, death due to any cause, or last follow-up visit was termed DFS. The period from the initiation of the initial neoadjuvant therapy cycle to the death of any individual owing to any cause or the final follow-up visit was designated as the OS. No residual tumor cells were present at the primary site of the surgical specimen or in the resected lymph nodes (ypT0N0M0), indicating pCR. MPR was defined as <10% of surviving tumor cells remaining in the pathological examination of the postoperative specimen.Citation26,Citation27 R0 resection was defined as the complete removal of the tumor with all microscopic margins negative, (ie, no residual tumor). In the failure model, recurrence was divided into LRR and DM, with LRR defined as a primary tumor or local lymph node recurrence and DM defined as non-regional lymph node metastases, systemic metastases, malignant pleural effusion, or peritoneal metastases. In terms of surgical complications, this study mainly recorded pneumonia, chylothorax, and hydropneumothorax, with other surgical complications categorized as others.
Statistical Analysis
PSM uses logistic regression to create a propensity score (PS) for individual patients using demographic and clinical variables. PSM was used to assemble a well-balanced queue using all the available explanatory factors.Citation28 Therefore, This study employed a 1:1 matching analysis between the NCT and NICT groups using the Nearest Neighbor Method without replacement and a caliper of 0.2 to control for potential confounding factors that might influence the outcomes. In addition to using PSM, we also applied IPTW to balance the baseline characteristics between the two groups. The variables used to estimate PS were age, sex, Karnofsky (KPS) score, percentage of the forced expiratory volume (FEV 1%), mode of lung resection, tumor location, history of smoking, history of alcohol consumption, concomitant disease, family history, number of cycles of neoadjuvant therapy, clinical stage, presence of tracheoscopy and presence of PET/CT. Each patient who received NCT was matched with the one who received NICT and had the closest PS. The Kruskal–Wallis, or independent samples t-test was used to compare R0 resection rates, pCR rates, MPR rates, surgical complications, and failure patterns. DFS and OS were assessed using the Kaplan–Meier technique, and the Log rank test was used for comparison. All statistical analyses were performed using the R software (version 4.2.1) and SPSS 25.0.
Results
Patients’ Baseline Characteristics
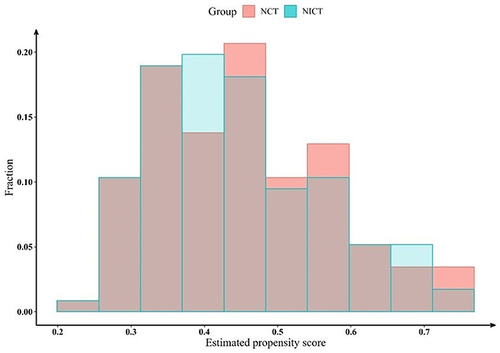
This study included 306 patients with resectable NSCLC who underwent either NCT or NICT combined with radical surgical resection between January 2014 and March 2023, at our institution, with 130 (42.5%) and 176 (57.5%) in the NCT and NICT groups, respectively. A 1:1 PSM of the NCT and NICT groups was performed balancing various factors that could potentially be biased, and 116 pairs of NSCLC patients receiving NCT and NICT were selected for the final analysis. Considering the distribution of propensity scores in both groups, as depicted by the histogram, we believe the ability of propensity scores to discriminate between the two groups is satisfactory (). summarizes the baseline traits of all patients before and after PSM and IPTW. The clinical characteristics of post-PSM patients were more balanced and included age, sex, KPS, FEV 1%, tumor location, family history, concomitant disease, history of smoking, history of alcohol consumption, clinical T stage, clinical N stage, clinical TNM stage, number of cycles of neoadjuvant therapy, presence of tracheoscopy, and presence of PET/CT.
