Abstract
Objective
The current study is conducted to investigate the potential prognostic value of the age-male-albumin-bilirubin-platelets (aMAP) score in breast cancer patients with liver metastasis after surgery.
Methods
This is a retrospective study of 178 breast cancer patients who developed liver metastasis after surgery. These patients were treated and followed up from 2000 to 2018 at our hospital. The aMAP risk score was estimated in accordance with the following formula: . The optimal cutoff value of the aMAP was evaluated via X-tile. Kaplan-Meier, Log-rank and Cox proportional hazards regression models were applied to determine the clinical influence of the aMAP score on the survival outcomes. The nomogram models were established by multivariate analyses. The calibration curves and decision curve analysis were applied to evaluate the estimated performance of the nomogram models.
Results
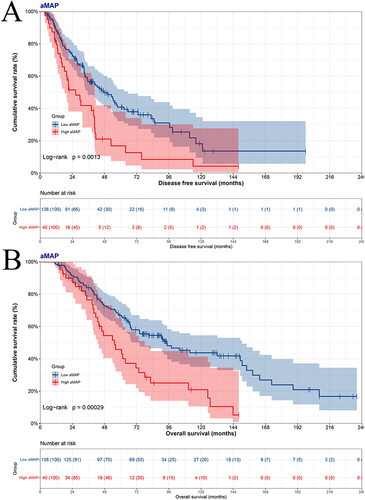
A total of 178 breast cancer patients were divided into low aMAP score group (<47.6) and high aMAP score group (≥47.6) via X-tile plots. The aMAP score was a potential prognostic factor in multivariate analysis. The median disease free survival (p=0.0013) and overall survival (p=0.0003) in low aMAP score group were longer than in high aMAP score group. The nomograms were constructed to predict the DFS with a C-index of 0.722 (95% CI, 0.673–0.771), and the OS with a C-index of 0.708 (95% CI, 0.661–0.755). The aMAP-based nomograms had good predictive performance.
Conclusion
The aMAP score is a potential prognostic factor in breast cancer with liver metastasis after surgery. The aMAP score-based nomograms were conducive to discriminate patients at high risks of liver metastasis and develop adjuvant treatment and prevention strategies.
Introduction
In the era of widespread application of early detection and diagnosis of tumors, breast cancer is the main cause of cancer-related deaths in females in the scope of world.Citation1 The treatment choice for cancer is decided by the stage of the disease.Citation2 For breast cancer, surgical resection and chemotherapy are first-line curative therapies.Citation3 Nevertheless, the two treatments also have a few restrictions.Citation4–6 After this treatment, more than half of breast cancer mortality give rise to distant metastasis.Citation7 Previous research reports have demonstrated that metastasis and recurrence might take its rise from primary tumors as a result of their invasive biological behavior and it was usually related to a variety of factors, such as tumor stage, pathological type, and biomarkers.Citation8–10 Hence, the key to materialize the aspiring global orientation is to diminish the metastasis, recurrence, and mortality of breast cancer.
A sensitive and efficient breast cancer monitoring plan can provide early diagnosis and improve prognosis. We go a step further by establishing an accurate and simple tool to discriminate breast cancer patients with different risks. In the past several years, some biomarkers and risk scores had been tested and verified to assess the risks of cancer progression, such as molecular classification,Citation11 C-reactive protein,Citation12 Naples prognostic score,Citation13 circulating tumor cells,Citation14 PAM50 risk of recurrence (ROR) score.Citation15 Although these indicators were favored by researchers in terms of the prognosis of breast cancer, there were few studies on breast cancer metastasis. For breast cancer, common metastatic sites include liver, lung, brain, and bone.Citation16 It is reported that liver metastasis causes about 20% to 35% of patients with metastatic breast cancer to die.Citation17 Studies have reported that breast cancer patients with liver metastasis had survived less than 9 months under no treatment.Citation18
In recent years, researchers have become more and more interested in creating predictive biomarkers for different cancers based on serological and hematology characteristics. Fan et al reported the age-male-ALBI-platelets (aMAP) score, which based on age, sex, ALBI, and platelets, acted as a novel model for evaluating 5-year liver cancer risk in patients with chronic hepatitis.Citation19 Moreover, albumin-bilirubin (ALBI) grade, based on serum bilirubin and albumin levels, acted as a novel model for objective measure of liver function.Citation20 For aMAP score, it can effectively predict the 5-year liver cancer risk regardless of aetiology or ethnicity. Furthermore, recent studies have performed that aMAP score had prognostic value in other diseases, such as HBV-related acute-on-chronic liver failure.Citation21 Another study performed that aMAP score was a model that predicts risk of hepatocellular carcinoma (HCC) development in patients with chronic hepatitis, and aMAP discrimination was greater for younger individuals.Citation22 As far as we know, the potential prognosis value of aMAP score in evaluating breast cancer, especially for liver metastasis from breast cancer, has not been reported. We presume that the aMAP score may furnish a well-prognostic value for breast cancer patients with liver metastasis after surgery. As a consequence, the current study will go deeply into the potential prognostic value of aMAP score, then, aMAP-based nomograms will apply to predict the probability of breast cancer patients with liver metastasis after surgery.
