Abstract
Purpose
Strategies therapy for hepatocellular carcinoma (HCC) beyond oligometastasis are limited. The optimal sequence of systemic treatment for advanced HCC is not yet clear. Our study aims to evaluate the effectiveness of simultaneous lenvatinib combined PD-1 inhibitor on advanced HCC beyond oligometastasis.
Patients and Methods
A total of 232 patients were enrolled in our retrospective study. Patients divided into three groups. (a) Lenvatinib plus simultaneous PD-1 inhibitor (Simultaneous group, n=58); (b) patients received PD-1 inhibitor before the tumor progression with continued lenvatinib administration (Before PD group, n=77); (c) patients received PD-1 inhibitor after the tumor progression (After PD group, n=97). To analyze overall survival (OS) and progression-free survival (PFS) among the three groups.
Results
The estimated 6-, 12-, 18- and 24-mon OS for Simultaneous group patients were 100%, 93.1%, 63.4%, 48.3%, whereas the OS rates were 100%, 78%, 36.3%, 23.6% in Before PD group, and 99%, 61.2%, 22.1%, 7.5% in After PD group. The OS rates were obviously improved with the use of simultaneous PD-1 inhibitor among the three groups (P <0.001). The estimated 3-, 6-, 9- and 12-month PFS rates for patients were 89.6%, 44.8%, 24.6%, 6% in After PD group, 90.9%, 59.7%, 27.3%, 12.4% in Before PD group and 98.3%, 81%, 51.7%, 39.7% in Simultaneous group, respectively. PFS rate was significantly different among the three groups (P <0.001).
Conclusion
Synchronous administration of lenvatinib and PD-1 inhibitors improved survival rate significantly. The synchronous combination could represent a promising strategy in HCC beyond oligometastasis.
Introduction
Hepatocellular carcinoma (HCC), typically occurs in chronic liver disease and cirrhosis patients, is an invasive tumor.Citation1 HCC treatments include resection, liver transplantation, local therapy and systemic therapy. Resection was recommended in early-stage HCC which is restricted in liver.Citation2 However, when patients experience or subsequently develop with distant metastasis, they would be classified into advanced stage.Citation3 As recommended by updated Barcelona Clinical Liver cancer (BCLC) treatment algorithms, systemic therapy is the mainstay treatment for advanced HCC.Citation4 Evidence showed that extrahepatic metastases and recurrence have been the main tumor-caused death.Citation5 The compliance rate between HCC and metastasis is relatively high.Citation6 Metastases are usually associated with relatively high tumor burden and vascular invasion. Recent studies have shown that those patient populations have a poor outcomes with locoregional therapies, thus may be more suitable for initial systemic treatment. Furthermore, the LAUNCH trial suggests that for patients with high tumor burden and/or macrovascular invasion, the combination of transarterial chemoembolization (TACE) and lenvatinib may have better outcomes compared to lenvatinib monotherapy.Citation7
Lungs are the most common sites of metastases, followed by lymph nodes, bones and adrenal gland.Citation8 Patients with five or fewer metastases were defined as oligometastasis, which may be suitable for local treatment.Citation9 The survival of HCC patients is often determined based on the degree of liver dysfunction. If they have good physical state and liver function, they may still not be considered as advanced, even with large tumor volume, vascular invasion and distant metastases.Citation10 The prognosis of patients with multi-metastases has improved with the emerging therapies.Citation11 Convincing studies have shown that after killing the foci within oligometastasis, local therapy could achieve long-term disease-free survival.Citation12 Compared to patients with oligometastasis, the prognosis was significantly worse in patients beyond oligometastasis.Citation13 At the present age of molecularly targeted systemic therapy, increasingly tolerable and effective choice is being made to control advanced HCC beyond oligometastasis.Citation14
Although lenvatinib is a first-line treatment in advanced HCC, new combinations are still needed to overcome the limitations of single therapy.Citation15 In terms of immune checkpoint inhibitors, including immune checkpoint related inhibitors targeting programmed cell death receptor 1 (PD-1) and its ligand 1 (PD-L1), cytotoxic T lymphocyte associated antigen (CTLA-4), has revealed promising results.Citation16 Currently, the combined use of PD-1 inhibitor and lenvatinib is a hot topic in clinical application, showed stronger anti-tumor effects in clinical trials.Citation17,Citation18 Lenvatinib could inhibit immunosuppressive effects and neovascularization of tumor microenvironments, by enhancing the antitumor immune response, would strengthen the clinical benefit of PD-1 antibodies.Citation18 In fact, the time patients received the PD-1 inhibitor and lenvatinib can be synchronous or different in the real world.Citation19 However, there was no study reporting synchronous or non-synchronous differences between lenvatinib and PD-1 inhibitor in advanced HCC. Thus, in this multi-center study, we aim to compare the effectiveness of synchronous and non-synchronous of PD-1 inhibitor and lenvatinib on the advanced HCC beyond oligometastasis.