Table 1 Baseline Characteristics Before and After PSM and IPTW
Pathological Response to Neoadjuvant Therapy
All patients underwent radical resection under general anesthesia 3–8 weeks after the last neoadjuvant treatment, with no delays in surgery due to treatment-related adverse events. As shown in , patients in the NICT group had better outcomes in terms of pathological response than those in the NCT group, with a pCR rate of 44.8% in the NICT group and 2.6% in the NCT group (P<0.001). In addition, the NICT group had a significantly higher rate of complete pathological remission of both primary lesions and lymph nodes compared to the NCT group (primary lesions 44.8% vs 2.6%, P<0.001; lymph nodes 75.9% vs 48.3%, P<0.001). The MPR rate was significantly higher in the NICT group than that in the NCT group (66.4% vs 20.7%, P<0.001). No statistically significant difference was observed in the R0 resection rates between the NCT and NICT groups (96.6% vs 97.4%, P=1.000). No statistical differences were observed between the NICT and NCT groups for both nerve invasion and lymphovascular invasion (LVSI) (0.0% vs 2.6% for nerve invasion, P=0.245; 5.2% vs 7.8% for LVSI, P=0.593). The IPTW method yielded similar pathologic results.
Table 2 Comparative Analysis of Pathological Outcomes Between NCT and NICT Before and After PSM and IPTW
Surgical Outcome
The surgical information for both groups of patients is shown in . VATS was selected by 54 patients (46.6%) in the NICT group and 60 patients (51.7%) in the NCT group, with no statistical difference (P=0.511). No significant difference was observed in intraoperative bleeding between the NICT and NCT groups (140.7 ± 161.8 vs 111.8 ± 79.7, P=0.086), and the surgical interval was the time interval between the last treatment and surgery, again without a statistical difference (37.2 ± 12.0 vs 35.9 ± 9.3, P=0.373). However, there was a noteworthy difference in the duration of surgery and length of surgical stay between the NICT and NCT groups (duration of surgery 122.4 ± 37.6 vs 137.8 ± 39.1, P=0.003; length of surgical stay 16.5 ± 6.4 vs 18.5 ± 5.8, P=0.048). In terms of postoperative complications, no significant difference was observed between the NICT and NCT groups (12.1% vs 9.5%, P=0.182). Deaths within a short period after surgery occurred only in the NCT group, with one death occurring within 30 days after surgery due to multiorgan failure.
Table 3 Comparative Analysis of Surgical Information Between NCT and NICT Before and After PSM and IPTW
Progression and Survival Outcomes
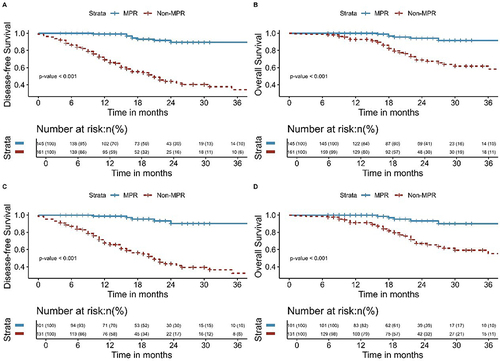
The median follow-up time for all patients in this study was 20 months (range, 8–117 months). The median follow-up time for patients in the NCT and NICT groups was 24 months (range, 8–117 months) and 18 months (range, 7–45 months), respectively. As shown in , the NICT group had significantly better DFS and OS than that of the NCT group ([3-year DFS: 75.2% vs 43.3%, P< 0.001] and [3-year OS: 91.5% vs 58.0%, P< 0.001]). Among all patients, those with postoperative pathology of pCR had better DFS (P< 0.001) and OS (P= 0.009) (). Patients with postoperative pathology of MPR had better DFS (P< 0.001) and OS (P< 0.001) than those without MPR (). This study also analyzed the failure modes of the two groups, with two (1.7%) patients in the NICT group and four (3.4%) patients in the NCT group experiencing LRR, with no significant difference observed (P= 0.679). The DM in the NICT group was significantly better than that of the NCT group (6.0% vs 32.8%, P<0.001) ().