Patients and Methods
Ethics Approval and Consent to Participate
The Institutional Review Board of Cancer Hospital Chinese Academy of Medical Sciences approved the retrospective study. This retrospective single-center study was conducted in line with the amended Declaration of Helsinki. The enrolled patients provided written informed consent for treatment. The personal information of these patients was kept confidential.
Patients
In the current study, breast cancer with liver metastasis after surgery was treated at our hospital from 2000 to 2018. A total of 178 breast cancer patients with liver metastasis were included in the current study, and the clinical and pathological data of these patients were gained from the electronic medical records. Inclusion requirements were that 1) breast cancer patients with liver metastasis after surgery were confirmed by CT and/or MR when followed up; 2) complete clinical and pathological data and follow-up information. Exclusion requirements were that 1) more than one region; 2) postoperative metastasis of breast cancer to more than one site, such as bone and lungs; 3) missing data and lost to follow up.
Calculation of aMAP Risk Score
aMAP, also known as the age-male-ALBI-platelets (aMAP), were calculated using age, sex, bilirubin, albumin and platelets. In our study, the aMAP risk score was estimated in accordance with the following formula: , referred Fan R’ study.Citation19 The albumin-bilirubin (ALBI) score was estimated in accordance with the following formula: (log10 bilirubin×0.66) - (albumin×0.085), where bilirubin is in μmol/L and albumin in g/L. The optimal cutoff value of aMAP was evaluated via X-tile. In this research, these patients were divided into low aMAP score group (<47.6) vs high aMAP score group (≥47.6).
Follow-Up
All enrolled patients were followed up regularly. The patients had routine checkups with a physical examination, hematology examination, breast and abdominal ultrasound, or CT every 3 months. The treatment and protocol were the same as described previously. Disease free survival (DFS) was defined as the time from surgery to progression with liver metastasis. Overall survival (OS) was defined as the time from surgery to the date of death from any cause or last follow-up.
Statistical Analysis
In this study, the categorical variables were compared using Chi-square test and Fisher’s exact test. X-tile software was applied to assess the optimal cut-off value for the variables. The cumulative DFS and OS were appraised using Kaplan-Meier and Log rank tests. The potential factors were examined using Cox proportional hazards models. Nomograms were constructed by multivariate analysis. The time-dependent receiver operating characteristic curve, concordance index, calibration curve, and decision curve analysis were used to graphically evaluate the accuracy of predictive performance of these models. Statistical analysis was performed using the R (http://www.R-project.org/). The P-value of <0.05 indicated statistical significance.
Results
Clinical Characteristics of the Patients
During this study, a total of 178 breast cancer patients with liver metastasis after surgery were included in the analysis. Patients were grouped into two groups by aMAP score. Then, 138 cases were divided into the low aMAP group, and 40 cases were divided into the high aMAP group. The expression of Ki67 was lower than 14%, divided into negative group. The expression of AR, CK5/6, E-cad, EGFR, P53, and TOP2A was evaluated by immunohistochemistry, and negative expression of these parameters was divided into negative group. As shown in , the demographic data were compared. Age, BMI, menopause, pathological tumor size, pathological TNM stage, and E-cad showed significant differences between the groups.
Table 1 Clinical Characteristics of the Breast Cancer Patients with Liver Metastasis After Surgery Based on aMAP Score
Comparison of Serological and Hematological Characteristics According to aMAP Score
During this study, these serological and hematological indicators were tested before surgery. The median value served as a grouping indicator for these characteristics’ markers. As shown in , the serological and hematological characteristic data of low aMAP group (n=138) and high aMAP group (n=40) were compared. The carcinoembryonic antigen (CEA) and platelet showed significant differences between the groups.
Table 2 Comparison of Serological and Hematological Characteristics According to aMAP Score in Breast Cancer Patients with Liver Metastasis After Surgery
The Potential Prognostic Factors for Breast Cancer Patients with Liver Metastasis After Surgery
In the univariate and multivariate Cox regression analysis, the aMAP, menarche age, C-reactive protein (CRP), monocyte, positive lymph nodes (PLN), PR, lymph vessel invasion were the potential prognostic factors for DFS (); the aMAP, menarche age, albumin (ALB), monocyte, postoperative endocrine therapy were the potential prognostic factors for OS ()
Table 3 The Potential Prognostic Indicators of Disease Free Survival for Breast Cancer Patients with Liver Metastasis After Surgery
Table 4 The Potential Prognostic Indicators of Overall Survival for Breast Cancer Patients with Liver Metastasis After Surgery
Clinical Impact of the aMAP Score on Survival
In accordance with the optimal cut-off value of aMAP score, there were 138 patients in low aMAP score group, and 40 patients in high aMAP score group. First, to probe into the effects of the aMAP score on survival. The median DFS and OS were 47.40 months and 94.60 months in low aMAP score group; 26.43 months and 55.43 months in high aMAP score group (DFS Log rank: p=0.0013, OS Log rank: p=0.0003) ( and ). On the basis of DFS, 1-year survival rate was 0.881 (95% CI, 0.827–0.937), 3-year survival rate was 0.565 (95% CI, 0.482–0.661), 5-year survival rate was 0.408 (95% CI, 0.322–0.518), 10-year survival rate was 0.181 (95% CI, 0.095–0.346), 15-year survival rate was 0.136 (95% CI, 0.058–0.321) in low aMAP score group; and 1-year survival rate was 0.822 (95% CI, 0.710–0.951), 3-year survival rate was 0.418 (95% CI, 0.282–0.620), 5-year survival rate was 0.168 (95% CI, 0.075–0.380), 10-year survival rate was 0.042 (95% CI, 0.006–0.277) in high aMAP score group. In light of OS, 1-year survival rate was 0.986 (95% CI, 0.966–1.000), 3-year survival rate was 0.840 (95% CI, 0.780–0.903), 5-year survival rate was 0.668 (95% CI, 0.594–0.753), 10-year survival rate was 0.437 (95% CI, 0.350–0.545), 15-year survival rate was 0.239 (95% CI, 0.143–0.398) in low aMAP score group; and 1-year survival rate was 1.000 (95% CI, 1.000–1.000), 3-year survival rate was 0.764 (95% CI, 0.641–0.912), 5-year survival rate was 0.458 (95% CI, 0.321–0.652), 10-year survival rate was 0.209 (95% CI, 0.105–0.413) in high aMAP score group.