Materials and Methods
Patients and Study Design
Patients who met the inclusion criteria would be enrolled: (1) Histologically or clinically confirmed HCC; (2) Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1; and Child-Pugh class A or B;(3) Barcelona Clinic Liver Cancer (BCLC) stage C beyond oligometastasis (more than five metastases); (4) prothrombin activity > 40%, international normalized ratio (INR) < 1.26; (5) serum creatinine concentration not exceeding 1.5 times the upper normal limit. Patients were not eligible for the following reasons: (a) advanced HCC with five or fewer metastasis; (b) under 18 or over 75 years old;(c) incomplete clinical data; (d) portal vein tumor thrombus (PVTT) were involving the superior mesenteric vein; (e) there was tumor thrombus in the atrium or vena cava.
This study was conducted in patients with advanced HCC beyond oligometastasis at Hunan Provincial People’s Hospital from January 2018 to December 2019. The study complied with the guidelines of the Declaration of HelsinkiCitation20 and approved by the ethics committee of Hunan Provincial People’s Hospital. Due to being a retrospective study, informed consent was waived. The data was maintained with confidentiality.
Treatment and Response Assessment
All individuals underwent magnetic resonance imaging (MRI) or contrast-enhanced computed tomography (CT) within 2 weeks prior to the lenvatinib application. Patients were divided into three groups. (a) Lenvatinib plus PD-1 inhibitor simultaneous (Simultaneous group); patients who received PD-1 inhibitor and lenvatinib at the same time, or patients received PD-1 inhibitor within 2 weeks of lenvatinib. (b) Patients received PD-1 inhibitor before the tumor progression (Before PD group); Patients received the PD-1 inhibitor more than 3 months after lenvatinib with continued administration at the condition that the tumor did not occur progression. (c) Patients received PD-1 inhibitor after the tumor progression (After PD group); patients received PD-1 inhibitor after tumor progression after lenvatinib stopped.
Information regarding of initiation, completion of treatment, and adverse events (AEs) during therapy was methodically recorded. The prescribed dosage of lenvatinib was 8 milligrams (for patients with a body weight <60 kg) or 12 milligrams (for patients with a body weight ≥60 kg) orally per day. The PD-1 inhibitor dose was applied based on the drug instruction (including nivolumab, pembrolizumab, tislelizumab, sintilimab, toripalimab, camrelizumab).
Outcomes and Definitions
Overall survival (OS) was the main objective, and progression-free survival (PFS) was the secondary endpoint. OS was defined as the duration from receiving lenvatinib to death or the last follow-up. PFS was defined as the duration from receiving lenvatinib to tumor progression or the last follow-up. Albumin-Bilirubin (ALBI) grade were used to evaluate liver function.Citation21 According to systemic imaging (either contrast-enhanced CT of the chest or bone scan, MRI or contrast-enhanced CT of abdomen or brain or positron emission tomography/computer tomography (PET/CT), tumor stage was assessed. According to Cheng’s criteria,Citation22 PVTT included four categories: type I, tumor thrombus involving the portal vein segmental branches or higher; type II, tumor thrombus involving either the right or left portal vein; type III, tumor thrombus affecting the main portal vein; type IV, the superior mesenteric vein was involved by tumor thrombus. Patients receiving TACE, ablation or surgery before lenvatinib were registered, and drug-related complications were also recorded.
Follow-Up
This study was followed up until April 30, 2021. Patients should undergo evaluation at least once every 6 weeks after therapy. Each follow-up included experimental tests and abdominal imaging examination (contrast-enhanced CT and/or MRI). Experimental tests of prothrombin time (PT), alanine transaminase (ALT), aspartate transaminase (AST), alfa-fetoprotein (AFP), albumin and bilirubin should be conducted every 6 weeks after treatment to evaluate liver function and treatment response. A maximum of two lesions per organ and five lesions in total were chosen as target tumors. Based on the Response Evaluation Criteria in Solid Tumors 1.1 (RECST 1.1), tumor imaging response was evaluated.Citation23 In brief, the outcome was divided into four types, including complete response (CR), partial response (PR), progressive disease (PD) and stable disease (SD). The CR was described as the disappearance of tumor arterial enhancement. PR was described as a targeted tumor diameter reduction of ≥ 30%. PD was described as new lesion appeared, or a minimum growth of 20% in the overall diameter of the targeted tumors. SD was neither CR nor PR and PD compliant. The sum of CR and PR were counted as objective response rate (ORR). The combination of CR, PR and SD were counted as the disease control rate (DCR).