Table 4 Failure Modes After Surgery
Figure 3 Kaplan-Meier survival analysis of DFS (A) and OS (B) between NCT and NICT before PSM; Kaplan-Meier survival analysis of DFS (C) and OS (D) between NCT and NICT after PSM.
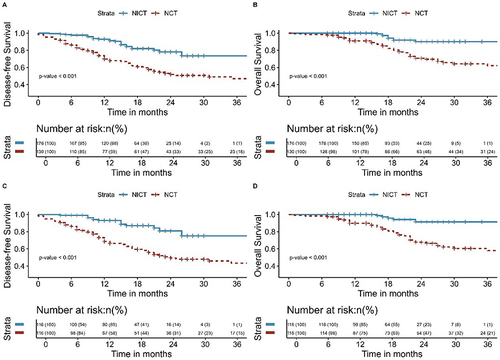
Figure 4 Kaplan-Meier survival analysis of DFS (A) and OS (B) between pCR and Non-pCR before PSM; Kaplan-Meier survival analysis of DFS (C) and OS (D) between pCR and Non-pCR after PSM.
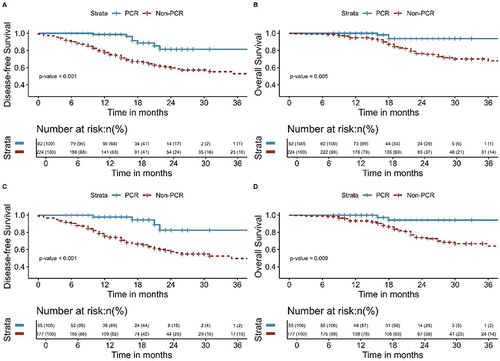
Figure 5 Kaplan-Meier survival analysis of DFS (A) and OS (B) between MPR and Non-MPR before PSM; Kaplan-Meier survival analysis of DFS (C) and OS (D) between MPR and Non-MPR after PSM.
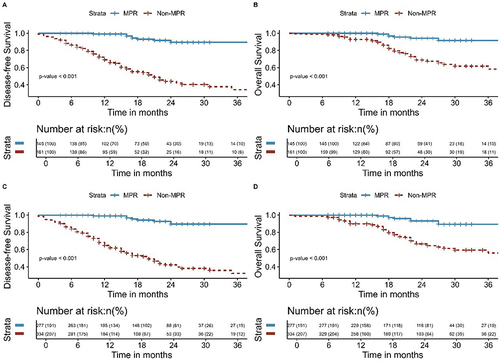
The Kaplan-Meier survival analyses for DFS and OS comparing NCRT and NICT, both pre and post-IPTW, are delineated in . Additionally, Kaplan-Meier survival analyses of DFS and OS for pCR and Non-pCR groups and MPR and Non-MPR groups before and after the application of IPTW are shown in and . The findings from this extensive analysis are bolstered by the coherence of results derived from two distinct statistical methodologies.
Figure 6 Kaplan-Meier survival analysis of DFS (A) and OS (B) between NCT and NICT before inverse probability of treatment weighting; Kaplan-Meier survival analysis of DFS (C) and OS (D) between NCT and NICT after inverse probability of treatment weighting.
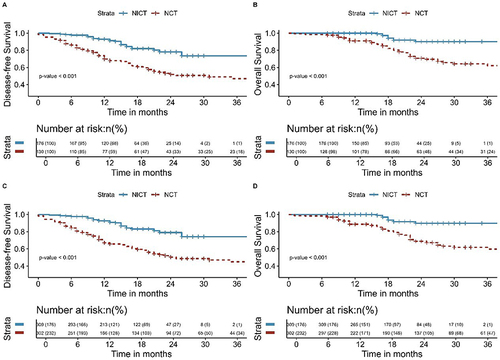
Figure 7 Kaplan-Meier survival analysis of DFS (A) and OS (B) between pCR and Non-pCR before inverse probability of treatment weighting; Kaplan-Meier survival analysis of DFS (C) and OS (D) between pCR and Non-pCR after inverse probability of treatment weighting.
