Abstract
Psoriasis is a chronic inflammatory skin disease characterized by the excessive proliferation of keratinocytes and heightened immune activation. Targeting pathogenic genes through small interfering RNA (siRNA) therapy represents a promising strategy for the treatment of psoriasis. This mini-review provides a comprehensive summary of siRNA research targeting the pathogenesis of psoriasis, covering aspects such as keratinocyte function, inflammatory cell roles, preclinical animal studies, and siRNA delivery mechanisms. It details recent advancements in RNA interference that modulate key factors including keratinocyte proliferation (Fibroblast Growth Factor Receptor 2, FGFR2), apoptosis (Interferon Alpha Inducible Protein 6, G1P3), differentiation (Grainyhead Like Transcription Factor 2, GRHL2), and angiogenesis (Vascular Endothelial Growth Factor, VEGF); immune cell infiltration and inflammation (Tumor Necrosis Factor-Alpha, TNF-α; Interleukin-17, IL-17); and signaling pathways (JAK-STAT, Nuclear Factor Kappa B, NF-κB) that govern immunopathology. Despite significant advances in siRNA-targeted treatments for psoriasis, several challenges persist. Continued scientific developments promise the creation of more effective and safer siRNA medications, potentially enhancing the quality of life for psoriasis patients and revolutionizing treatments for other diseases. This article focuses on the most recent research advancements in targeting the pathogenesis of psoriasis with siRNA and explores its future therapeutic prospects.
Introduction
Psoriasis is a chronic, non-infectious, and disabling disease, with a global prevalence of approximately 2–3% and an increasing incidence over recent years.Citation1,Citation2 It affects both genders equally, with an average age of onset at 33 years, though it generally occurs earlier in females.Citation3 Characterized by the excessive proliferation and abnormal differentiation of keratinocytes, along with immune cell infiltration into the epidermis and dermis, this abnormal immune activation leads to the development of raised, scaly, and erythematous skin patches. The pathogenesis of psoriasis remains complex and not yet fully elucidated. Currently, topical and systemic treatments represent the first-line therapeutic approaches for psoriasis. Topical treatments, typically used in the early stages, involve the administration of vitamin D3 or corticosteroids, supplemented by phototherapy.Citation4 Systemic treatments primarily comprise immunosuppressants, non-specific anti-inflammatory drugs, and molecular targeted therapies. In particular, the introduction of biologics targeting key inflammatory cytokines such as Tumor Necrosis Factor-Alpha (TNF-α) and Interleukin-17 (IL-17) has revolutionized the treatment of moderate to severe psoriasis over the past decade.Citation5 Compared to early-stage treatments, systemic therapies are costlier and may increase the risk of infections.Citation6,Citation7 The introduction of biologic therapies has markedly improved the treatment of moderate to severe psoriasis. Methods of administration have evolved from subcutaneous delivery to include oral and topical medications. For instance, Tapinarof (an AhR modulator) and Roflumilast (a PDE-4 inhibitor) have demonstrated favorable efficacy and safety outcomes, leading to their approval by the FDA for the topical treatment of plaque psoriasis.Citation8 Psoriasis still presents many unresolved issues, notably adverse drug reactions and disease recurrence after medication discontinuation. Novel therapies may offer a promising strategy for managing these challenges and maintaining treatment efficacy.
Recently, RNA interference (RNAi) has emerged as a promising therapeutic strategy, potentially offering distinct advantages over existing systemic treatments for psoriasis. RNAi is an endogenous cellular pathway through which small double-stranded RNA molecules, known as small interfering RNAs (siRNAs), induce sequence-specific post-transcriptional gene silencing. siRNAs, which are small double-stranded RNA molecules, silence genes by interrupting the translation of DNA into proteins, thereby selectively interfering with the expression of specific genes.Citation9 This process can selectively target and knock out almost any gene. Consequently, siRNA therapy possesses the potential for precise and effective suppression of specific targets crucial to the pathogenesis of psoriasis. The successful application of Onpattro (Patisiran), the first RNAi therapeutic drug approved by the FDA, is viewed as an acknowledgment of the immense potential of siRNA in treating numerous diseases.Citation10 In December 2021, the FDA approved inclisiran for the treatment of arteriosclerotic cardiovascular disease (ASCVD), characterized by elevated LDL-C levels or heterozygous familial hypercholesterolemia (HeFH). Research has confirmed that inclisiran effectively reduces LDL-C levels in patients for whom statin therapy alone is inadequate.Citation11 Furthermore, the development of efficient siRNA delivery systems, such as siRNA nanocarriers, in clinical trials has significantly advanced siRNA therapy.Citation12 This mini-review summarizes the latest advances and explores future prospects for understanding and treating psoriasis at the molecular level through RNAi technology. Specifically, it focuses on the key studies utilizing siRNA to target and silence key mediators of excessive proliferation in keratinocytes, inflammatory immune responses, cell signaling pathways, and the differentiation processes known to drive psoriasis formation. To date, no clinical trial reports on the treatment of psoriasis with siRNA have been identified. The available research is limited to in vitro cellular experiments (involving lesional and non-lesional skin tissues from psoriasis patients as well as normal skin tissues from healthy individuals) and animal studies (such as imiquimod-induced psoriasis in mice). Consequently, this article systematically summarizes the therapeutic prospects and challenges of siRNA in treating psoriasis, aiming to provide a theoretical reference for translating preclinical siRNA results into clinical therapies.