Statistical Analysis
For comparison of difference among three groups, the Pearson χ2 test was investigated to compare categorical variables and ANOVA was used to analyze continuous variables. According to the Kaplan-Meier method with the Log rank test, the survival curves overall survival (OS) and progression-free survival (PFS) were constructed. Determined the survival rates at 6, 12, 18, and 24 months using the z-test life table. The results were analyzed using the R software for Windows (Version 3.6.4 http://www.r-project.org) and Statistical Package for the Social Science (SPSS) software (version 22.0, SPSS Inc., Chicago, IL, USA) for Windows. All statistical tests were bilateral, and P <0.05 was considered significant.
Results
Baseline Characteristics
A total of 232 patients enrolled in the study (Supplementary Figure 1). Ninety-seven patients received PD-1 inhibitor after the tumor progression with stopping lenvatinib administration (After PD group); 77 patients received PD-1 inhibitor before the tumor progression with continued lenvatinib administration (Before PD group); 58 patients received lenvatinib plus PD-1 inhibitor simultaneous (Simultaneous group). The median follow-up time was 22.9 months. All patients in the primary analysis set were followed up for 6 months. A comparison of the baseline clinical and laboratory parameters was shown in . In this cohort, most patients were primary HCC, and a high proportion of patients were received TACE or sorafenib. About half of patients had different PVTT involvement, and most patients in three groups have more 3 tumors and a large HCC (>5 cm). Most patients had lung or lung-related metastasis. As showed in , except the age (P =0.028), there were no significant difference in baseline characteristics among the 3 groups.
Table 1 Clinical Characteristics of Patients with Advanced Hepatocellular Carcinoma (HCC) Beyond Oligometastasis by Type of Different Treatments: (1) Patients Received PD-1 Inhibitor After the Tumor Progression (After PD Group); (2) Patients Received PD-1 Inhibitor Before the Tumor Progression (Before PD Group); (3) Patients Received Lenvatinib Plus Simultaneous PD-1 Inhibitor (Simultaneous Group)
Overall Survival (OS) Analysis
During the follow-up period, 168 (72.4%) patients died, 72 (93.5%) patients occurring in the Before PD group, 58 (59.8%) patients in the After PD group, 32 (55.2%) patients in the Simultaneous group (P =0.031). The medium OS were 13.37±0.63 (95% CI: 12.51–14.96) months, 16.10±1.73 (95% CI: 12.70–19.50) months and 20.57±3.03 (95% CI: 14.64–26.50) months in After PD group, Before PD group and Simultaneous group, respectively. The estimated 6-, 12-, 18- and 24-month OS rates for patients in the Simultaneous group were 100.0%, 93.1%, 63.4%, 48.3%, whereas the OS rates were 100.0%, 78%, 36.3%, 23.6% in Before PD group, and 99.0%, 61.2%, 22.1%, 7.5% in After PD group (Supplementary Table 1). There was a statistically significant difference between Simultaneous group and Before PD (P <0.001) or After PD group (P <0.001) (). In addition, the difference was also statistically significant between the Before PD and After PD group (P =0.043).
Figure 1 Kaplan–Meier curves for overall survival (OS) in 232 patients with HCC beyond oligometastasis by different treatments (Patients received PD-1 inhibitor after the tumor progression (After PD group); patients received PD-1 inhibitor before the tumor progression (Before PD group); patients received lenvatinib plus simultaneous PD-1 inhibitor (Simultaneous group)). (A) The OS (P <0.001) and PFS (B) (P <0.001) curves of After PD group, Before PD group, Simultaneous group, respectively.
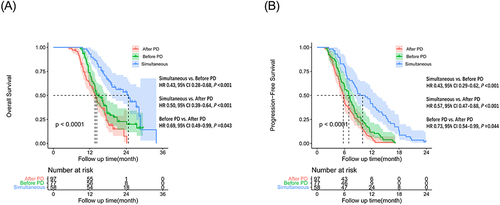
Supplementary Table 2 presented the univariate analysis of OS. Multivariate analysis exhibited that type of PVTT I (HR=3.63; 95% CI: 1.73–7.59; P =0.001), type of PVTT II (HR=1.93; 95% CI: 1.26–2.96; P =0.003), type of PVTT III (HR=1.78; 95% CI: 1.15–2.77; P =0.010), metastasis of other organs (HR=2.09; 95% CI: 1.39–3.14; P <0.001), metastasis of lung plus other organs (HR=1.56; 95% CI: 1.02–2.37; P =0.041), Before PD (HR=2.37; 95% CI: 1.5–3.74; P <0.001), After PD (HR=3.97; 95% CI: 2.49–6.35; P <0.001) were associated with poorer OS ().