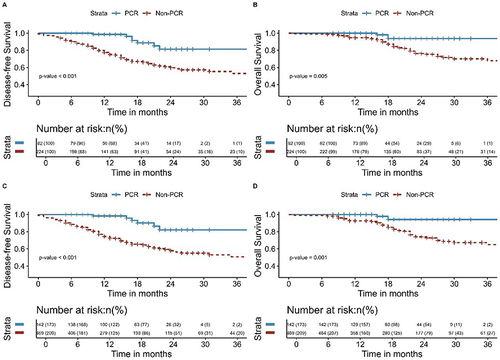
Figure 8 Kaplan-Meier survival analysis of DFS (A) and OS (B) between MPR and Non-MPR before inverse probability of treatment weighting; Kaplan-Meier survival analysis of DFS (C) and OS (D) between MPR and Non-MPR after inverse probability of treatment weighting.
Univariate and Multivariate Regression Analysis
Through a logistic regression analysis examining pCR, our results indicate that neoadjuvant regimens independently predict pCR with significance (P<0.001) as shown in .
Table 5 Logistical Regression Analysis for Predictors of pCR
In the Cox regression analysis assessing OS, both the neoadjuvant treatment regimen and MPR emerged as independent prognostic factors (P=0.023 and P=0.004, respectively). Meanwhile, in the analysis for DFS, MPR (P<0.001) and lymph nodes pCR (P=0.016) stood out as independent prognostic indicators. Notably, the neoadjuvant regimen did not demonstrate a significant prognostic distinction in DFS (P=0.106), as detailed in .
Table 6 Cox Regression Analysis for Predictors of OS and DFS
Discussion
In patients with resectable NSCLC, sometimes surgical treatment alone is ineffective or not radically resectable In recent years, a neoadjuvant therapy model jointly formulated by multiple disciplines has been widely used for NSCLC patients, and neoadjuvant therapy combined with radical resection of lung cancer has become the standard treatment for patients with resectable NSCLC. With the success of immune checkpoint inhibitors that block the PD-1 signaling pathway in the treatment of advanced tumors,Citation29–33 immunotherapy has also been added to neoadjuvant therapy and has significantly improved patient prognosis. Few studies have compared NICT with NCT in patients with resectable NSCLC; therefore, we retrospectively analyzed the efficacy and safety of NICT versus NCT for resectable NSCLC. To validate the reliability of our study, we employed two methods (PSM and IPTW) to control for confounding factors and conducted a thorough analysis. IPTW also requires the initial calculation of the PS. Through PS, each patient is assigned a weight, with the treatment group weight being 1/PS and the control group weight being 1/(1-PS). By standardizing each patient with PS weighting, a standardized population is ultimately obtained. Within this standardized cohort, the distribution of confounders in both the treatment and control groups tends to be consistent. This means that any difference in treatment outcomes between the groups can be attributed to the different treatment protocols used, allowing for a direct comparison. Compared to the sole use of PSM, an advantage of the IPTW method is that it does not result in a loss of sample size.Citation34 In this study, the pCR and MPR rates of the NICT group were significantly better than those of the NCT group both before and after matching, and the NICT group also had a significant advantage in terms of DFS and OS. Moreover, we observed that NICT did not increase the difficulty of surgery according to the duration of surgery or the amount of intraoperative bleeding, and there was also no difference in the occurrence of postoperative complications between the two groups, proving the efficacy and safety of NICT.
