Abstract
Objective
Serum-specific antibodies as a non-invasive means to effectively diagnose idiopathic membranous nephropathy and assess clinicopathology.
Methods
Immunofluorescence of anti-PLA2R and THSD7A antibodies and kidney tissue PLA2R, THSD7A and IgG4 expression in IMN and non-IMN (2020–2021) was detected to assess the efficacy of diagnosing IMN. IMN patients were divided into two groups, anti-PLA2R antibody positive (161 cases) and negative (26 cases), and two groups, kidney tissue PLA2R (40 cases) and PLA2R+THSD7A (6 cases), to compare the clinical and pathological features, and to carry out a prognostic analysis of THSD7A-positive patients, with a focus on correlation with malignancy.
Results
The positive rate of anti-PLA2R antibodies was significantly higher in IMN (P<0.05); anti-PLA2R antibodies, kidney tissue PLA2R and IgG4 and THSD7A had some diagnostic value. Anti-PLA2R antibodies correlated with proteinuria levels in IMN patients, and their levels were negatively correlated with blood albumin (r=−0.146, P=0.042); correlated with pathological stage and C3 and IgG4 immunodeposition; there was no significant difference in clinical pathology between kidney tissue THSD7A+PLA2R positive compared to kidney tissue PLA2R positive patients, but the probability of achieving complete remission was low and time longer, and no malignancy events were detected during follow-up.
Conclusion
Anti-PLA2R antibodies, kidney tissue PLA2R, THSD7A and IgG4 have high diagnostic efficacy for IMN; anti-PLA2R antibodies can be used as diagnostic markers to assist in the assessment of clinical and pathological features; co-expression of kidney tissue PLA2R and THSD7A is not significantly different from kidney tissue PLA2R in assessing the clinical features, pathological manifestations and prognosis, but requires long-term. However, long-term follow-up is needed to monitor the potential risk, and a larger multicentre study with long-term follow-up is expected to be conducted to comprehensively assess IMN characteristics.
Introduction
Idiopathic membranous nephropathy (MN) is the most common pathological type of nephrotic syndrome, accounting for approximately 13.3% of primary glomerular disease,Citation1 with a yearly trend of increase.Citation2 One third of patients with membranous nephropathy will achieve self-remission, one third of IMN patients will develop persistent proteinuria, and one third will progress to kidney failure. In 2009, Beck et alCitation3 found that the M-type phospholipase A2 receptor (PLA2R) coexisted with the IgG4 subtype in the glomerular immune deposits of IMN patients. In 2014, Tomas et alCitation4 found that 3–4% of the patients with PLA2R-negative MN had positive Thrombospondin type-1 domain-containing7 A (THSD7A) in the kidney tissue. This was accompanied by positive antibodies in the blood. Previous studies have demonstrated the usefulness of anti-PLA2R antibodies for the diagnostic efficacy and assessment of IMN,Citation5 but there is a lack of assessment of the diagnostic efficacy and relevance of the combination of anti-PLA2R and THSD7A antibodies to the clinical features and pathology of IMN; the association between THSD7A-associated membranous nephropathy and malignancy has been neglected and is less well reported.Citation6
Based on these considerations, this study analysed the clinical and histological characteristics of 195 cases of PLA2R-related and THSD7A-related IMN from 2020 to 2021, assessing the diagnostic effectiveness of antibodies in kidney tissues and blood-related antibodies, focusing on the prognosis of THSD7A-related membranous nephropathy, with a focus on the association of THSD7A with malignancy.
Materials and Methods
Inclusion and Exclusion Criteria
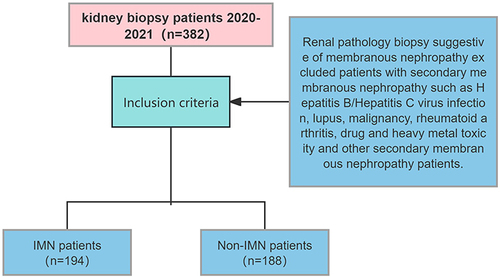
From January 2020 to December 2021, 194 patients were hospitalized at the Department of Nephrology, Beng Medical First Affiliated Hospital with kidney biopsy definite IMN; 188 patients with non-IMN, including 5 cases of lupus membranous nephritis, 6 cases of secondary membranous nephropathy, 98 cases of IgA nephropathy, 5 cases of proliferative glomerulonephritis, 14 cases of focal segmental glomerulosclerosis, 40 cases of podocytosis, 7 cases of metabolic-associated nephropathy, including diabetic nephropathy and obesity-related kidney disease. Hepatitis B-associated nephropathy in 1 case, and tumour-associated kidney damage (including kidney amyloidosis, light chain deposition disease and monoclonal immunoglobulinemia) in 12 cases. The study was approved by the Ethics Committee of the First Affiliated Hospital of Bengbu Medical College (Lunke Approval No. 117 [2020]) and written informed consent was obtained from all participants. The study was in accordance with the Declaration of Helsinki.
