Abstract
Background
Cytokines act a vital role in autoimmune neuroinflammatory diseases (ANDs) with undetermined causal relationships. Mendelian randomization (MR) analysis was performed to estimate the causal effects of circulating levels of cytokines on the risk of ANDs.
Methods
The causal relationship between 34 circulating cytokines and 4 kinds of ANDs, including multiple sclerosis (MS), neuromyelitis optica (NOM), chronic inflammatory demyelinating polyneuropathy (CIDP) and myasthenia gravis (MG) were explored using four methods of MR analysis. MR-PRESSO, MR-Egger regression methods and Cochran’s Q statistic were utilized to identify the instrumental variables (IVs) with potential pleiotropy and heterogeneity. The Bonferroni correction was used for multiple group comparisons. P-value less than 3.68E-04 (0.05/ (34*4)) was considered statistically significant.
Results
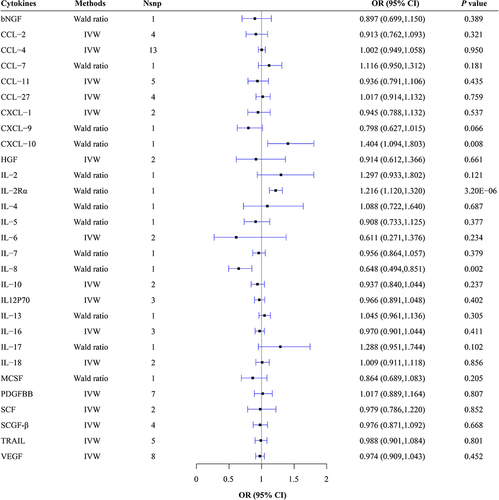
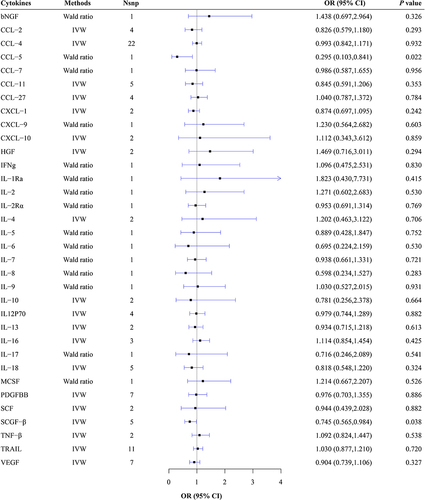
Negative causal effects of circulating levels of interleukin (IL)-8 (OR = 0.648, 95% CI: 0.494-0.851, P = 0.002) on risk of MS, chemokine (C–C Motif) ligand (CCL)-5 (OR = 0.295, 95% CI: 0.103-0.841, P = 0.022) and stem cell growth factor-beta (SCGF-β) (OR = 0.745, 95% CI: 0.565-0.984, P = 0.038) on risk of CIDP, as well as positive causal effects of circulating levels of IL-2 receptor α (IL-2Rα) (OR = 1.216, 95% CI: 1.120-1.320, P = 3.20E-06) and chemokine C-X-C motif ligand (CXCL)-10 (OR = 1.404, 95% CI: 1.094-1.803, P = 0.008) on MS were observed. Nevertheless, only IL-2Rα still had a causal effect on MS after Bonferroni correction.
Conclusion
The results identify a genetically predicted causal effect of IL-2Rα, IL-8 and CXCL-10 on MS, CCL-5 and SCGF-β on CIDP.
Introduction
Autoimmune neuroinflammatory diseases (ANDs) are a type of diseases characterized with the mistaken attack on its own nervous system by the immune system, leading to inevitable damage to the structure and function.Citation1 Excessive inflammation, immune dysregulation, and immune over activity are the three major features.Citation1 Compared with other well-known neuroinflammatory disorders such as Alzheimer’s disease and Parkinson’s disease, defects in self-tolerance resulting in autoimmunity make ANDs to be unique.Citation2 ANDs have a wide range of injuries as well as complex and diverse clinical manifestations, which can extensively affect the central nervous system (CNS), peripheral nervous system (PNS), and the neuromuscular junction (NMJ), resulting in neuronal or axonal damage, demyelination, nerve-muscle junction damage and other pathological changes. The representatives of ANDs in the CNS, PNS and NMJ are multiple sclerosis (MS) and neuromyelitis optica (NOM), Guillain–Barré syndrome (GBS) and chronic inflammatory demyelinating polyneuropathy (CIDP), as well as myasthenia gravis (MG), respectively.Citation3
ANDs have a serious impact on the quality of life of patients, making it crucial to understand their pathogenic mechanisms and search for new treatment methods. Cytokines play a key role in the pathogenesis of ANDs, involving important neural processes such as neuronal endocrine function, neurotransmitter metabolism, and neural plasticity.Citation1,Citation3,Citation4 Previous observational studies also have demonstrated increased circulating levels of some cytokines such as interleukin (IL)-2 in GBS, CIDP, MG and active MS patients,Citation5 interferon (IFN)-γ, tumor necrosis factor (TNF)-α, IL-1, IL-6 and IL-17 in MS and MG patients, IL-22 in MS patients,Citation6 IL-1β, IL-6, IL-17, and IL-19 in CIDP patients,Citation1,Citation2 as well as decreased IL-10 in MS and MG patients.Citation3
Despite the observed association between cytokines and ANDs in existing research, there are several shortcomings. Firstly, the limited sample size of existing observational studies focuses on a few cytokines or specific types of ANDs issues, and may be influenced by confounding factors.Citation4 Secondly, investigating the specific role of cytokines in the pathogenesis of ANDs also faces challenges, primarily due to the confounding effects of reverse causation bias. Reverse causation bias suggests that ANDs may be a cause of inflammation, rather than just a consequence, making it extremely difficult to determine the exact role of cytokines in the pathogenesis of ANDs.