Several studies have compared the efficiency of NCT combined with surgery for NSCLC patients to surgery alone and found that NCT significantly improved the prognosis of patients but with very limited efficacy. Roth et al demonstrated that preoperative 3-week chemotherapy significantly prolonged 3- and 5-year OS in patients with stage IIIA NSCLC compared with surgery alone (43% vs 19%; 36% vs 15%).Citation35 The 2003 JCOG9029 trial compared the survival outcomes of patients with stage IIIA (N2) NSCLC treated with preoperative NCT plus surgery versus surgery alone and concluded that there was little difference in OS between the two groups.Citation36 A meta-analysis of 15 randomized controlled NCT trials in 2385 patients showed a significant improvement in OS after preoperative chemotherapy compared with surgery alone (HR = 0.87, 95% CI: 0.78 to 0.96, P=0.007), with a 5% absolute improvement in conversion from preoperative chemotherapy to 5-year OS.Citation4
However, immunotherapy significantly improves the prognosis of patients with resectable NSCLC. The antitumor effect produced by preoperative neoadjuvant immunotherapy not only shrinks the tumor but also maximizes the activation of the antitumor effect before surgical lymph node dissection. After the primary lesion has been removed, activated T cells are still able to target potential metastatic lesions from “memory”, improving the prognosis.Citation37 The Checkmate 816 study concluded that in patients with resectable NSCLC, NICT prolonged DFS by 11 months and increased pCR rates compared with those associated with chemotherapy alone (24.0% vs 2.2% pCR rates, respectively).Citation23 However, existing studies show wide variations in the MPR rates, from 36.9% in the Checkmate 816 study to 82.9% in the NADIM study, hence the real-time scenario remains unclear.Citation16,Citation23
This study obtained similar results, with NICT significantly increasing pCR rates and MPR rates, which in turn significantly improved DFS and OS. Preclinical studies have shown that chemotherapeutic agents can promote immune response by disrupting the activity of immunosuppressive cells, causing immunogenic death, and upregulating of MHC class I molecule expression.Citation38–41 We did not perform further subgroup analyses of the immunological drugs in this study, which were all selected from Chinese domestic PD-1 inhibitors, and by default, the basic pharmacological mechanism of action of PD-1 inhibitors is the same. Patients with postoperative pathology up to pCR and MPR had a better prognosis than those with non-pCR and non-MPR, which is consistent with the results of previous studies.Citation42 Before matching, patients with postoperative pathology results of pCR had a significant advantage in both OS and DFS. After matching, we similarly saw a significant advantage, which reminds us that higher pCR rates remain a focus of neoadjuvant treatment strategies. MPR and pCR are suggested as surrogate endpoints for survival, as they are strongly associated with improved survival and may therefore provide a faster way to compare different neoadjuvant treatment options.Citation27,Citation43 However, a major limitation is the lack of precision in the MPR owing to inherent interobserver variability.Citation44 The relationship between pathological response and survival benefit requires further evaluation in ongoing neoadjuvant therapy trials involving NSCLC patients. For the failure mode, no significant difference was observed between the NICT and NCT groups in terms of LRR, suggesting that early implementation of both neoadjuvant treatments provided control of the tumor lesion and increased the success rate of the procedure. However, in terms of DM, NICT has a significant advantage over NCT, indicating that NICT is more advantageous in eliminating micrometastases that cannot be detected using conventional imaging.
In previous studies on neoadjuvant radiotherapy, although neoadjuvant chemoradiotherapy reduced tumor staging, the complexity of the procedure increased, possibly because neoadjuvant treatment can lead to local tissue adhesions and increased vascular fragility, thus making the procedure more difficult.Citation45,Citation46 However, in this study, no significant differences were found in the amount of intraoperative bleeding and R0 resection rates between the NICT and NCT groups, and the duration of surgery and surgical hospital stay were significantly lower in the NICT group than in the NCT group, indicating that the addition of immunotherapy not only did not increase the difficulty and unresectability of surgery but also reduced it. Our results were consistent with the findings of previous clinical studies. In the Checkmate 816 study, the nivolumab combined with the chemotherapy group had a shorter median duration of surgery and a higher R0 resection rate compared to the chemotherapy alone group (83.2% vs 77.8%), and adverse events of any cause occurred in 92.6% of the nivolumab combined with chemotherapy group and in 97.2% of the chemotherapy-only group. In the NADIM study, the addition of neoadjuvant nivolumab to chemotherapy was well-tolerated, with a similar frequency of treatment-related adverse events as in the chemotherapy-only arm. This could be attributed to the fact that immunotherapy enhances microcirculation and NICT can kill tumor cells more effectively, resulting in more significant tumor shrinkage, and reduced surgical staging. Again, we did not find an increased risk of delayed surgery with NICT. It is well known that early diagnosis and timely surgery can significantly improve the cure and survival rates of lung cancer.Citation47 In terms of postoperative complications, the addition of IT, which did not increase the incidence of complications such as postoperative pneumonia, demonstrated the feasibility and safety of NICT in treating resectable NSCLC.