Clinical Features
Various clinical information was obtained at the time of the patient’s kidney biopsy, including age, sex and weight; blood and fluid markers, including albumin blood creatinine, uric acid, lipids, 24 h urine protein volume, eGFR (calculated by the CKD-EPI formula).
Circulating anti-PLA2R antibodies and anti-THSD7A antibodies
PLA2R antibody levels were measured using a double antibody sandwich assay, following the kit instructions. Serum PLA2R antibody kit ≤ 14 RU/mL is considered negative. Serum THSD7A antibody was detected by indirect immunofluorescence and a non-specific fluorescence reaction at a serum dilution < 1:10 was defined as negative.
Kidney Pathology
Kidney pathological specimens were examined by light microscopy, immunofluorescence (IF) and electron microscopy (EM). Light microscopy was performed using 3um paraffin-embedded sections stained with hematoxylin eosin (H&E), Jones methyleneamine silver, Masson trichrome and periodate-Schiff’s reagent. Grade 0: no hyperplasia; Grade 1: mild hyperplasia; Grade 2: moderate hyperplasia; Grade 3: severe hyperplasia. Acute kidney tubular interstitial lesions include flattening of tubular epithelial cells, detachment of brush border and infiltration of interstitial inflammatory cells in non-tubular atrophic areas; kidney parenchymal tubular atrophy/interstitial fibrosis was classified as absent (T0), mild (T1) <25%, moderate (T2) 25% to 50% and severe (T3) >50%.Citation7 Direct immunofluorescence method: fresh kidney tissue specimens were embedded in OCT, made into frozen sections and detected by semi-quantitative methods according to fluorescence intensity (0 to 4+) for IgG (IgG1; IgG2; IgG3; IgG4); IgA; IgM; C3; C1q; PLA2R; THSD7A. Electron microscopy was performed for pathological staging with reference to the criteria of Ehrenreich and Churg.Citation8
Treatment and Follow-Up
The use of steroids and immunosuppressants in our centre is in accordance with the 2012 KDIGO. Efficacy determination: clinical remission, including complete and partial remission, using the 2012 Kidney Disease Improvement Global Prognosis Organization (KDIGO) guideline criteria: (1) complete remission (CR): 24 h urine protein quantification <0.3 g, serum albumin >35 g/L, and normal blood creatinine are required. (2) Partial remission (PR): 24 h urine protein quantification <3.5 g and >50% decrease, improved serum albumin and stable creatinine; (3) No remission (NR) means that the above criteria are not met. Follow-up data Serum albumin, 24-h urine protein, blood creatinine and eGFR were recorded during the follow-up period.
Statistical Analysis
Statistical software was SPSS 25.0. Measurement data were expressed as (x ± s) and t-test was used for comparison between groups. Count data were expressed as number of cases (percentage) and χ2 test was used for comparison between groups. Rank data were described as number of cases (percentage) and rank sum test was used for comparison between groups. The predictive power of the variables of interest for IMN was assessed using the subject ROC curve and the area under the curve. COX risk proportional regression models were used for univariate and multifactorial survival analysis, and KM survival curves and Logrank tests were used to assess differences in prognosis between subgroups for categorical indicators. Differences were considered statistically significant at P<0.05.