Fortunately, Mendelian randomization (MR) methods, as an emerging approach, may offer better solutions. It uses genetic variations that influence exposure as instrumental variables (IVs) to assess the causal relationship between exposure and outcomes. Given that the alleles are randomly allocated at conception, they are not influenced by confounding factors and the state of diseases. MR could lessen the confounding effects from environmental factors and avoid reverse causal bias by using genetic variants as IV.Citation5,Citation6
Three MR studies have explored the causal effects of some circulating levels of cytokines on the risk of MS.Citation7–9 These three studies assessed the causal association between five growth factors, six interleukins and the IL-6 signaling regulated by body mass index (BMI) with MS. However, the circulating cytokine factors analyzed in these studies were not comprehensive enough. There is also a lack of MR studies on the causal association between circulating cytokines and the other three ANDs, including NOM, GBS, CIDP and MG. Therefore, further MR studies encompassing a wider array of circulating cytokines and different ADs are necessary to gain a more comprehensive understanding of cytokine roles in various types of ADS, identify more potential causal relationships, and explore the molecular basis of cytokine regulatory mechanisms.
Herein, in order to comprehensively estimate the causal relation between circulating cytokines levels and ANDs, two-sample MR analyses were performed. Given the availability of GWAS data, genetic variants associated with thirty-four circulating cytokines as IVs were utilized to improve the inference for the possible influences of cytokines on the risk of four ANDs including MS, NOM, CIDP and MG.
Materials and Methods
Study Design
MR analysis is a statistical method used to assess causal relationships, often in observational data. It uses genetic variations that influence exposure as IVs to assess the causal relationship between exposure and outcomes. One advantage of MR analysis is that it can reduce biases caused by confounding factors and reverse causation, as genetic variations are generally not influenced by environmental or behavioral factors.Citation5,Citation6 MR studies will help address the current challenges in researching the relationship between circulating cytokines and different ANDs, enabling more precise investigation of direct causal associations between circulating cytokines and various ANDs.
Two-sample MR (TSMR) was utilized to investigate whether levels of thirty-four circulating cytokines are causally associated with risk of ANDs (including MS, NOM, CIDP and MG) in European populations. TSMR identifies IVs with a high genetic risk for high circulating levels of cytokines from genome-wide summary data and further explores their causal relationships with ANDs in another sample. The summary data were from published studies and had obtained institutional review committee.
Data Source
The largest publicly available genome-wide association study (GWAS) including as many as 8293 participants was used for extracting IVs of these thirty-four circulating cytokines ().Citation10 The cytokine GWAS has been adjusted for age, sex and body mass index (Supplementary Tables 1 and 2).
Table 1 The Thirty-Four Circulating Cytokines Included in This Study
Genome-wide summary data based on 47,429 cases and 68,374 controls from the European population for MS were obtained from the International MS Genetics Consortium (Supplementary Table 1).Citation11 The NMO-related GWAS data were retrieved from the recently and largest available NMO GWAS meta-analysis, which including 215 NMO cases and 1244 controls (Supplementary Table 1).Citation12 Genetic associations of CIDP were generated from 456,348 European ancestry individuals from UK Biobank using fast genome-wide association-generalized linear mixed model and were obtained from Catalog (Supplementary Table 1).Citation13 The GWAS data based on 232 cases and 217,056 controls of MG were from IEU GWAS pipeline (https://gwas.mrcieu.ac.uk/datasets/finn-b-G6_MYASTHENIA/) (Supplementary Table 1).
IVs Selection
To guarantee that the result was viable and undisturbed, we screened SNPs having significant association with exposure elements from the exposure GWAS (P < 5 × 10−8), deleted SNPs with minor allele frequency (MAF < 0.01), conducted clustering method (R2<0.001 within clumping distance at 10 Mkb) to erase linkage disequilibrium (LD) between SNPs. Additionally, we simultaneously assessed potential pleiotropy in instrumental variables (IVs) using two methods: MR Pleiotropy RESidual Sum and Outlier (MR-PRESSO) and MR-Egger regression techniques. MR-PRESSO not only detects horizontal pleiotropy but also identifies outliers among the IVs.Citation14 When the P-values for MR-PRESSO and MR-Egger are less than or equal to 0.05, it indicates the presence of pleiotropic IVs. After removing any anomalous values, we iteratively conducted MR-PRESSO and MR-Egger tests until no horizontal pleiotropy was detected in any IV. Additionally, Cochran’s Q statistic was utilized to measure the level of heterogeneity across all IVs. When the P-value of the Q-test is greater than 0.05, it indicates the absence of heterogeneous IVs.Citation15
At the genome wide significant threshold P < 5 × 10−8, twenty-six cytokines were found to have at least one SNP useable in our study, while eight cytokines had no useable SNP; therefore, we changed the threshold to P < 5 × 10−7 for the eight cytokines. The screened SNPs were paired from outcome GWAS, and if SNP cannot be searched in outcome data or was identified as a palindrome SNP, its proxy SNP with high LD (r2>0.8) take its place. Finally, the remaining SNPs after the elimination of outlier SNPs were used as the final IV. The information of the IVs is listed in Supplementary Table 2.