Model Performance and Validation of the Nomograms
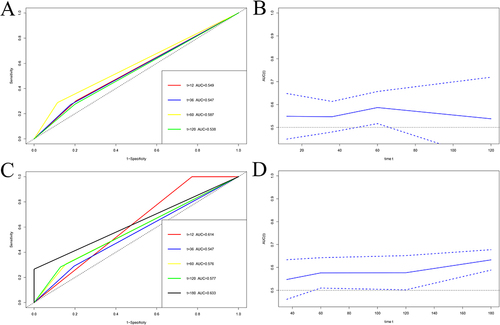
Nomograms were established to determine the DFS and OS probabilities for breast cancer patients with liver metastasis after surgery ( and ). In these nomograms, the potential risk factors for DFS that were determined through Cox multivariate analysis (aMAP, menarche age, C-reactive protein, monocyte, positive lymph nodes, PR, lymph vessel invasion); and for OS that were determined through Cox multivariate analysis (aMAP, menarche age, albumin, monocyte, postoperative endocrine therapy). Every enrolled factor was distributed a score, and the sum of these scores was located on the total score axis to achieve the DFS and OS probability. The C-index for a nomogram-based model based on DFS was 0.722 (95% CI, 0.673–0.771) (Figure S1A). The C-index for a nomogram-based model based on OS was 0.708 (95% CI, 0.661–0.755) (Figure S1B). In addition, the calibration curves of DFS at 1-year, 3-year, and 5-year after operation indicated that the best consistency between the actual and predicted observations (). The calibration curves of OS at 1-year, 3-year, 5-year, and 10-year after operation showed that the best agreement between the actual and predicted observations (). Moreover, the decision curve analysis of DFS at 1-year and 3-year after operation proved that the constructed nomograms had better predictive value than aMAP score (). The decision curve analysis of OS at 3-year and 5-year after operation performed that the established nomograms had better predictive value than aMAP score (). Besides, TDROC analysis showed that areas under the receiver operating characteristic curves (AUROCs) at 1-year, 3-year, 5-year, and 10-year of DFS after operation of follow-up were 0.549, 0.547, 0.587, and 0.538 for the aMAP score and at 1-year, 3-year, 5-year, 10-year, and 15-year of OS after operation of follow-up were 0.614, 0.547, 0.576, 0.577, and 0.633 for the aMAP score. Furthermore, the aMAP score showed that the AUROCs for survival time had the highest value at 5-year point (95% CI: 51.67–65.69%) for DFS ( and ), at 15-year point (95% CI, 58.84–67.74%) for OS ( and ).
Figure 2 The constructed of aMAP-based nomograms in breast cancer patients with liver metastasis after surgery ((A) nomogram of disease free survival; (B) nomogram of overall survival).
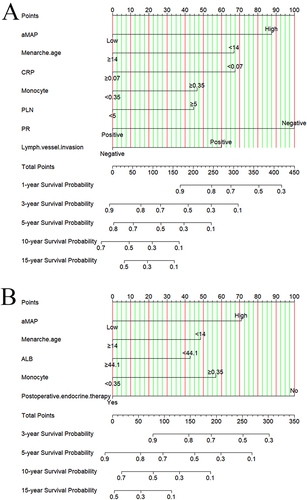
Figure 3 The calibration curves for aMAP-nomogram model in predicting different survival time. (A) 1-year DFS, (B) 3-year DFS, (C) 5-year DFS, (D) 1-year OS, (E) 3-year OS, (F) 5-year OS, (G) 10-year OS.