Results
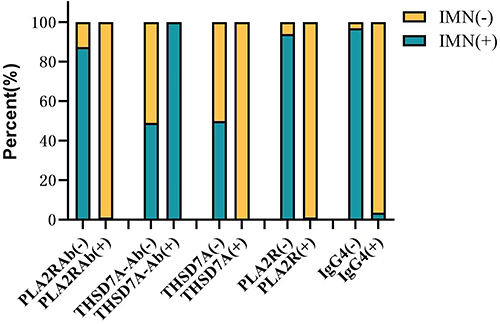
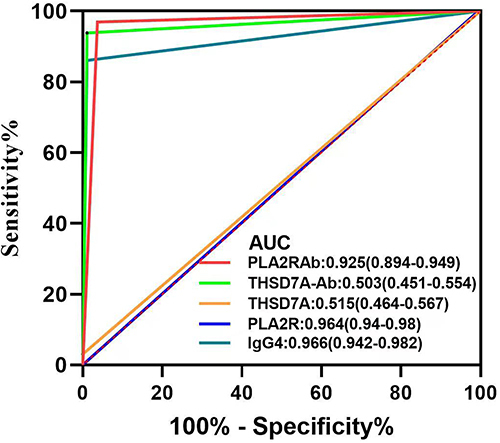
Comparison of serum anti-PLA2R antibody, anti-THSD7A antibody and glomerular PLA2R, THSD7A and IgG4 positivity rates and evaluation of their efficacy in diagnosing IMN
Serum anti-PLA2R antibodies, kidney tissue and PLA2R, THSD7A and IgG4 were distributed in significantly different proportions in the two groups of patients, with statistically significant differences. The AUC value for THSD7A in the ROC curve was 0.515, with low diagnostic efficacy but high diagnostic specificity (100%). (as shown in and )
Table 1 ROC Diagnostic Curves and Comparison of IMN and Non-IMN Indicators
Comparison of clinical features and pathological characteristics of IMN patients in the anti-PLA2R antibody-positive and negative groups
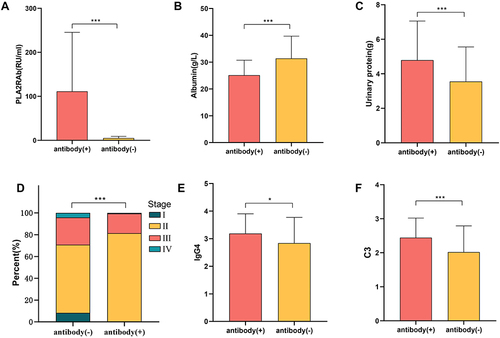
In the study, we found significant differences in PLA2RAb, Albumin and Urinary protein in antibody positive patients compared to the negative group. Also, in the positive group, patients had enhanced immunofluorescence of C3 and IgG4 in kidney tissue; the pathological staging was severe (P<0.01); while no significant differences were found in glomerular proliferation, acute tubular lesions and tubular atrophic fibrosis (P>0.05). No significant differences were found in the remaining pathological features. (shown in ) and )
Table 2 Analysis of Clinical Features and Pathological Characteristics of Antibody Positives and Negatives
Figure 4 (A) PLA2R antibody titers in the two groups of anti-PLA2R positive and negative patients; (B) blood albumin levels in the two groups of anti-PLA2R positive and negative patients; (C) 24h urine protein levels in the two groups of anti-PLA2R positive and negative patients; (D) percentage of pathological stages in the two groups of anti-PLA2R positive and negative patients; (E) immunofluorescence of renal IgG4 in the two groups of anti-PLA2R positive and negative patients (F)comparison of immunofluorescence of renal C3 in two groups of patients with positive and negative anti-PLA2R.
Comparison of clinical features, pathological characteristics and follow-up indicators of PLA2R-positive and PLA2R+THSD7A-positive kidney tissue patients
Patients were randomly selected for analysis using the caret package in the R software because of the large differences in their proportions. There were no significant differences in the clinical indicators ALB and UTP at the time of kidney biopsy between the two groups; there were no significant differences in the pathological features (shown in ).
Table 3 Comparison of Clinical Case Indicators Between Groups of Double Antibody Negatives and Positives
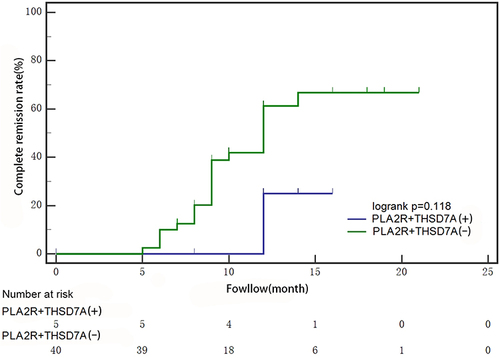
Survival analysis was performed on the follow-up records of the two groups, there were 6 cases in the kidney tissue PLA2R+THSD7A-positive group, with one case of loss of follow-up, and the median follow-up time was 12 months (IQR:10,15); there were 40 cases in the kidney tissue PLA2R+THSD7A-negative group, with no one loss of follow-up, and the median follow-up time was 12 months (IQR:10,16). After defining whether patients reached complete remission as the dependent variable, we found that blood creatinine level (mg/dL), blood creatinine level at the most recent follow-up (mg/dL), and serum protein level at the most recent follow-up (x±s, g/L) were all significantly associated with the time it took for patients to reach complete remission (see ). Subsequently, incorporating these three variables into a multivariate analysis, we identified the serum protein level at the most recent follow-up as an independent predictor of complete remission achievement (for detailed results, refer to ).