Mendelian Randomization Analysis
The total causal effects of cytokines on ANDs were estimated using univariable TSMR. This study utilized five methods for MR analysis. When only one IV was present, the analysis employed the Wald ratio method. With two IVs, the analysis is switched to the Inverse-Variance-Weighted (IVW) method. For cases with more than two IVs, four complementary methods were employed: MR Egger, Weighted Median, Weighted Mode, and IVW, with IVW serving as the primary basis for interpreting results.Citation16 The Bonferroni correction was applied to adjust for multiple comparisons in our analysis. We chose a significance threshold of α = 0.05/n, where n represents the number of comparisons, to account for the increased risk of false positives due to multiple testing.Citation17 This correction method helps maintain the overall Type I error rate at an acceptable level while controlling for the familywise error rate. Finally, P-value less than 3.68E-04 (0.05/(34*4)) was considered statistically significant. All statistical analyses were performed utilizing the packages of R version 4.1.1, including “TwoSampleMR” and “MRPRESSO”.
Sensitivity Analyses
A series of sensitivity analyses was performed. Firstly, the F-statistic was employed to assess the potential bias caused by possible weak instruments. An F-statistic less than 10 suggests the existence of weak instruments-caused bias. Secondly, the causal effects were further calculated by the MR-Egger regression and weighted median methods. Allowing for an unconstrained intercept, the MR-Egger regression method could estimate a robust causal effect after the adjustment of horizontal pleiotropy. In addition, the leave-one-out analysis, in which the instruments were in turn eliminated and causal associations between the residual SNPs and outcome were re-calculated, was performed to investigate the influence of each IV on the outcome. Notably, the MR-Egger, weight median and leave-one-out analysis were impracticable if there were less than three included IVs for the exposure. To evaluate whether the causal effects had comparability, the Cochran’s Q statistic was utilized.
Results
Causal Effects of Circulating Cytokine Concentrations on Risk of MS
IVs of Circulating Cytokines on MS
For MS, 81 SNPs functioned as IVs, but 31 SNPs were not found in the MS GWAS data and lacked corresponding proxy SNPs. In addition, some SNPs not found in GWAS data had proxy SNPs available for substitution. Specifically, rs113010081, rs7221878, rs9472168, rs4253283, rs1801020, rs116924815, rs77451439, rs9952273, rs6921438, rs74675876 were not existed and were substituted by their proxy SNPs, rs6782522, rs7210540, rs11757903, rs34899763, rs2731674, rs41275788, rs11875279, rs28653063, rs4513773, rs6914863. Furthermore, all IVs for MS exhibited F-values exceeding 10, indicating the exclusion of weak instrumental variables. Detailed information regarding the number, inclusion, substitution, exclusion, and cumulative F-statistic of each IV for cytokine concentration is available in Supplementary Table 2.
Circulating Cytokine Concentrations on MS
From the Wald Ratio Results, we observed a causal effect of 1 SD decrease in IL-8 content correlated with the risk of MS (Wald Ratio: OR = 0.648, 95% CI: 0.494–0.851, P = 0.002). Additionally, causal evidence was also detected for a diverse effect of 1 SD increase in IL-2Rα content (Wald Ratio: OR = 1.216, 95% CI: 1.120–1.320, P = 3.20E-06) and CXCL-10 content (Wald Ratio: OR = 1.404, 95% CI: 1.094–1.803, P = 0.008) (, Supplementary Table 3, Supplementary Figures 1–20). After Bonferroni correction, the causal effect of IL-2Rα on MS still exists, while the causal effects of IL-8 and CXCL-10 on MS disappear. The causal relationship between other circulating cytokines and the risk of MS was not observed. For detailed MR analysis, results regarding the causal relationship between other circulating cytokines and the risk of MS are presented in Supplementary Table 3.
Pleiotropy and Sensitivity Analysis for MS
Heterogeneity was observed among the IVs of CCL-2 (Q = 9.549, P = 0.023), CCL-11 (Q = 10.102, P = 0.039), CXCL-1 (Q = 7.704, P = 0.006) and hepatocyte growth factor (HGF) (Q = 4.962, P = 0.026) regarding their impact on MS. Pleiotropy tests indicated no outlier SNPs and no horizontal pleiotropy among IVs and outcomes. Leave-one-out analysis demonstrated that excluding any single SNP did not affect the results (Supplementary Figures 1–20) The results of the aforementioned analysis are listed in Supplementary Tables 3–6.
Causal Effects of Circulating Cytokine Concentrations on Risk of NMO
IVs of Circulating Cytokines on NMO
In the case of NMO, 99 SNPs were utilized IVs, but 13 SNPs were not present in the NMO GWAS data, with no corresponding proxy SNPs available. Furthermore, although the SNPs rs58704839 and rs55876513 were not found in the NMO GWAS data, their available proxy SNPs, rs913835 and rs1857821, were identified for substitution. Moreover, all IVs for NMO displayed F-statistic surpassing 10, suggesting the absence of weak IVs. Detailed information of each IV can be found in Supplementary Table 2.