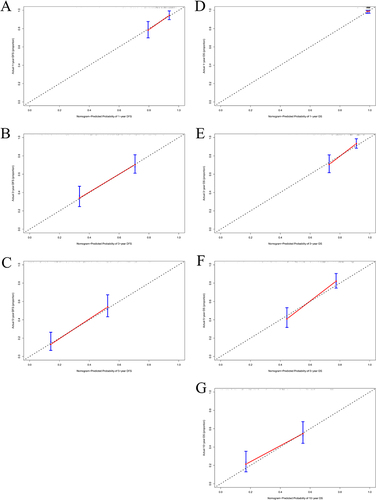
Figure 4 The decision curve analysis for the aMAP-nomogram and aMAP score model in evaluating the benefits for different survival time. (A) 1-year DFS, (B) 3-year DFS, (C) 5-year DFS, (D) 10-year DFS, (E) 3-year OS, (F) 5-year OS, (G) 10-year OS, (H) 15-year OS.
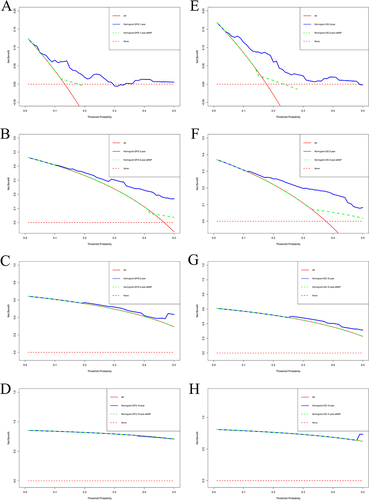
Figure 5 Time-dependent receiver operating characteristic (TDROC) analyzed the plots of area under the receiver operating characteristic curves (AUROCs) for aMAP score in breast cancer patients with liver metastasis after surgery of followup. (A) Time-dependent AUROCs for DFS, (B) 95% CI changes of AUROCs for DFS, (C) Time-dependent AUROCs for OS, (D) 95% CI changes of AUROCs for OS.
Subgroup Analyses to Assess the Effects of the aMAP Score on Survival
Age and platelets constituted part of aMAP score. Whereupon, we conducted a subgroup analysis to assess the impact of aMAP score on survival based on age and platelet. Kaplan–Meier curves indicated that the aMAP score was related to DFS (Log rank: p=0.015) and OS (Log rank: p=0.0022) in age condition (Figure S2A and B). Older patients with breast cancer with high aMAP score had poor prognosis and survival time. Higher age was also correlated with higher aMAP score. Kaplan–Meier curves showed that the aMAP score was connected with DFS (Log rank: p=0.014) and OS (Log rank: p=0.0022) in platelet condition (Figure S3A and B). Patients with high-level platelet and high aMAP score had poor prognosis and survival time. Higher level platelet was also correlated with higher aMAP score. Additionally, we also analyzed the effects of aMAP score on survival based on BMI and menopause. Kaplan–Meier curves showed that the aMAP score was significantly associated with DFS (Log rank: p=0.0016) and OS (Log rank: p=0.0011) in BMI status (Figure S4A and B). Obese breast cancer patients with high aMAP score had poor prognosis and survival time. Kaplan–Meier curves also indicated that the aMAP score was significantly related to DFS (Log rank: p=0.0022) and OS (Log rank: p=0.0011) in BMI status (Figure S5A and B). Premenopausal patients with breast cancer with high aMAP score had poor prognosis and survival time. Moreover, the pathological tumor size and pathological TNM stage were analyzed to evaluate the effects of aMAP score. Kaplan–Meier curves also indicated that the aMAP score was significantly related to DFS (Log rank: p=0.0063) and OS (Log rank: p=0.0028) in different pathological tumor size (Figure S6A and B). Patients with larger tumor sizes and higher aMAP scores had poor prognosis and survival time. Kaplan–Meier curves also showed that the aMAP score was significantly related to DFS (Log rank: p=0.045) and OS (Log rank: p=0.0037) in different pathological TNM stage (Figure S7A and B). Patients with higher TNM stage and high aMAP score had poor prognosis and survival time. Higher TNM stage was also correlated with higher aMAP score. Furthermore, we analyzed the effects of aMAP score on survival based on the E-cad and CEA. Kaplan–Meier curves also indicated that the aMAP score was significantly related to DFS (Log rank: p=0.0011) and OS (Log rank: p=0.00074) in E-cad status (Figure S8A and B). Patients with positive expression of E-cad and high aMAP score had poor prognosis and survival time. Kaplan–Meier curves also showed that the aMAP score was significantly related to DFS (Log rank: p=0.014) and OS (Log rank: p=0.0039) in CEA level (Figure S9A and B). Patients with high level of CEA and high aMAP score had poor prognosis and survival time.