Table 4 Univariate Analysis of Factors Affecting Complete Remission in Patients
Table 5 Multifactorial Analysis of Factors Affecting Complete Remission in Patients
When constructing KM survival curves for both groups, we found that patients who were double antibody positive were less likely to achieve complete remission and for longer than those who were negative for PLA2R antibodies alone, but the difference was not statistically significant. (p=0.118) (shown in ).
Six patients with positive THSD7A kidney tissue presented with nephrotic syndrome. Neither serum tumour-related markers nor imaging at the time of kidney puncture suggested a malignancy event. Five patients were treated with glucocorticoids (GCs) combined with cyclophosphamide. One patient was treated with glucocorticoids (GCs) combined with a calcineurin inhibitor. One patient was missed at follow-up and for the remaining 5 patients, 1 patient had a CR outcome, 2 patients had a PR outcome, and 2 patients had an NR outcome with no malignancy events at follow-up (based on patient symptoms, blood, body fluids and chest CT).
Discussion
Membranous nephropathy has become increasingly prevalent in recent years and is of interest to researchers. Although kidney biopsy is the gold standard for diagnosis, some patients are not eligible for kidney biopsy due to contraindications, so serum diagnostic markers play an important role in the diagnosis.Citation9 Foreign studiesCitation10–12 found that the positive rate of anti-PLA2R antibodies in IMN patients from different countries ranged from 52.6% to 72.3, and the positive rate of glomerular PLA2R deposition ranged from 50% to 83.3%. A current domestic study on 572 IMN patients reported a 68.5% anti-PLA2R antibody positivity rate and 89.9% glomerular PLA2R positive expression rate.Citation13 In our study, we found that the antibody positivity rate in IMN patients was 86.1%, and the kidney tissue PLA2R and IgG4 positivity rates were 93.8% and 96.9%, with a higher expression of positivity rates relative to previous studies, probably because first: some patients presented with nephrotic syndrome on admission, and the expression of tissue PLA2R and serum antibodies correlated with proteinuria in membranous nephropathy, and therefore higher levels of expression,Citation14 which is consistent with the findings of Qin et al; furthermore, most of the patients enrolled were first diagnosed, not receiving immunosuppressive therapy, and expression levels were not affected by drugs; also we defined anti-PLA2R antibodies greater than 14 RU/mL as positive, whereas previous studies mostly used 20 RU/mL as the cut-off value,Citation15 with a higher sensitivity of the test. Finally, there may be differences in genetics or environmental factors between Asia and other countries and these may contribute to differences in results.
In our study, we found that the sensitivity and specificity of anti-PLA2R antibodies, PLA2R and IgG4 immunodeposits in kidney tissues were significantly higher than in non-IMN patients, and that the combination of the three was of high diagnostic value for idiopathic IMN. The diagnostic specificity of anti-PLA2R antibodies was 98.94% and was specific for IMN. Two patients with positive anti-PLA2R antibodies, a patient with FSGS presented with nephrotic syndrome complicated by acute kidney injury (secondary factor seen with antinuclear antibodies 1:320) and received adequate hormonal therapy. One patient with secondary membranous nephropathy presented with nephrotic syndrome (no secondary factors). After 6 months of follow-up and treatment with ARBs, the patient had no significant improvement in proteinuria and creatinine and was persistently anaemic (Hb between 102–111 g/l). In patients who are unable to complete a kidney biopsy, the detection and follow-up of secondary nephritic factors, in addition to specific antibody testing, remains a concern.