Circulating Cytokine Concentrations on NMO
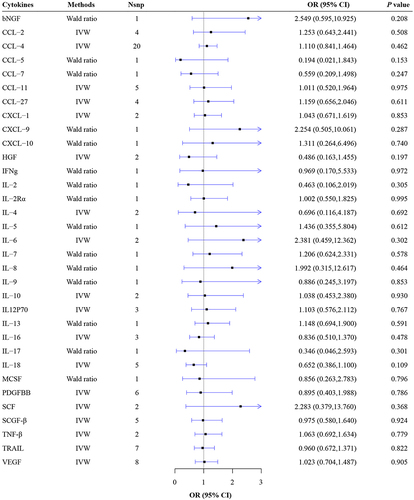
No causal effects of all thirty-four circulating cytokines on the risk of NMO were observed (all P>0.05) (, Supplementary Table 4, Supplementary Figures 1–20). From the Wald Ratio results, no causal association was found of the concentrations of CCL-5 (Wald ratio: OR = 0.194, 95% CI: 0.021–1.843, P = 0.153), CCL-7 (Wald ratio: OR = 0.559, 95% CI: 0.209–1.498, P=0.247), IL-2 (Wald ratio: OR = 0.463, 95% CI: 0.106–2.019, P = 0.305), IL-2Rα (Wald ratio: OR = 1.002, 95% CI: 0.55–1.825, P = 0.995), IL-5 (Wald ratio: OR = 1.436, 95% CI: 0.355–5.804, P = 0.612), IL-7 (Wald ratio: OR = 1.206, 95% CI: 0.624–2.331, P = 0.578), IL-8 (Wald ratio: OR = 1.992, 95% CI: 0.315–12.617, P = 0.464) on the risk of NMO were observed. From the IVW results, no causal association was observed of the concentrations of CCL-2 (IVW: OR = 1.253, 95% CI: 0.643–2.441, P = 0.508), CCL-4 (IVW: OR = 1.11, 95% CI: 0.841–1.464, P = 0.462), IL-4 (IVW: OR = 0.696, 95% CI: 0.116–4.187, P = 0.692), IL-6 (IVW: OR = 2.381, 95% CI: 0.459–12.362, P = 0.302), IL-10 (IVW: OR = 1.038, 95% CI: 0.453–2.38, P = 0.930) on the risk of NMO were observed. The detailed information from the MR analysis can be found in Supplementary Table 4.
Pleiotropy and Sensitivity Analysis for NMO
No heterogeneity was observed in IVs of all cytokines on NMO. The results of pleiotropy tests manifested that there were no outlier SNPs and no horizontal pleiotropy among IVs and outcomes. It was indicated by leave-one-out analysis that exclusion of any single SNP did not affect the results (Supplementary Figures 1–20). The results of the aforementioned analysis are listed in Supplementary Tables 3–6.
Causal Effects of Circulating Cytokine Concentrations on Risk of CIDP
IVs of Circulating Cytokines on CIDP
In the case of CIDP, 108 SNPs were employed as IVs, but 4 SNPs were missing in the CIDP GWAS data without proxy SNPs. In CIDP GWAS data, rs135564 and rs57396456 were not existed and were replaced by their proxy SNPs, rs135562 and rs75582879. Furthermore, the F-statistic for all selected IVs surpassed 10, indicating minimal influence from weak instruments. Supplementary Table 2 provides comprehensive details for each IV regarding cytokine concentration.
Circulating Cytokine Concentrations on CIDP
From the Wald Ratio results, a causal association was found of 1 SD decrease in concentrations of CCL-5 on the risk of CIDP (Wald Ratio: OR = 0.295, 95% CI: 0.103–0.841, P = 0.022). According to the results of IVW, the decreased concentrations of stem cell growth factor-beta (SCGF-β) was also observed to influence the risk of CIDP (IVW: OR = 0.745, 95% CI: 0.565–0.984, P = 0.038). However, these causal effects were not significant after Bonferroni correction, and there was insufficient evidence supporting the causal associations between other circulating cytokines and the risk of CIDP (, Supplementary Table 5, Supplementary Figures 1–20). No causal association was detected between additional circulating cytokines and the risk of CIDP. For a comprehensive overview of the MR analysis results, please refer to Supplementary Table 5.
Pleiotropy and Sensitivity Analysis for CIDP
Heterogeneity was found among the IVs of IL-10 (Q = 9.045, P = 0.003) and CXCL-10 (Q = 4.009, P = 0.045) on CIDP. Pleiotropy tests revealed no outlier SNPs and no horizontal pleiotropy among IVs and outcomes. Leave-one-out analysis indicated that excluding any single SNP did not affect the results (Supplementary Figures 1–20). The results of the aforementioned analysis are provided in Supplementary Tables 3–6.
Causal Effects of Circulating Cytokine Concentrations on Risk of MG
IVs of Circulating Cytokines on MG
For MG, 109 SNPs acted as IVs, yet 3 SNPs were missing in the MG GWAS data without any identified proxy SNPs. In MG GWAS data, rs58704839, rs9450351 and rs57396456 were not existed and were substituted by their proxy SNPs rs3176820, rs9359670, and rs75582879. Moreover, all selected IVs concerning CIDP demonstrated an F-statistic exceeding 10. Supplementary Table 2 furnishes exhaustive particulars concerning each IV pertaining to cytokine concentration.