Methods
The application of siRNAs has expanded significantly with advancing research into their physiological effects and mechanisms of action. This paper presents a comprehensive review to summarize and evaluate the potential of siRNAs in the treatment of psoriasis. For this review, two researchers independently conducted a systematic search in the PubMed database. The search was conducted using “siRNA” and “Psoriasis” as keywords, with the deadline set for November 2023. The search engine for this study retrieved a total of 158 publications; 21 were excluded after reviewing their titles, which included unrelated reviews and titles not pertinent to the study’s subject. One hundred thirty-seven documents were retained and analyzed for their abstracts and full texts; 62 were subsequently excluded due to irrelevance or incomplete information. Ultimately, 75 articles were included for analysis.
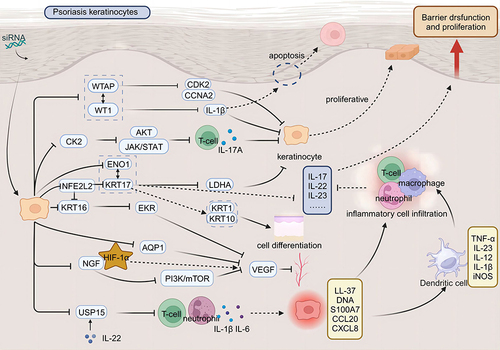
Targeting Keratinocytes
The pathogenesis of psoriasis is complex, involving intricate interactions among keratinocytes, immune cells, and other resident skin cells. It is characterized by the abnormal proliferation and differentiation of keratinocytes, alongside excessive infiltration of immune cells into the dermis and epidermis.Citation13 siRNA technology can inhibit the progression of psoriasis by targeting the proliferation, differentiation, apoptosis, and migration of keratinocytes.
Mediating the Proliferation and Apoptosis of Keratinocytes
The pathogenesis of psoriasis is complex, primarily due to the imbalance between the proliferation and apoptosis of keratinocytes. Abnormal proliferation of keratinocytes results in excessive cell accumulation on the skin surface, forming characteristic psoriatic lesions. Concurrently, aberrant apoptosis contributes further to this accumulation, exacerbating psoriasis symptoms.
Firstly, siRNA-mediated gene silencing exerts specific effects on the proliferation and apoptosis of keratinocytes. For instance, siRNA silencing of Fibroblast Growth Factor Receptor 2 (FGFR2) inhibits keratinocyte proliferation.Citation14 Similarly, knockdown of Nuclear Factor of Activated T Cells 2 (NFAT2) reduces keratinocyte proliferation and decreases epidermal thickness.Citation15 Additionally, TRAF3 Interacting Protein 2 (TRAF3IP2) knockdown diminishes the proliferation of keratinocytes and endothelial cells by promoting apoptotic signaling and blocking the G2/M cell cycle phase.Citation16 Knockdown of Interferon Alpha Inducible Protein 6 (G1P3) via siRNA promotes apoptosis.Citation17 Further, siRNA knockdown experiments have confirmed the role of Aldo-Keto Reductase Family 1 Member B10 (AKR1B10) in promoting excessive proliferation of keratinocytes.Citation18 siRNA silencing of Proteasome Maturation Protein (POMP) inhibits keratinocyte proliferation and induces apoptosis by disrupting proteasome assembly.Citation19 Circ-Insulin Like Growth Factor 1 Receptor (circIGF1R) silencing, through regulation of the MicroRNA-194-5p/Cyclin Dependent Kinase 1 (CDK1) axis, inhibits keratinocyte proliferation, migration, and invasion, and induces apoptosis.Citation20 Knockdown of Pituitary Tumor-Transforming 2 (PTTG2) leads to the downregulation of vimentin and upregulation of E-cadherin, thereby enhancing the vitality and migration of keratinocytes.Citation21 siRNA silencing of MIR31 Host Gene (MIR31HG) induces cell cycle arrest at the G2/M phase and inhibits proliferation.Citation22 Additionally, certain siRNA-mediated gene knockdowns exhibit inhibitory effects on cell proliferation under specific conditions. For example, siRNA-mediated inhibition of Karyopherin Subunit Alpha (KPNA) is crucial for significantly suppressing keratinocyte proliferation under starvation conditions.Citation23
Secondly, beyond individual gene siRNA silencing, analysis across multiple studies suggests that some genes exhibit inter-regulatory relationships. WT1 Associated Protein (WTAP) is linked to WT1 Transcription Factor (WT1), with both showing close functional and expression relationships. Low expression of WT1 inhibits keratinocyte proliferation and promotes apoptosis, whereas its overexpression has the opposite effects.Citation24 Similarly, overexpression of WTAP enhances keratinocyte proliferation, while its inhibition reduces proliferation.Citation25 Therefore, WTAP and WT1 may exhibit an upstream-downstream regulatory relationship, suggesting the need for further joint research. Early Growth Response 1 (EGR1) and Polo Like Kinase 2 (PLK2) are pro-proliferative factors with mutual regulation and interaction between EGR1 and WT1. In both physiological and pathological processes, they collaboratively participate in cell growth, differentiation, and tumorigenesis.Citation26 Targeting EGR1 and PLK2 with specific siRNA in keratinocytes significantly reduces their expression, leading to decreased keratinocyte proliferation.Citation27