In 2014, TomasCitation5 first reported that 15 of 154 IMN patients had positive anti-THSD7A antibodies, but no PLA2R expression. A Japanese studyCitation16 reported that glomerular THSD7A granular expression was detected in 9.1% of IMN patients. The current Asian findings differ from this Japanese study, considering the following reasons:Citation5,Citation16 firstly, genetic background and environmental conditions; differences in study methodology cannot be excluded: furthermore, the Japanese study focused on patients with positive anti-THSD7A antibodies, increasing the glomerular THSD7A positivity rate to some extent; and finally differences in the timing of testing, with sera from the Tomas study being collected from 0 to 87 months after biopsy months of follow-up patients, and they also found that serum THSD7A-Ab titres may be decreased in patients in immune inactivation, spontaneous remission, and that there are factors that interfere with THSD7A expression; whereas in Japan blood was collected at the same time as kidney biopsy, reducing the effect of subsequent medication and disease regression on THSD7A expression. Jia et alCitation17 found that THSD7A was detected in 12 (2.1%) of 578 IMN patients. Our cohort study also found a positive THSD7A immunodeposition rate of 3.1% in IMN patients, which was significantly different compared to non-IMN. In our study, we found that the clinical presentation of THSD7A-positive patients was consistent with the findings of Wang et al. Current studies have found that anti-THSD7A antibodies have promising clinical applications in the diagnosis of IMN and other aspects,Citation17 but they are mostly evaluated by the rate of positive THSD7A expression as an indicator, and there is a lack of systematic studies on the diagnostic efficiency of THSD7A in IMN patients. Given the practical clinical need for non-invasive diagnosis, in our study we excluded the bias of immunosuppressive treatment and found that the diagnostic sensitivity of THSD7A was 3.09%, but the specificity was 100%. Although the sensitivity was not high, there was a high diagnostic specificity, which is consistent with a meta-analysis of THSD7A diagnosis.Citation18 In our study, we focused on the diagnostic properties of THSD7A: despite its low diagnostic sensitivity, THSD7A has a high specificity. This finding offers a new perspective for diagnosing IMN, especially when kidney biopsy is not feasible. Additional studies have found that IMN patients with positive anti-THSD7A antibodies may be at a higher risk of developing cancer,Citation6,Citation19 with 6% to 25% of THSD7A-associated IMN patients having malignancies.Citation20 In addition, THSD7AmRNA has been found in malignancy such as gallbladder cancer,Citation16 malignant melanoma and in patients with a history of bladder cancer and non-malignant papillary bladder malignancy.Citation21 One study investigating 1276 patients with MN found 40 patients with positive serum THSD7A antibodies, eight of whom developed malignancy during a mean follow-up period of 3 months.Citation19 A multicentre study found that 14 (3%) of 469 IMN patients had positive kidney tissue for THSD7A, and most expressed nephrotic syndrome.Citation22 However, it has also been found that THSD7A-positive MN may not be complicated by malignancy.Citation23 Members of the study group were concerned about the correlation between THSD7A and malignancy, and we noted that THSD7A was not detected in the glomeruli of one patient with IMN combined with gastric cancer and five patients with myeloma nephropathy, while one patient with lupus nephritis (grade IV + V) was positive for anti-THSD7A antibodies and had no glomerular THSD7A expression, and no tumour-related events were seen at six months of follow-up to date. Considering the small number of patients with positive kidney tissue THSD7A expression, it is not possible to fully assess whether patients with malignancy combined with tissue THSD7A expression are more susceptible to nephropathy and whether THSD7A is a bridge between malignancy and IMN. However, most current studies suggest an association between cancer and anti-THSD7A antibodies and therefore recommend screening for malignancy in patients with THSD7A-associated MN, particularly gastrointestinal and genitourinary tract malignancy, which require a longer follow-up period to observe patients for oncological events.
In our study, we investigated the relationship between the level of anti-PLA2R antibodies and clinical features and pathological manifestations and found that anti-PLA2R antibodies were qualitatively associated with proteinuria and serum albumin. The study by Radice et alCitation5 showed a linear correlation between anti-PLA2R-antibody levels and increased proteinuria and decreased albumin, and a strong correlation between clinical indicators and antibody titer levels, which could predict disease activity based on antibody titer levels. It is therefore reasonable to speculate that high levels of antibodies may lead to more subepithelial immune complex deposition and more severe podocyte damage, and subsequently more proteinuria. In our study, we found that anti-PLA2R antibody levels were negatively correlated with blood albumin, which is consistent with previous findings,Citation24 and that their antibodies are useful in assessing disease status.
In this study, there were differences between the antibody negative and positive groups of patients in terms of pathological staging, C3 and IgG4 immunofluorescence deposition. It has been shownCitation25 that the intensity of C3 deposition and the staging of membranous nephropathy are associated with anti-PLA2R antibodies. It is thus hypothesized that autoantibodies are not only associated with clinical manifestations but are also involved in pathological changes. C3 particle staining correlates with proteinuria and kidney function in patients, implying that the deposition of anti-PLA2R-IgG in glomeruli may be the beginning of autoimmunity and the subsequent triggering of complement activation subsequently induces further kidney pathological damage. Considering the correlation between PLA2R and complement, the kidney tissue MBL was now studied in the concurrently enrolled IMN patients to further the correlation of the MBL pathway with PLA2R and its pathogenic role in IMN. There were significant differences in IgG4 immunofluorescence deposition between the two groups, considering that the anti-PLA2R autoantibodies in serum samples from patients with membranous nephropathy were mainly IgG4, the main immunoglobulin subclass in the glomerular deposits. PLA2R is expressed in the podocytes of normal human glomeruli and co-localises with IgG4 in glomerular immune deposits from patients with membranous nephropathy, so that IgG4 is predominantly expressed in the positive group. However, it is important to note that group differentiation is based on the characterisation of anti-PLA2R antibodies, and other autoantigenic indicators need to be considered in the negative group. The study group was limited by the sparse number of serum THSD7A and Nell positive patients and no further grouping based on other antibodies was performed, suggesting that glomerular IgG subclasses in the PLA2R-related/unrelated MN population could be studied by increasing the sample size. Furthermore, we observed no significant differences between the two groups in terms of glomerular proliferation, tubulointerstitial acute and chronic inflammation, but it has been shownCitation25 that PLA2R antibodies are an influential factor in the development of interstitial kidney injury in IMN patients and can predict the development of interstitial kidney injury in IMN patients. It has been demonstratedCitation26 that chronic kidney tubulointerstitial injury can be used to assess the clinical condition of patients with IMN nephropathy, including serum albumin and proteinuria levels. Current prognostic studiesCitation27,Citation28 have found that the severity of cell proliferation and chronic tubulointerstitial injury are independent risk factors for kidney prognosis in IMN and also for kidney insufficiency. In our study, we graded and accounted for detailed information on glomerular hyperplasia and tubulointerstitial damage in IMN kidneys, and will note the correlation between hyperplasia, chronic interstitial damage and kidney insufficiency during follow-up.