Discussion
High incidence of tumor recurrence and metastasis to critical organs is a critical factor affecting long-term survival of breast cancer patients after surgery.Citation23,Citation24 In the past few years, many studies have shown that breast cancer exhibited different heterogeneity in metastasis and the priority of metastasis varies among different organs, leading to different prognosis and treatment responses in breast cancer patients.Citation16,Citation23,Citation25 For instance, ductal carcinoma in situ (DCIS) is a heterogeneous disease and only some patients will progress to invasive breast cancer. However, patients with DCIS after breast-conserving surgery had clinically significant recurrence rates, and approximately half of these cases will be life-threatening invasive recurrences.Citation26 Histological subtype has emerged as an important tool in predicting prognosis for breast cancer and has been shown to correlate with differences in survival with invasive micropapillary carcinoma groups having poor outcomes.Citation27,Citation28 The bony skeleton, lung, liver, and brain are the primary targets of breast cancer metastasis.Citation29,Citation30 For breast cancer, the liver is the third most common metastatic site for solid cancer followed by lung and bony skeleton metastases, and accounts for 20% to 35% of the deaths of metastatic breast cancer patients.Citation31 Research also indicated that adipokines were related to proliferation, invasion, and metastasis of breast cancer cells, and were also associated with the prognosis of breast cancer.Citation32 Moreover, it is important to note that breast cancer patients with liver metastasis exhibited drug resistance and eventually develop resistance to systemic chemotherapy, endocrine therapy, and targeted therapy.Citation33–36 Nevertheless, study had performed that the survival time for breast cancer patients with liver metastasis is only 4–8 months without treatment.Citation37,Citation38 Recently, it is difficult to accurately predict the occurrence of liver metastasis from tumors. Some markers or scores are connected with the prognosis of breast cancer patients; however, it is not clear whether these indicators reflect prognosis in survival time.Citation39–41
Fan et al developed an objective and accurate hepatocellular carcinoma (HCC) surveillance programme tool (called the aMAP score) could predict the risk of HCC development and offer early diagnosis.Citation19 The aMAP score involves five factors, including age, sex, albumin, bilirubin, and platelets.Citation19 Gui et al also performed that the aMAP score accurately predicted the risk of HCC in at-risk patients with compensated cirrhosis undergoing antiviral therapy.Citation42 Although the aMAP score has been confirmed in some studies and proven to be the best performing liver cancer forecasting model, its role has not been determined in other tumors, such as breast cancer.Citation42,Citation43
In this study, we are attempting to explore the application of aMAP score on breast cancer patients with liver metastasis after surgery. The results showed that aMAP score was a potential prognostic factor for DFS and OS in breast cancer patients with liver metastasis after surgery. The results indicated that the aMAP score could furnish a well-discriminated risk stratification for liver metastasis after surgery as the low aMAP score group (median DFS time: 47.40 months, median OS time: 94.60 months) and high aMAP score group (median DFS time: 26.43 months, median OS time: 55.43 months). Chen et al found that the median OS time in the low-risk aMAP group was 26.2 months, in the medium-risk aMAP group was 23.3 months, and in the high-risk group was 10.4 months for HCC; and the Kaplan–Meier curve showed that aMAP score had a remarkable effect on OS (p < 0.0001).Citation44 Other study also indicated that the aMAP score was a detached risk factor for HBV-related hepatocellular carcinoma, and the high-risk group was related to the worst RFS and OS compared with medium-risk and low-risk groups.Citation45 Our findings were consistent with previous reports, and we also explored the clinical impact of aMAP score on patients with liver metastasis after breast cancer surgery.
In the current study, the aMAP score was noticeably associated with age, BMI, menopause, pathological tumor size, pathological TNM stage, E-cad, CEA, and platelet ( and ). In Wang R’s study, the multivariable analyses indicated that age was a detached prognostic factor for stage IV breast cancer patients with different metastatic sites, and the patients with liver metastasis were the lowest than those with other metastasis sites.Citation46 Our study also indicated that age was a potential prognostic factor for breast cancer with liver metastasis after surgery, and the older patients with high aMAP score had poor prognosis and survival time. A study had shown that obese breast patients tended to have large tumor size compared with normal weight breast cancer patients, whereas there was no significant difference of DFS between overweight and obese and normal weight premenopausal patients.Citation47 Obese breast cancer patients with high aMAP score had poor prognosis and survival time in our study. For menopause, postmenopausal estrogen activates EMT gene to stimulate breast cancer metastasis.Citation48 Our results also showed that premenopausal breast cancer patients with high aMAP score had poor prognosis and survival time. Furthermore, patients with larger tumor size and higher tumor staging had worse prognosis. Many studies indicated that TNM stage and tumor size were associated with the prognosis of survival time.Citation49–51 In various cancers, increased levels of E-cad have been found, and E-cad correlated dramatically with TNM stage, tumor grade, and lymph node metastasis; furthermore, E-cad level was a potential prognostic factor in Asian breast cancer patients.Citation52–54 In our study, patients with positive expression of E-cad and high aMAP score had poor prognosis and survival time. One study has shown that CEA can be used in the diagnosis of metastatic breast cancer, and different combinations of tumor markers had different diagnostic values.Citation55 In the current study, patients with high level of CEA and high aMAP score had poor prognosis and survival time. Garmi et al performed the study to show that platelets might be a prognostic marker for breast cancer and that patients who died in 1 year had low platelet counts before treatment.Citation56 Patients with high-level platelet and high aMAP score had poor prognosis and survival time in our study.
At the same moment, we also constructed aMAP-based nomograms to evaluate breast cancer patients with liver metastasis after surgery. The discriminated and calibrated nomograms based on potential prognostic factors of breast cancer liver metastasis with a C-index of 0.722 (95% CI, 0.673–0.771) to predict the probability of DFS, and 0.708 (95% CI, 0.661–0.755) to predict the probability of OS. Moreover, the calibration curves at 1-year, 3-year, and 5-year after surgery showed the best consistency between the actual and predicted observations. Decision curve analysis at 1-year and 3-year after surgery indicated that the constructed nomograms had better predictive value than aMAP score. Furthermore, the TDROC analysis performed showed that the AUROCs for aMAP score had the highest value at 5-year point for DFS and 15-year point for OS. These models may contribute to individualized risk stratification.