Keratin 16 (KRT16) and Keratin 17 (KRT17) are members of the same keratin gene family, with several genes potentially related to both KRT16 and KRT17. Both KRT16 and KRT17 exhibit high expression levels in keratinocytes. Silencing the KRT16 gene inhibits keratinocyte proliferation,Citation28 while suppression of KRT17 gene expression can both inhibit proliferation and induce apoptosis in these cells.Citation29 NFE2L2 enhances keratinocyte proliferation by upregulating Keratin 6 (KRT6), KRT16, and KRT17; locally applied NFE2L2 small interfering RNA subsequently reduces epidermal hyperplasia and decreases the expression of these keratins.Citation30 Fatty Acid Binding Protein 5 (E-FABP), highly expressed in the epidermis of psoriasis patients, shows that its significant inhibition markedly reduces cellular differentiation and upregulates psoriasis markers like KRT16.Citation31 siRNA-mediated knockdown of Enolase 1 (ENO1) significantly reduces both glycolysis and keratinocyte proliferation. Luo et al demonstrated the interaction between KRT17 and ENO1, which promotes glycolysis and proliferation in keratinocytes.Citation32
Many key pathogenic mediators of psoriasis are associated with the Janus kinase/Signal Transducer and Activator of Transcription (JAK-STAT) signaling pathway.Citation33 The JAK-STAT signaling pathway plays a crucial role in the transduction of cytokine and growth factor signals, regulating inflammatory responses, immune cell activation, keratinocyte proliferation, and abnormal differentiation.Citation34 Signal Transducer and Activator of Transcription 3 (STAT3) is highly expressed and activated in psoriatic keratinocytes, with its activity positively correlating with the severity of psoriasis. Silencing the STAT3 gene in psoriatic keratinocytes using siRNA, combined with ultrasound irradiation and microbubble technology, inhibits keratinocyte growth and induces apoptosis.Citation35 Transfection of keratinocytes, whether treated or untreated with avidin, with siRNA targeting Signal Transducer and Activator of Transcription 1 (STAT1) and STAT3, confirmed that avidin regulates psoriatic keratinocyte proliferation via the STAT signaling pathway.Citation36 Inhibiting Casein Kinase 2 (CK2) suppresses both the STAT3 and Threonine Kinase (AKT) signaling pathways in human keratinocytes, which inhibits epidermal hyperplasia, abnormal differentiation, and the inflammatory response.Citation37 Protein interaction networks have shown a strong interaction between Bcl-2 and STAT3, and further investigation of this relationship could reveal cellular regulatory mechanisms and provide new insights into the treatment of related diseases. Silencing BCL2L1 and IGF1R with siRNA induces growth inhibition, apoptosis, and increased UVB sensitivity in keratinocytes.Citation38
Mediating the Differentiation of Keratinocytes
In addition to excessive proliferation, abnormal differentiation of keratinocytes is a key characteristic of psoriasis.Citation39 Multiple studies have demonstrated that inhibiting genes regulating differentiation can shift the keratinocyte phenotype from psoriatic to normal. siRNA-mediated knockdown of Grainyhead Like Transcription Factor 2 (GRHL2) promotes normal differentiation in keratinocytes.Citation40 Silencing Sphingosine-1-Phosphate Lyase 1 (SGPL1) inhibits S1P cleavage enzymes in keratinocytes, thereby reducing cell proliferation and promoting normal differentiation.Citation41,Citation42 Silencing Cathepsin B (CTSB) rescues both the excessive proliferation and inflammatory responses induced by IL-17A and Serum Amyloid A (SAA), and corrects the deficiency in normal differentiation.Citation43 Exogenous CK2 promotes excessive proliferation and abnormal differentiation in human keratinocytes, which can be reversed by siRNA-mediated CK2 inhibition. CK2 downregulation reduces IL-17A expression and eliminates both proliferation and inflammatory cytokine expression in keratinocytes induced by IL-17A.Citation37 Furthermore, Gasdermin D gene knockout in mice exhibited normal keratinocyte proliferation and differentiation, and provided protection against psoriasiform inflammation. This suggests that siRNA-mediated gene silencing aimed at regulating differentiation may serve as a coordinated therapeutic approach for treating psoriasis symptoms.Citation44