Table 2 Multivariate Analyses of Prognostic Factors on Overall Survival (OS) and Progression-Free Survival (PFS) in 232 Patients with HCC Beyond Oligometastasis
Progression-Free Survival (PFS) Analysis
The medium PFS were 6.56±3.23 (95% CI: 5.92–7.19) months, 7.54±0.42 (95% CI: 6.71–8.37) months and 10.86±0.69 (95% CI: 9.50–12.22) months in After PD group, Before PD group, Simultaneous group, respectively. The estimated 3-, 6-, 9- and 12-month PFS rates for patients in After PD group were 89.6%, 44.8%, 24.6%, 6.0%, respectively, whereas the PFS rates were 90.9%, 59.7%, 27.3%, 12.4% in Before PD group and 98.3%, 81.0%, 51.7%, 39.7% in Simultaneous group, respectively. (Supplementary Table 1). The difference was statistically significant among Simultaneous group and Before PD or After PD group (P <0.001) (). In addition, there was also a significance between the Before PD and After PD group (P =0.044).
Supplementary Table 2 presented the univariate analysis of PFS. Multivariate analysis exhibited that other organs metastasis (HR=1.48; 95% CI: 1.04–2.11; P =0.032), lung plus other organs metastasis (HR=1.45; 95% CI: 1.04–2.02; P =0.027), AFP >400 ng/mL (HR=1.52; 95% CI: 1.07–2.16; P =0.021), After PD treatment (HR=3.43; 95% CI: 2.32–5.07; P <0.001), Before PD treatment (HR=2.41; 95% CI: 1.64–3.54; P <0.001) were associated with poorer PFS. ().
Efficacy Evaluation
Efficacy data was evaluated based on RECIST 1.1 evaluation. The following results were presented in . Here, we assessed the 3-month, 6-month tumor response in each group. In the 3-month evaluation, there was one patient achieved complete response in the Simultaneous group. ORR was 18.6%, 19.5%, 37.9%, and the DCR was 69.1%, 74.0%, 82.8% respectively in After PD group, Before PD group, Simultaneous group. The proportion of CR, PR, SD, PD in three groups was obvious difference (P =0.001) (). In the 6-month evaluation, there were two patients achieved CR in the Simultaneous group, and one patient in the Before PD group. ORR was 15.5%, 18.2%, 44.8% and the DCR was 41.2%, 46.8%, 67.2% in After PD group, Before PD group, Simultaneous group. The proportion of CR, PR, SD, PD in three groups was obvious difference (P <0.001) ().
Table 3 Efficacy Outcomes in 232 Patients with HCC Beyond Oligometastasis by Different Treatments: After PD Group; Before PD Group; Simultaneous Group
Subgroup Analysis
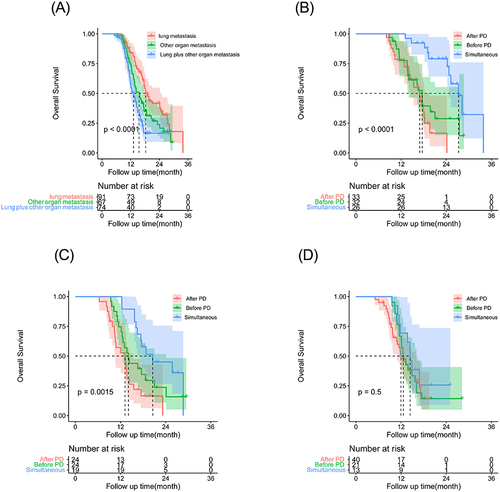
In order to further clarify the impact of different type of metastasis on the prognosis, patients were subdivided into other organs, lung metastasis, lung plus other organs. The OS rates were significantly increased with the use of simultaneous PD-1 inhibitor in three groups of patients () (). The medium OS of patients with lung metastases were 16.93±1.12 months, 17.60±1.00 months, 27.27±1.48 months in After PD group, Before PD group, Simultaneous group respectively, and there was obviously significant (P <0.001) among the three groups (). The medium OS for other organs were 13.20±1.95 months, 14.10±0.98 months, 20.57±4.43 months in After PD group, Before PD group, Simultaneous group. There was significant difference for patients with lung and other organs metastases among the three groups (P =0.002) () (). However, for patients with lung and other organs, there was limited improvements of OS, no difference was observed among the three groups (P =0.496) ().
Table 4 Medium Overall Survival (OS) and Progression-Free Survival (PFS) Rates of Different Metastases Location in in Patients with HCC Beyond Oligometastasis by Different Treatments (Simultaneous Group, After PD Group, Before PD Group)
Figure 2 Kaplan–Meier curves for overall survival (OS) in patients with HCC beyond oligometastasis by different treatments: After PD group; Before PD group; Simultaneous group. (A) The OS rates of lung, other organ, lung and other metastasis in the whole cohort (P <0.001). (B) The lung metastasis OS rates of the group of After PD group, Before PD group, Simultaneous group, respectively (P <0.001); (C) The other organ metastasis OS rates of the group of After PD group, Before PD group, Simultaneous group, respectively (P =0.002); (D) The lung metastasis and other metastasis OS rates of the group of After PD group, Before PD group, Simultaneous group, respectively (P = 0.496).