Circulating Cytokine Concentrations on MG
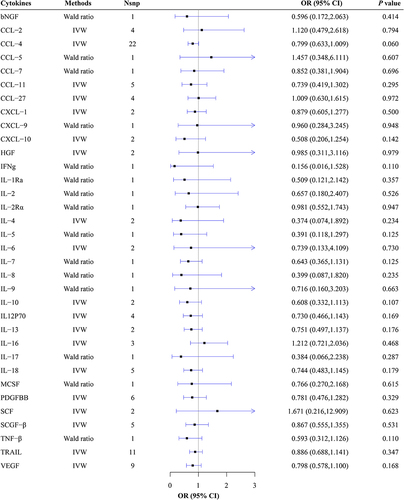
No evidence supporting the causal relationships between all thirty-four circulating cytokines and risk of MG was revealed (all P>0.05) (, Supplementary Table 6, Supplementary Figures 1–20). From the Wald Ratio results, no causal association was found of the concentrations of IL-2 (Wald ratio: OR = 0.657, 95% CI: 0.180–2.407, P = 0.526), IL-2Rα (Wald ratio: OR = 0.981, 95% CI: 0.552–1.743, P = 0.947), IL-5 (Wald ratio: OR = 0.391, 95% CI: 0.118–1.297, P = 0.125), IL-7 (Wald ratio: OR = 0.643, 95% CI: 0.365–1.131, P = 0.125), IL-8 (Wald ratio: OR = 0.399, 95% CI: 0.087–1.82, P = 0.235) on the risk of MG were observed. From the IVW results, no causal association was found of the concentrations of CCL-2 (IVW: OR = 1.120, 95% CI: 0.479–2.618, P = 0.794), CCL-4 (IVW: OR = 0.799, 95% CI: 0.633–1.009, P = 0.060), IL-4 (IVW: OR = 0.374, 95% CI: 0.074–1.892, P = 0.234), IL-6 (IVW: OR = 0.739, 95% CI: 0.133–4.109, P = 0.730), IL-10 (IVW: OR = 0.608, 95% CI: 0.332–1.113, P = 0.107) on the risk of MG were observed. The detailed MR analysis results are presented in Supplementary Table 6.
Pleiotropy and Sensitivity Analysis for MG
No heterogeneity in IVs of all cytokines concerning MG was observed. The results of pleiotropy tests manifested that there were no outlier SNPs and no horizontal pleiotropy among IVs and outcomes. It was indicated by leave-one-out analysis that exclusion of any single SNP did not alter the results (Supplementary Figures 1–20). Detailed results of the aforementioned analyses are listed in Supplementary Tables 3–6.
Discussion
As the MR results showed, the concentration of IL-8 was negatively correlated with MS, while IL-2Rα and CXCL-10 were positively correlated with MS, as well as the concentration of CCL-5 and SCGF-β were negatively correlated with CIDP. In particular, after the Bonferroni correction, IL-2Rα still has a causal effect on MS, which provides further evidence supporting the association between IL-2Rα and MS.
Recently, the relationship between some circulating cytokines and MS has been extensively investigated. The relationship between IL-2Rα and MS observed in previous observational studies was controversial. It was revealed that the serum level of IL-2Rα was elevated in MS patients compared to controls,Citation18–20 while another study found that the level of serum IL-2Rα was not different between MS patients and controls.Citation21 In this present study, we revealed the causal effects of IL-2Rα on MS. The elevated serum levels of IL-2Rα observed in MS patients compared to controls could be attributed to the dysregulated IL-2/IL-2Rα pathway, which plays a crucial role in the immune responses involved in MS pathogenesis. The IL-2/IL-2Rα pathway is known to regulate the expansion and apoptosis of T cells, which are key players in the immune response associated with MS.Citation22 Additionally, differences in the methylation of the IL2Rα promoter in T cells may contribute to the pathogenic process of MS, further highlighting the potential role of IL-2Rα in the development of MS.Citation23 Understanding the interplay between genetic and epigenetic factors in regulating IL-2Rα expression may provide valuable insights into the underlying mechanisms driving MS pathogenesis.
It has been revealed that the expression level of IL-8 was observed to be increased in CSF and decreased in serum of MS patients as compared with controls.Citation24 However, it was also proposed that the serum expression level of IL-8 and IL-8 secretion from PBMCs were higher in untreated MS patients than controls.Citation25 In this study, before Bonferroni correction, the concentration of IL-8 was negatively correlated with MS, suggesting that IL-8 have a weak causal association with MS. Currently, there is no conclusive evidence to suggest that IL-8 can reduce the risk of MS, but some studies indicate that it may play a role in immune regulation, which could affect the development of MS.Citation21,Citation26 IL-8 is a pro-inflammatory cytokine, and in some instances, it may promote the production of regulatory T cells.Citation26 These cells can suppress attacks on self-tissues, thereby reducing the risk of autoimmune diseases, including MS. However, more research is needed to further understand the exact role of IL-8 in the pathogenesis of MS, in order to clarify its potential protective or pro-inflammatory effects.