Inhibiting VEGF Secretion, Impeding Angiogenesis
Angiogenesis is abnormally active in psoriatic lesions and is closely related to disease progression.Citation45 Inflammatory cells promote endothelial cell migration, proliferation, and survival by releasing factors like Vascular Endothelial Growth Factor (VEGF). This activity induces angiogenesis, which in turn leads to the formation of vascular erythematous plaques, thereby exacerbating the condition.Citation46,Citation47
Aquaporin-1 (AQP1) functions as a water channel membrane protein. Nicchia et al employed siRNA to knock out AQP1, confirming its role as an angiogenic protein implicated in treating diseases related to angiogenesis, including cancer, psoriasis, endometriosis, arthritis, and atherosclerosis. The role of AQP1 in sustaining endothelial cell activity during angiogenesis may represent a potential target for anti-angiogenic therapies.Citation48 Nerve Growth Factor (NGF) regulates VEGF expression in human keratinocytes via the Phosphatidylinositol-4,5-Bisphosphate 3-Kinase/Mechanistic Target of Rapamycin Kinase (PI3K/MTOR) signaling pathway, and siRNA-mediated silencing of Hypoxia Inducible Factor 1 Subunit Alpha (HIF-1α) can block the NGF-induced transcriptional increase of VEGF.Citation49 Downregulation of TRAF3 Interacting Protein 2 (TRAF3IP2) alters both the expression and secretion of VEGF in keratinocytes and endothelial cells.Citation16 KRT16 gene silencing inhibits not only keratinocyte proliferation via the Extracellular Signal-Related Kinase (ERK) signaling pathway but also the secretion of VEGF by keratinocytes.Citation28 EGF Like Repeats And Discoidin Domains 3 (EDIL3) induce angiogenesis in endothelial cells through the αvβ3-FAK/MEK/ERK signaling pathway.Citation50
Regulating Inflammatory Cells, Reducing Inflammatory Cell Infiltration
Psoriasis is a chronic inflammatory skin disease that is closely associated with the activity of inflammatory cells. Histopathological features of psoriatic skin include excessive proliferation and abnormal differentiation of epidermal keratinocytes, which lead to epidermal thickening, accompanied by the infiltration of immune cells.Citation51 Extensive infiltration of lymphocytes, macrophages, and neutrophils into the skin characterizes the hallmark of psoriatic lesions.Citation52
Neutrophil infiltration, a marker of psoriasis, is significantly reduced following treatment with mesenchymal stem/stromal cells (MSCs)-IT. Mesenchymal stem cells transduced with TNF Alpha Induced Protein 6 (TSG-6) siRNA lose their therapeutic effect; however, applying recombinant TSG-6 alone can decrease neutrophil infiltration and alleviate psoriatic lesions. This finding confirms the potential therapeutic application of blocking neutrophil recruitment via MSCs-IT or TSG-6 in the treatment of psoriasis.Citation53 Knockout of Ubiquitin Specific Peptidase 15 (USP15) results in reduced inflammation in psoriatic keratinocytes, as well as impaired vitality and clonogenicity. Topical application of USP15 small interfering RNA significantly improves imiquimod (IMQ)-induced psoriatic dermatitis by reducing the infiltration of T cells and neutrophils.Citation54 Experimental research on the therapeutic effects of KRT17-specific siRNA in IMQ-induced psoriasiform dermatitis in mice demonstrated a significant reduction in the severity of ear dermatitis, characterized by a reduced inflammatory phenotype, including decreased ear thickness and reduced infiltration of inflammatory cells (CD3+ T cells and neutrophils).Citation55
Preclinical Animal Research Progress
Preclinical animal experiments are a crucial step in translating gene therapy into clinical research. Consequently, effectively silencing genes through single-gene siRNA represents an important focus for future in-depth studies. Silencing NFKB Inhibitor Zeta (NFKBIZ) via siRNA has revealed its role as a key regulatory factor for specific psoriasis-related genes and proteins induced by IL-17F.Citation56,Citation57 Additionally, local intradermal injection of NFKBIZ siRNA to ablate NFKBIZ function can eliminate psoriasiform skin inflammation,Citation58 suggesting that NFKBIZ antagonists may offer a targeted approach for treating psoriasis. Experimental research on KRT17-specific siRNA in IMQ-induced psoriasiform dermatitis in mice has shown it reduces inflammatory cell infiltration and decreases expression levels of IL-17, IL-22, and IL-23, 55 indicating KRT17’s potential as a new target for future psoriasis treatments. The isoflavonoid compound Lignum vitae can