NICT has an irreplaceable role in the treatment of patients with resectable NSCLC owing to its clear advantages in terms of pathological response and long-term prognosis, as well as its ability to eliminate distant micrometastatic lesions; however, NICT remains controversial. First, not all patients will benefit from NICT; therefore, finding markers that screen for patients who will benefit from NICT and establish a definitive relationship with clinical benefit can provide a basis for clinical decision-making and provide patients with more confidence in immunotherapy. This requires not only screening for more clinically relevant markers of immunotherapy but also a combination of multiple markers to allow more accurate screening of those who will benefit from neoadjuvant immunotherapy. Second, the optimal number of neoadjuvant treatment cycles was determined. The neoSCORE study, which compared 2-cycle and 3-cycle neoadjuvant therapies, initially showed that more cycles of NICT resulted in higher MPR and pCR rates for patients with resectable NSCLC and were well tolerated.Citation48 However, the optimal number of cycles of neoadjuvant therapy is inconclusive and needs to be confirmed using evidence-based medical evidence.
This study had certain limitations: (1) The efficacy and safety of different chemotherapeutic and immunological drugs vary slightly, and the long duration of this study might have introduced variations, therefore we used PSM to minimize the impact of bias; (2) the sample size was not sufficiently large and the follow-up period was relatively short.
Conclusion
Patients with resectable NSCLC who underwent NICT exhibited improved pathological responses and prognosis compared to those treated with NCT. Additionally, the safety profiles between NICT and NCT were comparable.
Ethical Approval and Consent to Participate
This study has been approved by the Ethics Committee of Cancer Hospital Affiliated to Shandong First Medical University. Given its retrospective nature, the committee has waived the informed consent requirement for this study. We declare that patients information will be kept confidential and that we adhere to the principles of the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi:10.3322/caac.21708
- Carnio S, Novello S, Papotti M, et al. Prognostic and predictive biomarkers in early stage non-small cell lung cancer: tumor based approaches including gene signatures. Transl Lung Cancer Res. 2013;2:372–381. doi:10.3978/j.issn.2218-6751.2013.10.05
- Uramoto H, Tanaka F. Recurrence after Surgery in Patients with NSCLC. Transl Lung Cancer Res. 2014;3:242–249. doi:10.3978/j.issn.2218-6751.2013.12.05
- Group NM-aC. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet. 2014;383:1561–1571. doi:10.1016/S0140-6736(13)62159-5
- de Miguel M, Calvo E. Clinical Challenges of Immune Checkpoint Inhibitors. Cancer Cell. 2020;38:326–333. doi:10.1016/j.ccell.2020.07.004
- Ribas A, Wolchok J. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi:10.1126/science.aar4060