In this study, it was observed that prior to Bonferroni correction, there was a positive correlation between the concentration of CXCL-10 and MS, indicating a weak causal relationship between CXCL-10 and MS. A previous review study noted that conclusions regarding the association between serum expression levels of CXCL-10 and MS were inconsistent.Citation27 CXCL-10 is a chemokine factor that typically participates in immune responses and inflammatory processes. It has the ability to attract and activate immune cells, particularly Th1-type lymphocytes, playing a crucial role in the immunopathology of MS.Citation28 By increasing the infiltration of these cells into the central nervous system, CXCL-10 may promote the autoimmune response in MS, leading to nerve damage and inflammation. Additionally, CXCL-10 may increase the risk of MS by affecting the blood–brain barrier. It could facilitate the breakdown and increased permeability of the blood–brain barrier, making it easier for immune cells to enter the central nervous system and trigger inflammatory responses.Citation29 A thorough investigation of these mechanisms contributes to a better understanding of the pathogenesis of MS and provides new targets for prevention and treatment.
In this study, the concentration of CCL-5 and SCGF-β were negatively correlated with CIDP, but this causal relationship disappeared after Bonferroni correction. These results suggest a weak causal association between CCL-5, SCGF-β and CIDP, which is a novel finding for CIDP. Previous studies have found a higher expression of TNF-α, HGF, CCL-4, IL-1β, IL-6, IL-2, and IL-4 in the serum of CIDP patients compared to controls,Citation30,Citation31 but the relationship between CCL-5, SCGF-β and CIDP is still lacking.
CCL-5 acts as a chemotactic factor, playing a crucial role in regulating the migration, infiltration, and activation of immune cells.Citation32 Elevated levels of CCL-5 may boost immune cell activity, dampening abnormal immune responses and thus reducing the risk of CIDP. It may also mitigate CIDP risk by inhibiting the infiltration and activation of inflammatory cells, thereby reducing inflammatory reactions. Additionally, CCL-5 exerts regulatory effects within the nervous system, potentially participating in neural development, repair, and protection.Citation33 Higher levels of CCL-5 could help maintain normal neural function, further lowering the risk of CIDP. The role of CCL-5 and SCGF-β in neuroimmune pathology remains insufficiently studied, and the understanding of its mechanisms in CIDP is still exploratory. This new finding from our study provides valuable insights for further exploration of SCGF-β’s role in CIDP pathogenesis, offering potential clues for discovering novel therapeutic targets in CIDP.
In addition, compared to patients with non-inflammatory chronic polyneuropathy and functional neurological disorders, serum IL-1β, IL-6, and IL-17 were highly expressed in patients with CIDP, as inflammatory factors downstream of NLRP3, which were involved in the pathogenesis of CIDP by influencing Th17/Treg (regulatory T cell) balance.Citation1 Unfortunately, no association between these above circulating cytokines and CIDP was found in this study. The reason for the inconsistency with previous studies may be that the studies are mostly observational studies, which may be susceptibly affected by confounding factors and reverse causality.
No causal effects of all circulating cytokines on NMO and MG were observed in this current study, which is inconsistent with the results of previous studies. In NMO patients, the serum concentration of IL-6, IL-17, IL-21, and IL-36 were revealed to be involved in the pathology of NMO.Citation34 Moreover, patients with NMO spectrum disorders (NMOSD) had higher serum CCL-2, CCL-13, CXCL-13 as compared with other noninflammatory neurological diseases (OND).Citation35,Citation36 MG patients have also been observed to have increased serum levels of IL-2, IL-5, IL-12p70 and VEGF as compared with controls.Citation37,Citation38 However, the conclusion from previous studies about whether the serum levels of IL-4 were different between MG patients and controls was inconsistent.Citation37–39 The inconsistency in the conclusions of these studies also reflects some of the limitations of previous studies. At first, previous studies exploring the associations between the circulating levels of cytokines and ANDs are mostly observational studies, which may be susceptibly affected by confounding factors and reverse causality.Citation4 Secondly, the sample sizes of previous studies are usually small, and the conclusions of them are not completely consistent. Finally, the circulating cytokine levels are probably influenced by detection methods and treatment for patients.
This study has several strengths: First, we employed a novel MR analysis method, which not only helped circumvent the influence of confounding factors on the causal association but also enhanced the precision and reliability of our findings. Second, we conducted a comprehensive analysis of the causal associations between 34 circulating cytokines and 4 types of ANDs, providing a thorough understanding of their interrelationships. Third, to ensure the robustness and validity of the results, we implemented an extremely strict correction method, mitigating the potential for false positives and strengthening the credibility of our conclusions.
However, there are also some limitations of this study. First, the study population is limited to individuals of European descent, which may impose certain restrictions when extrapolating the results to other racial or ethnic groups. Second, because the correction method used was extremely rigorous, it may have excluded some weaker causal associations. To address this limitation, when interpreting the MR results, causal associations that were meaningful before correction but meaningless after correction were considered as weak causal associations, and causal associations that were meaningful both before and after correction were considered as strong causal associations. Third, although MR analysis can provide results closer to causal inference, it cannot address all potential confounding and bias issues. Even after controlling for multiple confounding factors, there may still be residual confounding effects that are unknown or not considered. In order to minimize the impact of residual confounding, this study adopted a series of measures. Firstly, widely validated strongly associated genes were chosen as instrumental variables to ensure their independence from other factors in influencing the exposure. Secondly, multiple sensitivity analyses were conducted to examine the robustness of the results to potential residual confounding factors.