In the cohort study, we found a low prevalence of THSD7A-associated IMN patients, with six patients with positive glomerular THSD7A deposits with PLA2R deposits and no patients with membranous nephropathy with positive THSD7A alone; these six patients were analysed comparatively in terms of etiology, clinical features, pathology and prognosis. We first found in terms of etiology: no secondary nephropathic factors and no tumour-related symptoms, signs, chemistry and imaging; Our findings suggest that there is no direct association between THSD7A and malignancy, which is inconsistent with the possibility that patients with THSD7A-associated membranous nephropathy may have a higher risk of cancer. However, due to the short follow-up period, it is necessary to be vigilant for potential malignancy events in future follow-up to further assess any correlation. Furthermore, there was no significant difference in ALB and UTP between the two groups compared to PLA2R of kidney tissue alone. Previous studies have shownCitation16 that anti-THSD7A antibodies do not correlate well with serum creatinine, albumin and proteinuria levels. THSD7A was also found to be expressed in PLA2R-positive IMN patients, and its clinicopathological features did not differ according to single or double antibody positivity.Citation29 Finally the two groups of patients showed differences in glomerular immunofluorescence IgG4 and no differences in the remaining pathological parameters. IgG4 and THSD7A were co-localised in kidney biopsies from THSD7A-positive patients.Citation23 THSD7A and PLA2R have similar structural and biochemical properties, both are expressed on podocyte membranes and their corresponding antibodies are predominantly IgG4. In a national study, two patients with IMN were found to be double positive for PLA2R and THSD7A, with immunofluorescence showing antigen co-localisation and both being involved in immune complex formation. A previous studyCitation16 also found no significant difference in the clinicopathological features of THSD7A membranous nephropathy and PLA2R membranous nephropathy. This is consistent with our study. However, it should be noted that when comparing the mean age of patients with THSD7A-associated membranous nephropathy in the two studies, we noticed a difference in age. According to our study data, the mean age of the patients was 50 years. In contrast, the mean age of patients in the studyCitation16 was 42 years. This difference in age may have some impact on the results of the study, as it may be related to the clinical manifestations of the disease, response to treatment, and prognosis.
We then followed up six patients, one of whom was lost to follow-up; of the remaining five patients, one achieved complete remission, two achieved partial remission and two had no remission. We performed a multifactorial analysis of the follow-up data from both groups and found that kidney tissue THSD7A was not an influential factor in patient prognosis. Previous studies have also found no correlation between kidney tissue PLA2R, THSD7A, IgG4 staining and response to treatment.Citation30 However, using KM survival analysis curves, we found that the probability and time to complete remission was lower in the double antibody-positive patients than in the double antibody-negative group. Although the differences were not statistically significant, given the inadequacy of the study sample, further studies with an expanded sample are necessary to clarify the relationship between THSD7A and disease activity and prognosis. Our study showed no significant difference in clinical and pathological features between dual antigen positivity (PLA2R and THSD7A) and single antigen positivity (PLA2R only) in kidney tissues. In our study, the observation that patients with dual-antigen positivity (PLA2R and THSD7A) were less likely to achieve complete remission compared with PLA2R antibody-negative patients provides a new perspective on the clinical management of IMN. Although the impact of PLA2R and THSD7A co-expression on the clinical and pathological characteristics and prognosis of patients is understudied, our preliminary study presents this concept.