- Reck M, Remon J, Hellmann MD. First-line immunotherapy for non-small-cell lung cancer. J Clin Oncol. 2022;40:586–597. doi:10.1200/JCO.21.01497
- Ettinger DS, Wood DE, Aggarwal C, et al. NCCN guidelines insights: non-small cell lung cancer, version 1.2020. J Natl Compr Canc Netw. 2019;17:1464–1472. doi:10.6004/jnccn.2019.0059
- Uprety D, Mandrekar SJ, Wigle D, et al. Neoadjuvant Immunotherapy for NSCLC: current Concepts and Future Approaches. J Thorac Oncol. 2020;15:1281–1297. doi:10.1016/j.jtho.2020.05.020
- Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020;367:eaax0182. doi:10.1126/science.aax0182
- Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378:1976–1986. doi:10.1056/NEJMoa1716078
- Eichhorn F, Klotz LV, Kriegsmann M, et al. Neoadjuvant anti-programmed death-1 immunotherapy by pembrolizumab in resectable non-small cell lung cancer: first clinical experience. Lung Cancer. 2021;153:150–157. doi:10.1016/j.lungcan.2021.01.018
- Wislez M, Mazieres J, Lavole A, et al. Neoadjuvant durvalumab for resectable non-small-cell lung cancer (NSCLC): results from a multicenter study (IFCT-1601 IONESCO). J Immunother Cancer. 2022;10:e005636. doi:10.1136/jitc-2022-005636
- Gao S, Li N, Gao S, et al. Neoadjuvant PD-1 Inhibitor (Sintilimab) in NSCLC. J Thorac Oncol. 2020;15:816–826. doi:10.1016/j.jtho.2020.01.017
- Tong BC, Gu L, Wang X, et al. Perioperative outcomes of pulmonary resection after neoadjuvant pembrolizumab in patients with non-small cell lung cancer. J Thorac Cardiovasc Surg. 2022;163:427–436. doi:10.1016/j.jtcvs.2021.02.099
- Provencio M, Nadal E, Insa A, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21:1413–1422. doi:10.1016/S1470-2045(20)30453-8
- Rothschild SI, Zippelius A, Eboulet EI, et al. Sakk 16/14: durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non-small-cell lung cancer-a multicenter single-arm phase II trial. J Clin Oncol. 2021;39:2872–2880. doi:10.1200/JCO.21.00276
- Zhao ZR, Yang CP, Chen S, et al. Phase 2 trial of neoadjuvant toripalimab with chemotherapy for resectable stage III non-small-cell lung cancer. Oncoimmunology. 2021;10:1996000. doi:10.1080/2162402X.2021.1996000
- Yang CJ, McSherry F, Mayne NR, et al. Surgical outcomes after neoadjuvant chemotherapy and ipilimumab for non-small cell lung cancer. Ann Thorac Surg. 2018;105:924–929. doi:10.1016/j.athoracsur.2017.09.030
- Wang J, Li J, Cai L, et al. The safety and efficacy of neoadjuvant programmed death 1 inhibitor therapy with surgical resection in stage IIIA non-small cell lung cancer. Ann Transl Med. 2021;9:486. doi:10.21037/atm-21-670
- Shu CA, Gainor JF, Awad MM, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21:786–795. doi:10.1016/S1470-2045(20)30140-6
- Tfayli A, Al Assaad M, Fakhri G, et al. Neoadjuvant Chemotherapy and Avelumab in Early Stage Resectable Nonsmall Cell Lung Cancer. Cancer Med. 2020;9:8406–8411. doi:10.1002/cam4.3456
- Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386:1973–1985. doi:10.1056/NEJMoa2202170
- Reuss JE, Anagnostou V, Cottrell TR, et al. Neoadjuvant nivolumab plus ipilimumab in resectable non-small cell lung cancer. J Immunother Cancer. 2020;8:e001282.