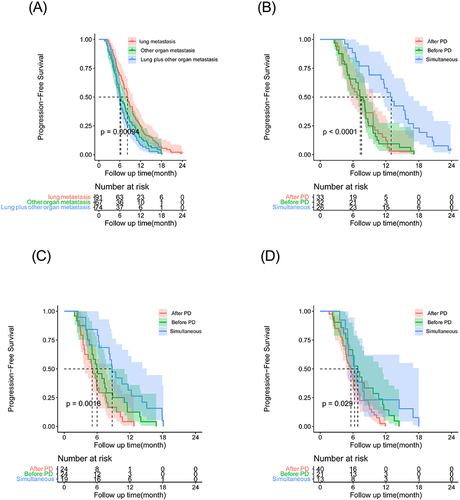
The PFS rates were significantly improved with the use of simultaneous PD-1 inhibitor among the three groups () (). The medium PFS of lung metastasis were 7.33±1.30 months, 7.23±0.83 months, 12.23±0.96 months in After PD group, Before PD group, Simultaneous group respectively, and the difference is significant (P <0.001) () (). Similar results were observed in the other organs metastasis and lung and other organs () ( and ).
Figure 3 Kaplan–Meier curves for progression-free survival (PFS) in patients with HCC beyond oligometastasis by different treatments: After PD group; Before PD group; Simultaneous group. (A) The PFS rate of lung, other organ, lung and other metastasis in the whole cohort (P <0.001); (B) The PFS rate of lung metastasis in After PD group, Before PD group, Simultaneous group, respectively (P <0.001); (C) The PFS rates of other organ metastasis the in After PD group, Before PD group, Simultaneous group, respectively (P =0.002); (D) The PFS rates of lung metastasis and other metastasis in After PD group, Before PD group, Simultaneous group, respectively (P = 0.029).
Safety
Most patients experienced treatment-related adverse events, with the main adverse events recorded in Supplementary Table 3. Hypertension and decreased appetite were the most common of all adverse events. There were 99 (42.6%, [99/232]) patients with hypertension, 82 (82.8%, [82/99]) patients with grade 1–2 and 17 (17.2%, [17/99]) patients with grade 3–4 hypertension. A total of 98 (42.2%, [98/232]) patients with decreased appetite, and all patients had grade 1–2 decreased appetite. No treatment-related deaths occurred in the three groups. Patients with grade 1–2 adverse events can improve their condition after accepting symptomatic treatment or dose reduction. Patients with grade 3–4 adverse events temporarily stop the lenvatinib and PD-1 inhibitor treatment until the adverse effects were relieved or disappeared.
Discussion
Treatment options for advanced HCC have rapidly evolved currently. The currently approved first-line targeted drugs for treatment include sorafenib, lenvatinib, cabozantinib, ramolizumab, apatinib, and donafinib.Citation24 The current effectiveness of monotherapy immune checkpoint inhibitor treatment is limited, with a maximum of no more than 30% or even lower. In addition, there is still room for improvement in targeted therapy for HCC. Clinical evidences reporting on the combination of lenvatinib plus pembrolizumab in advanced HCC were satisfactory and promising.Citation17 This kind of tyrosine kinase inhibitors and immune checkpoint inhibitors represented a new era for advanced HCC therapy. Antiangiogenic drugs can block tumor angiogenesis, thereby cutting off its nutritional supply and causing tumor cell death. In this study, we compared the synchronous and non-synchronous application of PD-1 inhibitor in advanced HCC patients beyond oligometastasis, and we found that synchronous combination of lenvatinib with PD-1 inhibitors lead to survival improvements significantly.
In a carefully designed prospective trials, lenvatinib and PD-1 inhibitor were administered simultaneously.Citation17 The optimal sequence of systemic treatment for advanced HCC is not yet clear. There is no literature reporting of lenvatinib and PD-1 inhibitor administration in real world study. Due to the rapid development and changes in new therapies (especially systemic therapies) and indications for various treatments, and only few studies exploring the sequence of effective treatment options or combining modern systemic therapies with liver targeted therapies, it is difficult to develop a procedural approach for the treatment of HCC. In fact, not all patients accepted both medications at the same time, and it involved the patients’ choice, financial affordability and doctor’s recommendation.