Conclusion
The results of this study showed that: First, IMN patients had a higher rate of positive anti-PLA2R antibodies and higher positive glomerular PLA2R, IgG4 and THSD7A staining, with the former two having an extremely high diagnostic value for IMN and the latter having a lower diagnostic sensitivity but higher specificity; second, anti-PLA2R antibodies assessed the clinical features and pathological characteristics of patients to some extent; then, kidney with double positivity (PLA2R+THSD7A) and single positive (PLA2R) did not differ significantly in terms of clinical features and pathological characteristics; finally, no correlation was found between THSD7A and malignancy, but one patient with lupus had positive antibodies that need to be evaluated with care. The current study is limited to a small sample size of patients and a multicentre study is recommended, along with an extended follow-up period.
In our study, we focused on the importance of serum-specific antibodies in the diagnosis of IMN, as well as the significance of specific antibodies and antigens in guiding the clinical and pathological assessment; for patients who cannot undergo kidney biopsy, attention should be paid to the assessment of secondary nephritis factors in addition to specific antibody testing; especially for patients with THSD7A, attention should be paid to the detailed assessment of malignancy events. We recommend further studies to expand the sample size and extend the follow-up period, in addition to multicentre studies, and to further improve the examination of other new specific indicators of IMN according to the current research progress.
Statement of Ethics
Study approval statement: This research protocol was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Bengbu Medical College under the approval number Lunke Approval [2020] No. 117.
Consent to Participate Statement
Written informed consent was obtained from the participants for this research project. The study was in accordance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest to declare in this work.
Acknowledgment
We are deeply grateful to all participants in this study and thank our colleague Xu Peng for his help.
Data Sharing Statement
Data supporting the results of this study are not publicly available and can be obtained from the first author Yan Pan ([email protected]).
Additional information
Funding
References
- Zhou FD, Zhao MH, Zou WZ, et al. The changing spectrum of primary glomerular diseases within 15 years: a survey of 3331 patients in a single Chinese centre. Nephrol Dial Tran. 2009;24:870–876. doi:10.1093/ndt/gfn554
- Zhu P, Zhou FD, Wang SX, et al. Increasing frequency of idiopathic membranous nephropathy in primary glomerulardisease: a 10-year renal biopsy study from a single Chinese nephrology centre. Nephrology. 2015;20:560–566. doi:10.1111/nep.12542
- Beck LH Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11–21. doi:10.1056/NEJMoa0810457
- Tomas NM, Beck LH Jr, Meyer-Schwesinger C, et al. Thrombospondin type-1 domain containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2014;372(24):2277–2287. doi:10.1056/NEJMoa1409354
- Radice A, Trezzi B, Maggiore U, et al. Clinical usefulness of autoantibodies to M-type phospholipase A2 receptor (PLA2R) for monitoring disease activity in idiopathic membranous nephropathy (IMN). Autoimmun Rev. 2016;15(2):146–154. doi:10.1016/j.autrev.2015.10.004
- Hoxha E, Wiech T, Stahl PR, et al. A mechanism for cancer-associated membranous nephropathy. N Engl J Med. 2016;374(20):1995–1996. doi:10.1056/NEJMc1511702
- Hoxha E, Harendza S, Zahner G, et al. An immunofluorescence test forphospholipaseA2-receptor antibodies and its clinical usefulness in patients with membranous glomeruli. Nephrol Dial Trans. 2011;26(8):2526–2532. doi:10.1093/ndt/gfr247
- Churg J, Ehrenreich T. Membranous nephropathy. Perspect Nephrol Hyper. 1973;1:443–448.
- Troyanov S, Roasio L, Pandes M, et al. Renal pathology in idiopathic membran ous nephropathy: a new perspective. Kidney Int. 2006;69(9):1641–1648. doi:10.1038/sj.ki.5000289
- Segarra-Medrano A, Jatem-Escalante E, Quiles-Pérez MT, et al. Prevalence, diagnostic value and clinical characteristics associated with the presence of circulating levels and renal deposits of antibodies against the Mtype phospholipase A2 receptor in idiopathic membranous nephropathy. Nefrologia. 2014;34(3):353–359. doi:10.3265/Nefrologia.pre2013.Dec.12291
- Ramachandran R, Sharma V, Verma A. PLA2R in Glomerular deposit and specific antibodies and membranous nephropathyanti PLA2R antibodies in Indian patients with active IMN. Nephrology. 2014;19(2):23–76.
- Hihara K, Iyoda M, Tachibana S, et al. AntiPhospholipase A2 Receptor (PLA2R) antibody and Glomerular PLA2R Expression in Japanese Patients with Membranous Neph-ropathy. PLoS One. 2016;11(6):e0158154. doi:10.1371/journal.pone.0158154
- Qin HZ, Zhang MC, Le WB, et al. Combined assessment of phospholipase a2 receptor autoantibodies and glomerular deposits in membranous nephropathy. J Am Soc Nephrol. 2016;27(10):3195–3203. doi:10.1681/ASN.2015080953
- Guan Y, Li H, Duan L, et al. Serum anti-phospolipase A2 receptor antibodies and glomerular IgG4 in the diagnosis of membranous nephropathy. Chinese J Nephrol. 2015;31(003):198–202.