Conclusion
The results of this study reveal a strong causal association between IL-2Rα concentration and MS, suggesting a potential role for IL-2Rα in the pathogenesis of MS. This finding highlights the need for further exploration into the specific mechanisms of action of IL-2Rα in the pathophysiological processes of MS, providing an important theoretical basis for the development of more effective treatment strategies in the future.
Abbreviations
ANDs, autoimmune neuroinflammatory diseases; CNS, central nervous system; PNS, peripheral nervous system; NMJ, the neuromuscular junction; MS, multiple sclerosis; MR-PRESSO, MR Pleiotropy RESidual Sum and Outlier; NOM, neuromyelitis optica, GBS, Guillain–Barré syndrome; CIDP, chronic inflammatory demyelinating polyneuropathy; MG, myasthenia gravis; IL, interleukin; IFN, interferon; TNF, tumor necrosis factor; MR, Mendelian randomization; IV, instrumental variable; GDF1, growth differentiation factor 1; IGF1, insulin growth factor 1; IGFBP3, insulin-like growth factor binding proteins 3; VEGF, vascular endothelial growth factor; IL-1Ra, IL-1 receptor antagonist; IL-2Rα, IL-2 receptor α; FGF, fibroblast growth factor; TSMR, two-sample MR; GWAS, genome-wide association study; LD, linkage disequilibrium; IVW, Inverse-Variance-Weighted; CSF, cerebrospinal fluid; NIND, non-inflammatory neurological diseases; NMOSD, NMO spectrum disorders; OND, noninflammatory neurological diseases; AChR, anti-acetylcholine receptor; oMG, ocular MG; gMG, generalized MG.
Ethics Approval
The data in this study were obtained from published studies, of which all data had been approved by the institutional review committee. The ethical application for this study was approved by the Medical Ethics Committee of Anhui Medical University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no competing interests to declare in this work.
Data Sharing Statement
The publicly available datasets used in the present study are included in the article/Supplementary material.
Additional information
Funding
References
- Zhou ZJ, Xia P. Elevated levels of NLRP3 inflammasome in serum of patients with chronic inflammatory demyelinating polyradiculoneuropathy are associated with disease severity. Neurol Sci. 2021;42(8):3383–3387. doi:10.1007/s10072-020-04949-7
- Sangsefidi S, Ghafouri-Fard S, Komaki A, et al. High Levels of Il-19 in Patients with Chronic Inflammatory Demyelinating Polyneuropathy. J Mol Neurosci. 2020;70(12):1997–2000. doi:10.1007/s12031-020-01602-y
- Danikowski KM, Jayaraman S, Prabhakar BS. Regulatory T cells in multiple sclerosis and myasthenia gravis. J Neuroinflammation. 2017;14(1):117. doi:10.1186/s12974-017-0892-8
- Boyko EJ. Observational research--opportunities and limitations. J Diabetes Complications. 2013;27(6):642–648. doi:10.1016/j.jdiacomp.2013.07.007
- Smith GD, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1–22. doi:10.1093/ije/dyg070
- Zheng J, Baird D, Borges MC, et al. Recent Developments in Mendelian Randomization Studies. Curr Epidemiol Rep. 2017;4(4):330–345. doi:10.1007/s40471-017-0128-6
- Vandebergh M, Becelaere S, Dubois B, et al. Body mass index, interleukin-6 signaling and multiple sclerosis: a Mendelian randomization study. Front Immunol. 2022;13:834644. doi:10.3389/fimmu.2022.834644
- Lu H, Wu PF, Zhang W, et al. Circulating interleukins and risk of multiple sclerosis: a Mendelian randomization study. Front Immunol. 2021;12:647588. doi:10.3389/fimmu.2021.647588
- Lu H, Wu PF, Ma DL, et al. Growth factors and their roles in multiple sclerosis risk. Front Immunol. 2021;12:768682. doi:10.3389/fimmu.2021.768682
- Ahola-Olli AV, Würtz P, Havulinna AS, et al. Genome-wide association study identifies 27 loci influencing concentrations of circulating cytokines and growth factors. Am J Hum Genet. 2017;100(1):40–50. doi:10.1016/j.ajhg.2016.11.007
- International Multiple Sclerosis Genetics Consortium*† and ANZgene and IIBDGC and WTCCC2. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science. 2019;365(6460):eaav7188.
- Estrada K, Whelan CW, Zhao F, et al. A whole-genome sequence study identifies genetic risk factors for neuromyelitis optica. Nat Commun. 2018;9(1):1929. doi:10.1038/s41467-018-04332-3
- Jiang L, Zheng Z, Fang H, et al. A generalized linear mixed model association tool for biobank-scale data. Nat Genet. 2021;53(11):1616–1621. doi:10.1038/s41588-021-00954-4
- Verbanck M, Chen CY, Neale B, et al. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–698. doi:10.1038/s41588-018-0099-7
- Greco MF, Minelli C, Sheehan NA, et al. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. 2015;34(21):2926–2940. doi:10.1002/sim.6522
- Sekula P, Del Greco MF, Pattaro C, et al. Mendelian Randomization as an Approach to Assess Causality Using Observational Data. J Am Soc Nephrol. 2016;27(11):3253–3265. doi:10.1681/ASN.2016010098
- Sedgwick P. Multiple hypothesis testing and Bonferroni’s correction. BMJ. 2014;349(oct20 3):g6284. doi:10.1136/bmj.g6284
- Maier LM, Anderson DE, Severson CA, et al. Soluble IL-2RA levels in multiple sclerosis subjects and the effect of soluble IL-2RA on immune responses. J Immunol. 2009;182(3):1541–1547. doi:10.4049/jimmunol.182.3.1541
- Greenberg SJ, Marcon L, Hurwitz BJ, et al. Elevated levels of soluble interleukin-2 receptors in multiple sclerosis. N Engl J Med. 1988;319(15):1019–1020.