alleviate psoriasiform inflammation in mice by inhibiting the STAT3 and Nuclear Factor Kappa B (NF-κB) signaling pathways.Citation59 Fan et al demonstrated that silencing REL in mice not only prevents and improves IMQ-induced psoriasis but also significantly blocks the expression of various inflammatory cytokines.Citation60 Increased expression of Phospholipase A2 Group IVB (PLA2G4B) in the early stages of inflammation triggers the release of inflammatory factors like IL-17 and IL-36 by CD8+ naive and Th17 T cells, exacerbating inflammation and keratinocyte proliferation at lesion sites; however, knockdown of PLA2G4B improves inflammation.Citation61 Oxymatrine alleviates itching and inflammation in psoriasis by inhibiting Heat Shock Protein 90 (HSP90) and Heat Shock Protein 60 (HSP60) in keratinocytes via the Mitogen-Activated Protein Kinase (MAPK) signaling pathway.Citation62 In the piclidenoson-induced psoriasis mouse model, local application of Interferon Alpha Inducible Protein 27 (IFI27) siRNA reduces epidermal thickness, rete ridge formation, and Proliferating Cell Nuclear Antigen (PCNA) expression.Citation63
Optimizing siRNA Delivery - Novel Drug Development
Although siRNA therapy holds great promise, delivering intact siRNA across the stratum corneum barrier poses a significant challenge for topical treatments. Advanced drug delivery systems are currently being developed to overcome these challenges and optimize local siRNA delivery for psoriasis. For instance,Citation9 laser-assisted siRNA delivery methods have improved in vivo psoriasiform lesion models by silencing epidermal IL-6, thereby improving psoriatic plaques; Solid silicon microneedle arrays have perforated and delivered cholesterol-modified GAPDH siRNA into mouse ear skin, reducing GAPDH expression by up to 66% without major organ accumulation. This demonstration marks the first effective, non-invasive delivery of siRNA to relevant skin areas using solid microneedle arrays;Citation64 Effective skin penetration of siRNA has been achieved through non-covalent complexation with hydrophobic cationic or choline-geranic acid ionic liquids (CAGE);Citation65 Transdermal IL platforms, utilizing ionic liquids, not only achieved significant gene ablation in an imiquimod-induced psoriasiform skin inflammation model but also permitted repeated application and scalability;Citation66 Furthermore, SPACE peptides combined with siRNA have enhanced both skin absorption of siRNA and the knockdown of corresponding protein targets.Citation67 Additionally, techniques like nanostructured lipid carriers, nanoparticles, and SECosome technology have enhanced siRNA delivery and its anti-psoriatic effects. In summary, the development of numerous siRNA delivery platforms is expected to gradually overcome penetration issues, enabling more precise and effective siRNA delivery, leading to clinical translation.
Advantages and Limitations
The advantages of siRNA therapy in treating psoriasis are becoming increasingly evident with the continuous advancement of technology. For instance, combining siRNA therapy with other treatments, such as topical medications and phototherapy, is evolving in clinical practice to enhance efficacy and reduce side effects. This novel therapeutic approach may hold considerable potential for addressing disease relapse and maintaining treatment effects. Furthermore, the ongoing development of new delivery systems, including nanoparticles, nano-lipid carriers, and solid microneedle arrays, shows promise in potentially resolving drug delivery challenges. However, it remains an undeniable fact that the application of siRNA therapy in psoriasis treatment is still at a preliminary exploration stage. Current research is largely confined to in vitro cell studies and animal experiments, with a lack of convincing clinical trial evidence. Although most existing studies have achieved satisfactory outcomes, concerns persist that targeting gene silencing could lead to severe consequences. For example, studies have shown that a significant proportion of mice with targeted silencing of NFKBIZ did not survive,Citation56 highlighting potential risks associated with siRNA technology. Although optimizing delivery platforms may address drug delivery difficulties, their application in clinical trials has not yet been realized, complicating the assessment of long-term effects and risks associated with carrier materials.Citation68 Additionally, the cost of applying this novel treatment approach presents a significant barrier to transitioning from in vitro experiments to clinical applications.