Here, we compared the prognosis of different combination of lenvatinib and PD-1 inhibitors in practical applications comprehensively. We classified patients into three groups: (a) lenvatinib plus simultaneous PD-1 inhibitor (Simultaneous group); (b) Patients received PD-1 inhibitor before the tumor progression (Before PD group); (c) Patients received PD-1 inhibitor after the tumor progression (After PD group). Our study revealed that lenvatinib with synchronous administration of PD-1 inhibitor were superior to treatment with the non-synchronous therapy (including the After PD and Before PD group) in patients with advanced HCC beyond oligometastasis. The OS and PFS were significantly longer than patients with non-synchronous therapy. The medium OS of Simultaneous group were 20.57 months, which was 4.47 months longer than Before PD group (16.10 months), 7.20 months longer than After PD group (13.37 months). The medium PFS of Simultaneous group were 10.86 months, which was 3.32 months longer than Before PD group (7.54 months), 4.3 months longer than After PD group (6.56 months).
The results of LEAP-002 are consistent with the efficacy and safety of early studies, and there is no significant statistical difference between lenvatinib plus pembrolizumab and the placebo group.Citation25 Another Retrospective multicenter study revealed that the combination of lenvatinib and carlizumab showed better efficacy than lenvatinib alone, the medium PFS were 10.3 and 7.5 months.Citation26 In the recent clinic trial of lenvatinib plus pembrolizumab administrated simultaneously on unresectable HCC, the medium OS and PFS were 22.0 and 9.3 months respectively.Citation17 Our result in Simultaneous group was a little shorter than that reported, the reason may that in our study, the patients were all with multi-metastases. However, the 3 months evaluation of response (ORR and DCR) in Simultaneous group were not obvious lower than reported (37.9% vs 41.0%), (82.8% vs 86.0%).Citation17 Although in our investigation, more advanced stage of HCC was reported, most individuals accepted the radiotherapy, TACE or ablation before enrolled for analysis, and the primary tumor and metastases were controlled to a certain extent. Besides, TACE, ablation or radiotherapy caused the tumor necrosis, which stimulated the immune response of the system. Scientific research suggested that the immunomodulatory effect of lenvatinib complemented PD-1 inhibitor activity through reversed the immunosuppressive effects of VEGF in the tumor microenvironment, thus increasing tumors sensitivity to combination therapy.Citation27
The REFLECT study of lenvatinib in the treatment of unresectable HCC showed medium OS and PFS were 13.6 and 8.9 months respectively.Citation28 In our research, the After PD group OS and PFS were 13.37 and 6.56 months. The OS was similar with REFLECT study, and the PFS was shorter than it. In our study, the patients enrolled were more advanced, and a shorter PFS is acceptable After lenvatinib progression, patients benefited more after adding the PD-1 inhibitor than REFLECT. There was almost 10 months post-PFS survival. In KEYNOTE-224 trial, patients with advanced HCC were progression of sorafenib or intolerant to it, then these patients received pembrolizumab, the OS was 12.9 months.Citation29 Similarly, in CheckMate 040 trial, the OS was 15 months.Citation30 It was not surprising that the OS of these results were better than the After PD group due to the more advanced stage of patients in our study. The outcome in our After PD group provided preliminary exciting result that PD-1 inhibitor demonstrated considerable antitumor effects after lenvatinib failure.
In our study, patients with PVTT, other organs metastasis, lung plus other organs metastasis were associated with lower survival rates in the multivariate analysis. An increasing number of studies have reported the efficacy and safety of lenvatinib in patients with HCC complicated with PVTT.Citation31–35 Many studies had revealed that PVTT as a crucial factor associated with poorer OS,Citation36 and in advanced HCC, extrahepatic metastases had also been shown to be a poor prognostic factor. Lung is the most common extrahepatic metastatic site in advanced HCC patients, accounting almost 20–40% of HCC metastases (followed by lymph nodes, bones, and adrenal glands).Citation37,Citation38 Nevertheless, there are few literatures reports on the prognosis of different kind of extrahepatic metastases. We divided metastases into lung, other organs, lung plus other organ. And analysis was conducted on the prognosis of different therapy (Synchronous, Before PD, After PD) for different types of metastatic tumors. The result showed that patients with lung metastases or patients with other organs metastases, both OS and PFS were the best with the use of simultaneous PD-1 inhibitor among the three group. However, for patients with lung and other organs, there was no difference among the three groups. This observation revealed that for patients with lung and other organs, there was limited improvement of OS among the different treatments.
Several limitations that need to be clarified. Firstly, this was a retrospective study, which inevitably has selection bias even we conduct multi-center research. Secondly, it is impossible to completely exclude interference from the doctor and patient self-choice in terms of patient enrollment and medication, since it was a real-world study about the application of lenvatinib and PD-1 inhibitor. Further prospective randomized could be designed to prove our results. Thirdly, although it was a multi-centers study, the insufficient sample size may have some impact on the results. Last but not least, we did not compare the differences between targeted therapy combined immunotherapy and local treatment. The combination of targeted drugs and immune checkpoint inhibitors, or the combination of two immune checkpoint inhibitor drugs, combined with comprehensive treatment, including tumor ablation therapy, interventional therapy, radiotherapy. These are all treatment plans that we need to constantly explore in the future to find more effective and accurate treatment methods to combat HCC.