- Gallo P, Piccinno MG, Pagni S, et al. Immune activation in multiple sclerosis: study of IL-2, sIL-2R, and gamma-IFN levels in serum and cerebrospinal fluid. J Neurol Sci. 1989;92(1):9–15. doi:10.1016/0022-510X(89)90171-8
- Khaibullin T, Ivanova V, Martynova E, et al. Elevated Levels of Proinflammatory Cytokines in Cerebrospinal Fluid of Multiple Sclerosis Patients. Front Immunol. 2017;8:531. doi:10.3389/fimmu.2017.00531
- Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4(9):665–674. doi:10.1038/nri1435
- Field J, Fox A, Jordan MA, et al. Interleukin-2 receptor-α proximal promoter hypomethylation is associated with multiple sclerosis. Genes Immun. 2017;18(2):59–66. doi:10.1038/gene.2016.50
- Matejčíková Z, Mareš J, Sládková V, et al. Cerebrospinal fluid and serum levels of interleukin-8 in patients with multiple sclerosis and its correlation with Q-albumin. Mult Scler Relat Disord. 2017;14:12–15. doi:10.1016/j.msard.2017.03.007
- Lund BT, Ashikian N, Ta HQ, et al. Increased CXCL8 (IL-8) expression in multiple sclerosis. J Neuroimmunol. 2004;155(1–2):161–171. doi:10.1016/j.jneuroim.2004.06.008
- Ortega SB, Kashi VP, Tyler AF, et al. The disease-ameliorating function of autoregulatory CD8 T cells is mediated by targeting of encephalitogenic CD4 T cells in experimental autoimmune encephalomyelitis. J Immunol. 2013;191(1):117–126. doi:10.4049/jimmunol.1300452
- Vazirinejad R, Ahmadi Z, Kazemi Arababadi M, et al. The biological functions, structure and sources of CXCL10 and its outstanding part in the pathophysiology of multiple sclerosis. Neuroimmunomodulation. 2014;21(6):322–330. doi:10.1159/000357780
- Balashov KE, Rottman JB, Weiner HL, et al. CCR5 + and CXCR3 + T cells are increased in multiple sclerosis and their ligands MIP-1α and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci U S A. 1999;96(12):6873–6878. doi:10.1073/pnas.96.12.6873
- Sørensen TL, Trebst C, Kivisäkk P, et al. Multiple sclerosis: a study of CXCL10 and CXCR3 co-localization in the inflamed central nervous system. J Neuroimmunol. 2002;127(1–2):59–68. doi:10.1016/S0165-5728(02)00097-8
- Beppu M, Sawai S, Misawa S, et al. Serum cytokine and chemokine profiles in patients with chronic inflammatory demyelinating polyneuropathy. J Neuroimmunol. 2015;279:7–10. doi:10.1016/j.jneuroim.2014.12.017
- Dziadkowiak E, Moreira H, Wieczorek M, et al. Correlations between electrophysiological parameters, lymphocyte distribution and cytokine levels in patients with chronic demyelinating inflammatory polyneuropathy. J Pers Med. 2021;11(8):766. doi:10.3390/jpm11080766
- Castellino F, Huang AY, Altan-Bonnet G, et al. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440(7086):890–895. doi:10.1038/nature04651
- Ramesh G, MacLean AG, Philipp MT. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm. 2013;2013:480739. doi:10.1155/2013/480739
- Khan AW, Farooq M, Hwang MJ, et al. Autoimmune neuroinflammatory diseases: role of interleukins. Int J Mol Sci. 2023;24(9):7960. doi:10.3390/ijms24097960
- Du L, Chang H, Xu W, et al. Elevated chemokines and cytokines for eosinophils in neuromyelitis optica spectrum disorders. Mult Scler Relat Disord. 2021;52:102940. doi:10.1016/j.msard.2021.102940
- Yang X, Peng J, Huang X, et al. Association of circulating follicular helper T Cells and Serum CXCL13 With neuromyelitis optica spectrum disorders. Front Immunol. 2021;12:677190. doi:10.3389/fimmu.2021.677190
- Uzawa A, Kawaguchi N, Himuro K, et al. Serum cytokine and chemokine profiles in patients with myasthenia gravis. Clin Exp Immunol. 2014;176(2):232–237. doi:10.1111/cei.12272
- Huan X, Zhao R, Song J, et al. Increased serum IL-2, IL-4, IL-5 and IL-12p70 levels in AChR subtype generalized myasthenia gravis. BMC Immunol. 2022;23(1):26. doi:10.1186/s12865-022-00501-8
- Wang L, Zhang Y, Zhu M, et al. Effects of follicular helper t cells and inflammatory cytokines on myasthenia gravis. Curr Mol Med. 2019;19(10):739–745. doi:10.2174/1566524019666190827162615