Discussions
As a novel therapeutic approach, siRNA offers notable advantages, including high specificity and minimal side effects. It holds considerable potential in the treatment of psoriasis (). Recent studies have elucidated siRNA’s pivotal role in modulating the pathogenesis of psoriasis. The application of siRNA can inhibit a range of mediators responsible for psoriasis development, including overproliferative agents, inflammatory cytokines, cellular signaling molecules, and differentiation regulators ().
Table 1 Therapeutic Role of siRNA in Psoriasis and Its Potential Applications
Figure 1 Therapeutic applications of siRNA in targeting pathogenic pathways underlying psoriasis. Therapeutic application of small interfering RNA (siRNA) targets the pathogenic pathways of psoriasis. In a physiological state, skin keratinocytes proliferate normally and fulfill a barrier function. In the pathological state of psoriasis, immune cells activate keratinocytes, leading to their rapid proliferation, abnormal differentiation, and inhibited apoptosis; this also promotes angiogenesis and increases inflammatory cell infiltration. Such activation disrupts the skin barrier function and causes a dysregulation in the normal proliferative capacity of keratinocytes, exacerbating the condition. siRNA therapy represents a promising strategy for treating psoriasis by silencing disease-causing genes. siRNA targets keratinocytes, silencing genes such as Casein Kinase 2 (CK2), WT1 Associated Protein (WTAP), WT1 Transcription Factor (WT1), and Enolase 1 (ENO1), which inhibits their proliferation. This action induces their normal differentiation and promotes apoptosis. Additionally, silencing genes such as Aquaporin 1 (AQP1), Nerve Growth Factor (NGF), and Keratin 16 (KRT16) inhibits Vascular Endothelial Growth Factor (VEGF), thereby hindering angiogenesis in psoriasis; Furthermore, targeting genes such as Ubiquitin Specific Peptidase 15 (USP15) and Keratin 17 (KRT17) can reduce inflammatory cell infiltration and attenuate the inflammatory response.
Some genes identified in studies positively affect disease progression by slowing it down and thus, cannot be targeted for gene silencing. Studies have demonstrated that Serum Response Factor (SRF) expression is strongly downregulated in the hyperproliferative epidermis of wounded and psoriatic skin. Further, siRNA-mediated knockdown of SRF in primary human keratinocytes revealed that cytoskeletal abnormalities and adhesion defects were direct consequences of SRF loss.Citation81 Inhibition of Polycystin 1 (PC1) function was associated with increased cell proliferation and migration in human keratinocytes.Citation82 Furthermore, overexpression of Granulin Precursor (PGRN) has been shown to inhibit keratinocyte inflammation by negatively regulating the production of inflammatory factors and positively influencing autophagy.Citation83 Therefore, the selection of genes for siRNA silencing requires further investigation.
Psoriasis is one of the autoimmune diseases that significantly affects the quality of life of patients.Citation84 With skin manifestations that may be associated with psoriatic arthritis, cardiovascular diseases, and inflammatory bowel disease. Therapeutic approaches should target not only psoriasis itself but also adopt a holistic perspective that includes the treatment of associated comorbidities. Given that psoriasis and certain comorbidities share similar pathogenic and inflammatory pathways or cytokines, this holistic approach has prompted experimental studies to repurpose drugs originally developed for other conditions to treat diseases with shared pathogenic mechanisms.Citation85 Due to variability in disease manifestation and comorbidity profiles among patients, future treatment strategies may involve selecting personalized siRNA therapies tailored to individual needs and the specific comorbidities each patient faces. This potential direction in developing novel treatment approaches underscores the importance of personalized and holistic care in managing psoriasis and its related conditions.
Conclusions
Compared to current systemic biologics, siRNA therapy may offer a more targeted approach and potentially reduce side effects. Looking forward, priority areas include evaluating the synergistic effects of siRNA combinations on interconnected molecular targets and conducting clinical trials to translate this promising preclinical research into viable psoriasis treatments. Additionally, personalizing siRNA therapy to accommodate various disease courses and types will be a key direction for future research. Furthermore, reducing the toxicity, immunogenicity, and cost of siRNA carrier materials is crucial for translating RNAi technology from preclinical research to clinical applications. With ongoing in-depth research and continuous technological innovation, siRNA is expected to become a significant tool in the treatment of psoriasis. It is also anticipated to be used in treating other diseases as delivery technologies advance and application costs decrease.