Notwithstanding, our study also has a few limitations. Firstly, this research was a retrospective study with small sample size, which may be potentially subject to selection bias. Secondly, our study requires prospective cohort studies to evaluate the prognostic accuracy of aMAP score in breast cancer. Finally, the established nomogram models should be performed for further validation.
Conclusion
In conclusion, aMAP score has a high prognostic ability for DFS and OS in breast cancer with liver metastasis after surgery. The aMAP score is a risk score that helps simple, particularity, dependable prediction of the risk of breast cancer with liver metastasis after surgery. The constructed nomogram models are conducive to discriminate breast cancer patients at high risks of liver metastasis.
Institutional Review Board Statement
This retrospective single-center study was conducted in accordance with the amended Declaration of Helsinki and was approved by the ethics committee of Cancer Hospital Chinese Academy of Medical Sciences (No.82173328).
Data Sharing Statement
The material supporting the conclusion of this article has been included within the article.
Informed Consent Statement
The enrolled patients provided written informed consent for using their data in this retrospective study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflict of interest.
Additional information
Funding
References
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi:10.3322/caac.21763
- Walsh EM, Nunes R, Wilkinson MJ, Santa-Maria CA. Extended endocrine therapy for early-stage breast cancer: how do we decide? Curr Oncol Rep. 2020;22(12):123. doi:10.1007/s11912-020-00988-7
- Wöckel A, Albert US, Janni W, Scharl A, Kreienberg R, Stüber T. The screening, diagnosis, treatment, and follow-up of breast cancer. Dtsch Arztebl Int. 2018;115(18):316–323. doi:10.3238/arztebl.2018.0316
- Tollan CJ, Pantiora E, Valachis A, Karakatsanis A, Tasoulis MK. A systematic review and meta-analysis on the role of repeat breast-conserving surgery for the management of ipsilateral breast cancer recurrence. Ann Surg Oncol. 2022;29(10):6440–6453. doi:10.1245/s10434-022-12197-6
- Giuliano M, Schettini F, Rognoni C, et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: a systematic review and network meta-analysis. Lancet Oncol. 2019;20(10):1360–1369. doi:10.1016/S1470-2045(19)30420-6
- Trapani D, Gandini S, Corti C, et al. Benefit of adjuvant chemotherapy in patients with lobular breast cancer: a systematic review of the literature and metanalysis. Cancer Treat Rev. 2021;97:102205. doi:10.1016/j.ctrv.2021.102205
- Padmanaban V, Krol I, Suhail Y, et al. E-cadherin is required for metastasis in multiple models of breast cancer. Nature. 2019;573(7774):439–444. doi:10.1038/s41586-019-1526-3
- Castaneda M, den Hollander P, Kuburich NA, Rosen JM, Mani SA. Mechanisms of cancer metastasis. Semin Cancer Biol. 2022;87:17–31. doi:10.1016/j.semcancer.2022.10.006
- Lv H, Niu L, Zhang M, Zeng H, Zhao S, Yan M. Diagnostic diversity and heterogeneity of tumors: a real-world study of metastasis re-biopsy in advanced breast cancer. Chin Med J. 2022;135(17):2076–2082. doi:10.1097/CM9.0000000000001969
- Kavan S, Kruse TA, Vogsen M, Hildebrandt MG, Thomassen M. Heterogeneity and tumor evolution reflected in liquid biopsy in metastatic breast cancer patients: a review. Cancer Metastasis Rev. 2022;41(2):433–446. doi:10.1007/s10555-022-10023-9
- Jiang YZ, Ma D, Suo C, et al. Genomic and transcriptomic landscape of triple-negative breast cancers: subtypes and treatment strategies. Cancer Cell. 2019;35(3):428–440.e5. doi:10.1016/j.ccell.2019.02.001
- Mikkelsen MK, Lindblom NAF, Dyhl-Polk A, Juhl CB, Johansen JS, Nielsen D. Systematic review and meta-analysis of C-reactive protein as a biomarker in breast cancer. Crit Rev Clin Lab Sci. 2022;59(7):480–500. doi:10.1080/10408363.2022.2050886
- Chen Y, Guan Z, Shen G. Naples prognostic score: a novel predictor of survival in patients with HER2-positive breast cancer. Future Oncol. 2022;18(21):2655–2665. doi:10.2217/fon-2022-0212
- Chantzara E, Xenidis N, Kallergi G, Georgoulias V, Kotsakis A. Circulating tumor cells as prognostic biomarkers in breast cancer: current status and future prospects. Expert Rev Mol Diagn. 2021;21(10):1037–1048. doi:10.1080/14737159.2021.1962710