Conclusion
In conclusion, our research showed that synchronous combination of lenvatinib with PD-1 inhibitors can significantly improve advanced HCC beyond oligometastasis. The simultaneous combination of lenvatinib and PD-1 inhibitors for the treatment of HCC with multi-metastases may be a promising strategy, as these agents induce the optimal tumor response in a complementary manner.
Abbreviations
HCC, hepatocellular carcinoma; BCLC, Barcelona Clinic Liver Cancer staging system; TACE, transcatheter arterial chemoembolization; PD-1, programmed death receptor-1 (PD-1); AFP, alpha-fetoprotein; ALBI, albumin-bilirubin; ALT, alanine transaminase; AST, aspartate aminotransferase; OS: overall survival; PFS, progression-free survival.
Ethics Approval and Informed Consent
The Ethics Committee Board of Hunan Provincial People’s Hospital approved this retrospective study and waived the requirement for patient consent for this retrospective review. We solemnly promise that this study will strictly abide by relevant laws and regulations, and will not disclose patient personal information and related information to any other personnel and organizations to ensure the security and confidentiality of patient information.
Disclosure
The authors declare no conflicts of interest in this work.
Acknowledgments
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Data Sharing Statement
It is available by contacting the corresponding author.
References
- The cancer of the liver Italian program. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the cancer of the liver Italian program (CLIP) investigators. Hepatology. 1998;28(3):751–755. doi:10.1002/hep.510280322
- Xu XL, Liu XD, Liang M, et al. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: systematic review of randomized controlled trials with meta-analysis and trial sequential analysis. Radiology. 2018;287:461–472. doi:10.1148/radiol.2017162756
- Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156:477–491.e471. doi:10.1053/j.gastro.2018.08.065
- Ko KL, Mak LY, Cheung KS, et al. Hepatocellular carcinoma: recent advances and emerging medical therapies. F1000Res. 2020;9:620. doi:10.12688/f1000research.24543.1
- Uchino K, Tateishi R, Shiina S, et al. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer. 2011;117:4475–4483. doi:10.1002/cncr.25960
- Natsuizaka M, Omura T, Akaike T, et al. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005;20:1781–1787. doi:10.1111/j.1440-1746.2005.03919.x
- Peng Z, Fan W, Zhu B, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: A Phase III, randomized clinical trial (LAUNCH). J Clin Oncol. 2023;41:117–127. doi:10.1200/jco.22.00392
- Lee HS. Management of patients with hepatocellular carcinoma and extrahepatic metastasis. Dig Dis. 2011;29:333–338. doi:10.1159/000327572
- Beckham TH, Yang TJ, Gomez D, et al. Metastasis-directed therapy for oligometastasis and beyond. Br J Cancer. 2021;124:136–141. doi:10.1038/s41416-020-01128-5
- Hsu CY, Liu PH, Ho SY, et al. Metastasis in patients with hepatocellular carcinoma: prevalence, determinants, prognostic impact and ability to improve the Barcelona Clinic Liver Cancer system. Liver Int. 2018;38:1803–1811. doi:10.1111/liv.13748
- Pinter M, Scheiner B, Peck-Radosavljevic M. Immunotherapy for advanced hepatocellular carcinoma: a focus on special subgroups. Gut. 2021;70:204–214. doi:10.1136/gutjnl-2020-321702
- Wong AC, Watson SP, Pitroda SP, et al. Clinical and molecular markers of long-term survival after oligometastasis-directed stereotactic body radiotherapy (SBRT). Cancer. 2016;122:2242–2250. doi:10.1002/cncr.30058
- Salama JK, Milano MT. Radical irradiation of extracranial oligometastases. J Clin Oncol. 2014;32:2902–2912. doi:10.1200/JCO.2014.55.9567
- Feng Z, Rong P, Wang W. Meta-analysis of the efficacy and safety of PD-1/PD-L1 inhibitors administered alone or in combination with anti-VEGF agents in advanced hepatocellular carcinoma. Gut. 2020;69:1904–1906. doi:10.1136/gutjnl-2019-320116
- Liu Z, Lin Y, Zhang J, et al. Molecular targeted and immune checkpoint therapy for advanced hepatocellular carcinoma. J Experim Clin Can Res. 2019;38:447. doi:10.1186/s13046-019-1412-8
- Zhu XD, Sun HC. Emerging agents and regimens for hepatocellular carcinoma. J Hematol Oncol. 2019;12:110. doi:10.1186/s13045-019-0794-6
- Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38:2960–2970. doi:10.1200/jco.20.00808
- Bedrose S, Miller KC, Altameemi L, et al. Combined lenvatinib and pembrolizumab as salvage therapy in advanced adrenal cortical carcinoma. J ImmunoTher Can. 2020;8:e001009. doi:10.1136/jitc-2020-001009
- Wang Y, Jiang M, Zhu J, et al. The safety and efficacy of lenvatinib combined with immune checkpoint inhibitors therapy for advanced hepatocellular carcinoma. Biomed Pharmacothe. 2020;132:110797. doi:10.1016/j.biopha.2020.110797
- World Medical A. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi:10.1001/jama.2013.281053
- Hiraoka A, Kumada T, Michitaka K, et al. Newly Proposed ALBI Grade and ALBI-T Score as Tools for Assessment of Hepatic Function and Prognosis in Hepatocellular Carcinoma Patients. Liver Cancer. 2019;8:312–325. doi:10.1159/000494844
- Lu J, Zhang XP, Zhong BY, et al. Management of patients with hepatocellular carcinoma and portal vein tumour thrombosis: comparing east and west. Lancet Gastroenterol Hepatol. 2019;4:721–730. doi:10.1016/S2468-1253(19)30178-5
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. doi:10.1016/j.ejca.2008.10.026
- Qin S, Li Q, Gu S, et al. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): a multicentre, double-blind, randomised, placebo-controlled, Phase 3 trial. Lancet Gastroenterol Hepatol. 2021;6:559–568. doi:10.1016/S2468-1253(21)00109-6
- Llovet JM, Kudo M, Merle P, et al. Lenvatinib plus pembrolizumab versus lenvatinib plus placebo for advanced hepatocellular carcinoma (LEAP-002): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24:1399–1410. doi:10.1016/S1470-2045(23)00469-2
- Li Q, Cao M, Yuan G, et al. Lenvatinib plus camrelizumab vs. lenvatinib monotherapy as first-line treatment for unresectable hepatocellular carcinoma: A multicenter retrospective cohort study. Front Oncol. 2022;12:809709. doi:10.3389/fonc.2022.809709
- Gunda V, Gigliotti B, Ashry T, et al. Anti-PD-1/PD-L1 therapy augments lenvatinib’s efficacy by favorably altering the immune microenvironment of murine anaplastic thyroid cancer. Int j Canc. 2019;144:2266–2278. doi:10.1002/ijc.32041
- Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi:10.1016/S0140-6736(18)30207-1
- Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label Phase 2 trial. Lancet Oncol. 2018;19:940–952. doi:10.1016/S1470-2045(18)30351-6
- El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, Phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi:10.1016/s0140-6736(17)31046-2
- Takahashi K, Kim J, Takahashi A, et al. Conversion hepatectomy for hepatocellular carcinoma with main portal vein tumour thrombus after lenvatinib treatment: a case report. World j hepato. 2021;13:384–392. doi:10.4254/wjh.v13.i3.384
- Luo F, Li M, Ding J, et al. The progress in the treatment of hepatocellular carcinoma with portal vein tumor thrombus. Front Oncol. 2021;11:635731. doi:10.3389/fonc.2021.635731
- Mukozu T, Nagai H, Matsui D, et al. Adaptation of lenvatinib treatment in patients with hepatocellular carcinoma and portal vein tumor thrombosis. Canc Chemother Pharm. 2022;89:11–20. doi:10.1007/s00280-021-04359-2
- Chuma M, Uojima H, Hiraoka A, et al. Analysis of efficacy of lenvatinib treatment in highly advanced hepatocellular carcinoma with tumor thrombus in the main trunk of the portal vein or tumor with more than 50% liver occupation: a multicenter analysis. Hepat rese. 2021;51:201–215. doi:10.1111/hepr.13592
- Kuzuya T, Ishigami M, Ito T, et al. Sorafenib vs. lenvatinib as first-line therapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Anticanc Res. 2020;40:2283–2290. doi:10.21873/anticanres.14193
- Kaneko S, Tsuchiya K, Yasui Y, et al. Strategy for advanced hepatocellular carcinoma based on liver function and portal vein tumor thrombosis. Hepat rese. 2020;50:1375–1385. doi:10.1111/hepr.13567
- Yan B, Bai DS, Zhang C, et al. Characteristics and risk differences of different tumor sizes on distant metastases of hepatocellular carcinoma: a retrospective cohort study in the SEER database. Int j Surg. 2020;80:94–100. doi:10.1016/j.ijsu.2020.06.018
- Mokdad AA, Singal AG, Marrero JA, et al. Vascular Invasion and Metastasis is Predictive of Outcome in Barcelona Clinic Liver Cancer Stage C Hepatocellular Carcinoma. J Natiol Compreh Canc Net. 2017;15:197–204. doi:10.6004/jnccn.2017.0020