Consent to Participate
All the authors listed in this manuscript have participated in the whole writing process of the manuscript and have informed consent to the publication of the manuscript.
Consent for Publication
The research article is original, has not already been published in any other journal (medical, or otherwise) and is not currently under consideration for publication by another journal, and does not infringe any existing copyright or any other rights prescribed by law.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Funding
References
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Cad Dermatol VenereoAl. 2017;31(2):205–212. doi:10.1111/jdv.13854
- Ghoreschi K, Balato A, Enerbäck C, et al. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet. 2021;397(10275):754–766. doi:10.1016/S0140-6736(21)00184-7
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370(9583):263–271. doi:10.1016/S0140-6736(07)61128-3
- Shen Q, Liu R, Tan S, et al. Advances in pathogenesis and nanoparticles (NPs)-mediated treatment of psoriasis. Front Immunol. 2022;13:1089262. doi:10.3389/fimmu.2022.1089262
- Yiu ZZ, Griffiths CE. Interleukin 17-A inhibition in the treatment of psoriasis. Expert Rev Clin Immunol. 2016;12(1):1–4. doi:10.1586/1744666X.2016.1112739
- D’Souza LS, Payette MJ. Estimated cost efficacy of systemic treatments that are approved by the US Food and Drug Administration for the treatment of moderate to severe psoriasis. J Am Acad Dermatol. 2015;72(4):589–598. doi:10.1016/j.jaad.2014.11.028
- Galloway JB, Hyrich KL, Mercer LK, et al. Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology. 2011;50(1):124–131. doi:10.1093/rheumatology/keq242
- Carmona-Rocha E, Rusiñol L, Puig L. New and emerging oral/topical small-molecule treatments for psoriasis. Pharmaceutics. 2024;16(2):239. doi:10.3390/pharmaceutics16020239
- Lee WR, Lin YK, Alalaiwe A, et al. Fractional laser-mediated siRNA delivery for mitigating psoriasis-like lesions via IL-6 silencing. Mol Ther Nucleic Acids. 2020;19:240–251. doi:10.1016/j.omtn.2019.11.013
- Saw PE, Song EW. siRNA therapeutics: a clinical reality. Sci China Life Sci. 2020;63(4):485–500. doi:10.1007/s11427-018-9438-y
- Lamb YN. Inclisiran: first Approval [published correction appears in Drugs. 2021 Jun;81(9):1129]. Drugs. 2021;81(3):389–395. doi:10.1007/s40265-021-01473-6
- Zhu L, Luo J, Ren K. Nucleic acid-based artificial nanocarriers for gene therapy. J Mater Chem B. 2023;11(2):261–279. doi:10.1039/D2TB01179D
- Ni X, Lai Y. Keratinocyte: a trigger or an executor of psoriasis? J Leukoc Biol. 2020;108(2):485–491. doi:10.1002/JLB.5MR0120-439R
- Xu N, Brodin P, Wei T, et al. MiR-125b, a microRNA downregulated in psoriasis, modulates keratinocyte proliferation by targeting FGFR2. J Invest Dermatol. 2011;131(7):1521–1529. doi:10.1038/jid.2011.55
- Hampton PJ, Jans R, Flockhart RJ, et al. Lithium regulates keratinocyte proliferation via glycogen synthase kinase 3 and NFAT2 (Nuclear Factor of Activated T Cells 2). J Cell Physiol. 2012;227(4):1529–1537. doi:10.1002/jcp.22872
- Song Y, Chen L, Li Y, et al. Knockdown of TRAF3IP2 suppresses the expression of VEGFA and the proliferation of keratinocytes and vascular endothelial cells. Heliyon. 2019;5(5):e01642. doi:10.1016/j.heliyon.2019.e01642
- Szegedi K, Sonkoly E, Nagy N, et al. The anti-apoptotic protein G1P3 is overexpressed in psoriasis and regulated by the non-coding RNA, PRINS. Exp Dermatol. 2010;19(3):269–278. doi:10.1111/j.1600-0625.2010.01066.x
- Gao Y, Yi X, Ding Y. Combined transcriptomic analysis revealed AKR1B10 played an important role in psoriasis through the dysregulated lipid pathway and overproliferation of keratinocyte. Biomed Res Int. 2017;2017:8717369. doi:10.1155/2017/8717369
- Zieba BA, Henry L, Lacroix M, et al. The proteasome maturation protein POMP increases proteasome assembly and activity in psoriatic lesional skin. J Dermatol Sci. 2017;88(1):10–19. doi:10.1016/j.jdermsci.2017.04.009
- Fang Y, C E, Wu S, et al. Circ-IGF1R plays a significant role in psoriasis via regulation of a miR-194-5p/CDK1 axis. Cytotechnology. 2021;73(6):775–785. doi:10.1007/s10616-021-00496-x
- Liu XB, Li F, Li YQ, et al. Pituitary tumor transforming gene PTTG2 induces psoriasis by regulating vimentin and E-cadherin expression. Int J Clin Exp Pathol. 2015;8(9):10887–10893.