- Bobart SA, Han H, Tehranian S, et al. Noninvasive Diagnosis of PLA2R-associated membranous nephropathy: A validation study. Clin J Am Soc Nephrol. 2021;16(12):1833–1839. doi:10.2215/CJN.05480421
- Iwakura T, Ohashi N, Kato A, et al. Prevalence of Enhanced Granular Expression of Thrombospondin Type-1 Domain-Containing 7A in the Glomeruli of Japanese Patients with Idiopathic Membranous Nephropathy. PLoS One. 2015;10(9):e0138841. doi:10.1371/journal.pone.0138841
- Wang J, Cui Z, Lu J, et al. Circulating Antibodies against Thrombospondin Type-I Domain-Containing 7A in Chinese Patients with Idiopathic Membranous Nephropathy. Clin J Am Soc Nephrol. 2017;12(10):1642–1651. doi:10.2215/CJN.01460217
- Liu Y, Zheng S, Ma C, et al. Meta-Analysis of the Diagnostic Efficiency of THSD7A-AB for the Diagnosis of Idiopathic Membranous Nephropathy. Glob Chall. 2020. 4(11):1900099. doi:10.1002/gch2.201900099
- Hoxha E, Beck LH, Wiech T, et al. An indirect immunofluorescence method facilitates detection of thrombospondin type 1 domain-containing 7A- specific Antibodies in membranous nephropathy. J Am Soc Nephrol. 2017;28(2):520–531. doi:10.1681/ASN.2016010050
- Ren S, Wu C, Zhang Y, et al. An update on clinical significance of use of THSD7A in diagnosing idiopathic membranous nephropathy: a systematic review and meta analysis of THSD7A in IMN. Ren Fail. 2018;40(1):306–313. doi:10.1080/0886022X.2018.1456457
- WeinmannMenke J, Holtz S, Sollinger D, et al. Treatment of membranous nephropathy in patients with THSD7A antibodies using immunoadsorption. Am J Kidney Dis. 2019;74(6):849–852. doi:10.1053/j.ajkd.2019.05.021
- Hara S, Tsuji T, Fukasawa Y, et al. Clinicopathological characteristics of thrombospondin type 1 domain-containing 7A-associated membranous. Virchows Arch. 2019;474(6):735–743. doi:10.1007/s00428-019-02558-0
- Zhang C, Zhang M, Chen D, et al. Features of phospholipase A2 receptor and thrombospondin type-1 domain-containing 7A in malignancy-associated membranous nephropathy. J Clin Pathol. 2019;72(10):705–711. doi:10.1136/jclinpath-2019-205852
- Gong Z, Yuan S, Zhu X, et al. Clinical significance of M-type phospholipase A2 receptor and thrombospondin Type 1 domain-containing 7A in primary membranous nephropathy. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2020;45(6):693–700. doi:10.11817/j.issn.1672-7347.2020.190109
- Zhang XD, Cui Z, Zhang MF, et al. Clinical implications of pathological features of primary membranous nephropathy. BMC Nephrol. 2018;19(1):215. doi:10.1186/s12882-018-1011-5
- Horvatic I, Ljubanovic DG, Bulimbasic S, et al. Prognostic significance of glomerular and tubulointerstitial morphometry in idiopathic membranous nephropathy. Pathol Res Pract. 2012;208(11):662–667. doi:10.1016/j.prp.2012.08.004
- Zhang BO, Cheng M, Yang M, et al. Analysis of the prognostic risk factors of idiopathic membranous nephropathy using a new surrogate endpoint. Biomed Rep. 2016;4(2):147–152. doi:10.3892/br.2015.555
- Wei C, He Y, Li T, et al. Glomerulosclerosis predicts poor renal outcome in patients with idiopathic membranous nephropathy. Int Urol Nephrol. 2021;53(3):505–514. doi:10.1007/s11255-020-02641-5
- Xin W, Cheng C, Guohua D, et al. Clinical and Pathological Characteristics of THSD7A Related Idiopathic Mem Branous Nephropathy. J Med Res. 2020;49(8):42–46.
- Kaya B, Paydas S, Balal M, et al. Renal expression of PLA2R, THSD7A, and IgG4 in Patients with membranous nephro-pathy and correlation with clinical findings. Int J Clin Pract. 2021;75(4):e13855. doi:10.1111/ijcp.13855