- Chansky K, Detterbeck FC, Nicholson AG, et al. The IASCL lung cancer staging project: external validation of the revision of the TNM stage groupings in the eighth edition of the TNM classification of lung cancer. J Thorac Oncol. 2017;12:1109–1121. doi:10.1016/j.jtho.2017.04.011
- Travis WD, Dacic S, Wistuba I, et al. IASCL multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy. J Thorac Oncol. 2020;15:709–740. doi:10.1016/j.jtho.2020.01.005
- Hellmann MD, Chaft JE, William WN, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol. 2014;15:e42–50. doi:10.1016/S1470-2045(13)70334-6
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi:10.1080/00273171.2011.568786
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi:10.1056/NEJMoa1716948
- Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–2350. doi:10.1056/NEJMoa1809697
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi:10.1056/NEJMoa1810865
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi:10.1056/NEJMoa1801005
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi:10.1056/NEJMoa1606774
- Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. 2016;35:5642–5655. doi:10.1002/sim.7084
- Roth JA, Atkinson EN, Fossella F, et al. Long-term follow-up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA Non-small-cell lung cancer. Lung Cancer. 1998;21:1–6. doi:10.1016/S0169-5002(98)00046-4
- Nagai K, Tsuchiya R, Mori T, et al. A randomized trial comparing induction chemotherapy followed by surgery with surgery alone for patients with stage IIIA N2 non-small cell lung cancer (JCOG 9209). J Thorac Cardiovasc Surg. 2003;125:254–260. doi:10.1067/mtc.2003.15
- El Kadmiri MAA, Rajan A. Neoadjuvant immunotherapy for non-small cell lung cancer: can early intervention result in durable clinical benefit? J Thorac Dis. 2018;10:S3203–S6. doi:10.21037/jtd.2018.08.39
- Wu J, Waxman DJ. Immunogenic chemotherapy: dose and schedule dependence and combination with immunotherapy. Cancer Lett. 2018;419:210–221. doi:10.1016/j.canlet.2018.01.050
- Kepp O, Galluzzi L, Martins I, et al. Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metastasis Rev. 2011;30:61–69. doi:10.1007/s10555-011-9273-4
- Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. 2015;3:436–443. doi:10.1158/2326-6066.CIR-15-0064
- Pol J, Vacchelli E, Aranda F, et al. Trial watch: immunogenic cell death inducers for anticancer chemotherapy. Oncoimmunology. 2015;4:e1008866. doi:10.1080/2162402X.2015.1008866
- Qu Y, Emoto K, Eguchi T, et al. Pathologic assessment after neoadjuvant chemotherapy for NSCLC: importance and implications of distinguishing adenocarcinoma from squamous cell carcinoma. J Thorac Oncol. 2019;14:482–493. doi:10.1016/j.jtho.2018.11.017
- Mouillet GME, Milleron B, Puyraveau M, et al. Pathologic complete response to preoperative chemotherapy predicts cure in early-stage non-small-cell lung cancer: combined analysis of two IFCT randomized trials. J Thor Oncol. 2012;7:841–849. doi:10.1097/JTO.0b013e31824c7d92
- Ulas EB, Dickhoff C, Schneiders FL, et al. Neoadjuvant immune checkpoint inhibitors in resectable non-small-cell lung cancer: a systematic review. ESMO Open. 2021;6:100244. doi:10.1016/j.esmoop.2021.100244
- Edelman MJ, Suntharalingam M, Burrows W, et al. Phase I/II trial of hyperfractionated radiation and chemotherapy followed by surgery in stage III lung cancer. Ann Thorac Surg. 2008;86:903–910. doi:10.1016/j.athoracsur.2008.06.022
- Albain KS, Swann RS, Rusch VW, et al. radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374:379–386. doi:10.1016/S0140-6736(09)60737-6
- Si H, Du D, Li W, et al. Sputum-based tumor fluid biopsy: isolation and high-throughput single-cell analysis of exfoliated tumor cells for lung cancer diagnosis. Anal Chem. 2021;93:10477–10486. doi:10.1021/acs.analchem.1c00833
- Qiu F, Fan J, Shao M, et al. Two cycles versus three cycles of neoadjuvant sintilimab plus platinum-doublet chemotherapy in patients with resectable non-small-cell lung cancer (neoSCORE): a randomized, single center, two-arm phase II trial. J Clin Oncol. 2022;40:16. doi:10.1200/JCO.2022.40.16_suppl.8500