- Lænkholm AV, Jensen MB, Eriksen JO, et al. PAM50 risk of recurrence score predicts 10-year distant recurrence in a comprehensive Danish cohort of postmenopausal women allocated to 5 years of endocrine therapy for hormone receptor-positive early breast cancer. J Clin Oncol. 2018;36(8):735–740. doi:10.1200/JCO.2017.74.6586
- Liang Y, Zhang H, Song X, Yang Q. Metastatic heterogeneity of breast cancer: molecular mechanism and potential therapeutic targets. Semin Cancer Biol. 2020;60:14–27. doi:10.1016/j.semcancer.2019.08.012
- Yang A, Xiao W, Zheng S, et al. Predictive nomogram of subsequent liver metastasis after mastectomy or breast-conserving surgery in patients with nonmetastatic breast cancer. Cancer Control. 2021;28:1073274821997418. doi:10.1177/1073274821997418
- Zuo Q, Park NH, Lee JK, Madak Erdogan Z. Liver metastatic breast cancer: epidemiology, dietary interventions, and related metabolism. Nutrients. 2022;14(12):2376. doi:10.3390/nu14122376
- Fan R, Papatheodoridis G, Sun J, et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J Hepatol. 2020;73(6):1368–1378. doi:10.1016/j.jhep.2020.07.025
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-The ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi:10.1200/JCO.2014.57.9151
- Sun Y, Li Z, Liao G, et al. aMAP score as a predictor for long-term outcomes in patients with HBV-related acute-on-chronic liver failure. Int J Gen Med. 2022;15:407–415. doi:10.2147/IJGM.S343457
- Johnson PJ, Innes H, Hughes DM, Kalyuzhnyy A, Kumada T, Toyoda H. Evaluation of the aMAP score for hepatocellular carcinoma surveillance: a realistic opportunity to risk stratify. Br J Cancer. 2022;127(7):1263–1269. doi:10.1038/s41416-022-01851-1
- Yeeravalli R, Das A. Molecular mediators of breast cancer metastasis. Hematol Oncol Stem Cell Ther. 2021;14(4):275–289. doi:10.1016/j.hemonc.2021.02.002
- Demicheli R, Dillekås H, Straume O, Biganzoli E. Distant metastasis dynamics following subsequent surgeries after primary breast cancer removal. Breast Cancer Res. 2019;21(1):57. doi:10.1186/s13058-019-1139-7
- Li X, Huang R, Ma L, Liu S, Zong X. Locoregional surgical treatment improves the prognosis in primary metastatic breast cancer patients with a single distant metastasis except for brain metastasis. Breast. 2019;45:104–112. doi:10.1016/j.breast.2019.03.006
- Akrida I, Mulita F. The clinical significance of HER2 expression in DCIS. Med Oncol. 2022;40(1):16. doi:10.1007/s12032-022-01876-9
- Verras GI, Mulita F, Tchabashvili L, et al. A rare case of invasive micropapillary carcinoma of the breast. Prz Menopauzalny. 2022;21(1):73–80. doi:10.5114/pm.2022.113834
- Verras GI, Tchabashvili L, Mulita F, et al. Micropapillary breast carcinoma: from molecular pathogenesis to prognosis. Breast Cancer. 2022;14:41–61. doi:10.2147/BCTT.S346301
- Yousefi M, Nosrati R, Salmaninejad A, Dehghani S, Shahryari A, Saberi A. Organ-specific metastasis of breast cancer: molecular and cellular mechanisms underlying lung metastasis. Cell Oncol. 2018;41(2):123–140. doi:10.1007/s13402-018-0376-6
- Liu H, Li X, Li H, et al. Potential molecular mechanisms and clinical progress in liver metastasis of breast cancer. Biomed Pharmacother. 2022;149:112824. doi:10.1016/j.biopha.2022.112824
- Cummings MC, Simpson PT, Reid LE, et al. Metastatic progression of breast cancer: insights from 50 years of autopsies. J Pathol. 2014;232(1):23–31. doi:10.1002/path.4288
- Verras GI, Tchabashvili L, Chlorogiannis DD, Mulita F, Argentou MI. Updated Clinical Evidence on the Role of Adipokines and Breast Cancer: a Review. Cancers. 2023;15(5):1572. doi:10.3390/cancers15051572
- Fisusi FA, Akala EO. Drug Combinations in Breast Cancer Therapy. Pharm Nanotechnol. 2019;7(1):3–23. doi:10.2174/2211738507666190122111224
- Ferrari P, Scatena C, Ghilli M, Bargagna I, Lorenzini G, Nicolini A. Molecular mechanisms, biomarkers and emerging therapies for chemotherapy resistant TNBC. Int J Mol Sci. 2022;23(3):1665. doi:10.3390/ijms23031665
- Hanker AB, Sudhan DR, Arteaga CL. Overcoming Endocrine Resistance in Breast Cancer. Cancer Cell. 2020;37(4):496–513. doi:10.1016/j.ccell.2020.03.009
- Bai X, Ni J, Beretov J, Graham P, Li Y. Triple-negative breast cancer therapeutic resistance: where is the Achilles’ heel? Cancer Lett. 2021;497:100–111. doi:10.1016/j.canlet.2020.10.016
- Rashid NS, Grible JM, Clevenger CV, Harrell JC. Breast cancer liver metastasis: current and future treatment approaches. Clin Exp Metastasis. 2021;38(3):263–277. doi:10.1007/s10585-021-10080-4
- Adam R, Aloia T, Krissat J, et al. Is liver resection justified for patients with hepatic metastases from breast cancer? Ann Surg. 2006;244(6):897–908. doi:10.1097/01.sla.0000246847.02058.1b
- Jaskulski S, Jung AY, Behrens S, et al. Circulating enterolactone concentrations and prognosis of postmenopausal breast cancer: assessment of mediation by inflammatory markers. Int J Cancer. 2018;143(11):2698–2708. doi:10.1002/ijc.31647
- Gunnarsdottir FB, Bendahl PO, Johansson A, et al. Serum immuno-oncology markers carry independent prognostic information in patients with newly diagnosed metastatic breast cancer, from a prospective observational study. Breast Cancer Res. 2023;25(1):29. doi:10.1186/s13058-023-01631-6
- Bownes RJ, Turnbull AK, Martinez-Perez C, Cameron DA, Sims AH, Oikonomidou O. On-treatment biomarkers can improve prediction of response to neoadjuvant chemotherapy in breast cancer. Breast Cancer Res. 2019;21(1):73. doi:10.1186/s13058-019-1159-3
- Gui H, Huang Y, Zhao G, et al. External Validation of aMAP Hepatocellular Carcinoma Risk Score in Patients With Chronic Hepatitis B-Related Cirrhosis Receiving ETV or TDF Therapy. Front Med. 2021;8:677920. doi:10.3389/fmed.2021.677920
- Shiha G, Mikhail N, Soliman R. External validation of aMAP risk score in patients with chronic hepatitis C genotype 4 and cirrhosis who achieved SVR following DAAs. J Hepatol. 2021;74(4):994–996. doi:10.1016/j.jhep.2020.10.008
- Chen Y, Shi Y, Lu L, et al. The aMAP Score is an independent risk factor for intermediate-stage hepatocellular carcinoma: a large retrospective cohort study. J Cancer. 2023;14(8):1272–1281. doi:10.7150/jca.79377
- Xin Y, Zhang X, Yang Y, et al. Prediction of late recurrence after radiofrequency ablation of HBV-related hepatocellular carcinoma with the age-male-albumin-bilirubin-platelets (aMAP) risk score: a multicenter study. J Gastrointest Oncol. 2021;12(6):2930–2942. doi:10.21037/jgo-21-506
- Wang R, Zhu Y, Liu X, Liao X, He J, Niu L. The Clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Cancer. 2019;19(1):1091. doi:10.1186/s12885-019-6311-z
- Wang K, Wu YT, Zhang X, et al. Clinicopathologic and Prognostic Significance of Body Mass Index (BMI) among breast cancer patients in western china: a retrospective multicenter cohort based on Western China Clinical Cooperation Group (WCCCG). Biomed Res Int. 2019;2019:3692093. doi:10.1155/2019/3692093
- Qureshi R, Picon-Ruiz M, Sho M, et al. Estrone, the major postmenopausal estrogen, binds ERa to induce SNAI2, epithelial-to-mesenchymal transition, and ER+ breast cancer metastasis. Cell Rep. 2022;41(7):111672. doi:10.1016/j.celrep.2022.111672
- Fouad TM, Barrera AMG, Reuben JM, et al. Inflammatory breast cancer: a proposed conceptual shift in the UICC-AJCC TNM staging system. Lancet Oncol. 2017;18(4):e228–e232. doi:10.1016/S1470-2045(17)30192-4
- Fan R, Chen Y, Nechuta S, et al. Prediction models for breast cancer prognosis among Asian women. Cancer. 2021;127(11):1758–1769. doi:10.1002/cncr.33425
- Liu Y, He M, Zuo WJ, Hao S, Wang ZH, Shao ZM. Tumor size still impacts prognosis in breast cancer with extensive nodal involvement. Front Oncol. 2021;11:585613. doi:10.3389/fonc.2021.585613
- Liang Z, Sun XY, Xu LC, Fu RZ. Abnormal expression of serum soluble E-cadherin is correlated with clinicopathological features and prognosis of breast cancer. Med Sci Monit. 2014;20:2776–2782.
- Chang K, Jiang L, Sun Y, Li H. Effect of E-cadherin on prognosis of colorectal cancer: a meta-analysis update. Mol Diagn Ther. 2022;26(4):397–409. doi:10.1007/s40291-022-00593-3
- Zhai X, Zhu H, Wang W, Zhang S, Zhang Y, Mao G. Abnormal expression of EMT-related proteins, S100A4, vimentin and E-cadherin, is correlated with clinicopathological features and prognosis in HCC. Med Oncol. 2014;31(6):970. doi:10.1007/s12032-014-0970-z
- Wang W, Xu X, Tian B, et al. The diagnostic value of serum tumor markers CEA, CA19-9, CA125, CA15-3, and TPS in metastatic breast cancer. Clin Chim Acta. 2017;470:51–55. doi:10.1016/j.cca.2017.04.023
- Garmi N, Nasrallah S, Baram Y, et al. Platelets and breast cancer. Isr Med Assoc J. 2020;22(10):613–617.