- Gao J, Chen F, Hua M, et al. Knockdown of lncRNA MIR31HG inhibits cell proliferation in human HaCaT keratinocytes. Biol Res. 2018;51(1):30. doi:10.1186/s40659-018-0181-8
- Umegaki-Arao N, Tamai K, Nimura K, et al. Karyopherin alpha2 is essential for rRNA transcription and protein synthesis in proliferative keratinocytes. PLoS One. 2013;8(10):e76416. doi:10.1371/journal.pone.0076416
- Liao Y, Su Y, Wu R, et al. Overexpression of Wilms tumor 1 promotes IL-1β expression by upregulating histone acetylation in keratinocytes. Int Immunopharmacol. 2021;96:107793. doi:10.1016/j.intimp.2021.107793
- Kong Y, Wu R, Zhang S, et al. Wilms’ tumor 1-associating protein contributes to psoriasis by promoting keratinocytes proliferation via regulating cyclinA2 and CDK2. Int Immunopharmacol. 2020;88:106918. doi:10.1016/j.intimp.2020.106918
- Adamo P, Ladomery MR. The oncogene ERG: a key factor in prostate cancer. Oncogene. 2016;35(4):403–414. doi:10.1038/onc.2015.109
- Kristl J, Slanc P, Krasna M, et al. Calcipotriol affects keratinocyte proliferation by decreasing expression of early growth response-1 and polo-like kinase-2. Pharm Res. 2008;25(3):521–529. doi:10.1007/s11095-007-9388-z
- Chen JG, Fan HY, Wang T, et al. Silencing KRT16 inhibits keratinocyte proliferation and VEGF secretion in psoriasis via inhibition of ERK signaling pathway. Kaohsiung J Med Sci. 2019;35(5):284–296. doi:10.1002/kjm2.12034
- Chang T, Sun L, Wang Y, et al. Inhibition of keratin 17 expression with antisense and RNAi strategies: exploring novel therapy for psoriasis. Exp Dermatol. 2011;20(7):555–560. doi:10.1111/j.1600-0625.2010.01235.x
- Yang L, Fan X, Cui T, et al. Nrf2 Promotes Keratinocyte Proliferation in Psoriasis through Up-Regulation of Keratin 6, Keratin 16, and Keratin 17. J Invest Dermatol. 2017;137(10):2168–2176. doi:10.1016/j.jid.2017.05.015
- Dallaglio K, Marconi A, Truzzi F, et al. E-FABP induces differentiation in normal human keratinocytes and modulates the differentiation process in psoriatic keratinocytes in vitro. Exp Dermatol. 2013;22(4):255–261. doi:10.1111/exd.12111
- Luo Y, Pang B, Hao J, et al. Keratin 17 covalently binds to alpha-enolase and exacerbates proliferation of keratinocytes in psoriasis. Int J Biol Sci. 2023;19(11):3395–3411. doi:10.7150/ijbs.83141
- Yu J, Zhao Q, Wang X, et al. Pathogenesis, multi-omics research, and clinical treatment of psoriasis. J Autoimmun. 2022;133:102916. doi:10.1016/j.jaut.2022.102916
- Zhou X, Chen Y, Cui L, et al. Advances in the pathogenesis of psoriasis: from keratinocyte perspective. Cell Death Dis. 2022;13(1):81. doi:10.1038/s41419-022-04523-3
- Ran L-W, Wang H, Lan D, et al. Effect of RNA interference targeting STAT3 gene combined with ultrasonic irradiation and SonoVue microbubbles on proliferation and apoptosis in keratinocytes of psoriatic lesions. Chin Med J. 2018;131(17):2097–2104. doi:10.4103/0366-6999.239297
- Qin X, Chen C, Zhang Y, et al. Acitretin modulates HaCaT cells proliferation through STAT1- and STAT3-dependent signaling. Saudi Pharm J. 2017;25(4):620–624. doi:10.1016/j.jsps.2017.04.034
- Huang W, Zheng X, Huang Q, et al. Protein Kinase CK2 promotes proliferation, abnormal differentiation, and proinflammatory cytokine production of keratinocytes via regulation of STAT3 and Akt pathways in psoriasis. Am J Pathol. 2023;193(5):567–578. doi:10.1016/j.ajpath.2023.01.016
- Lerman G, Volman E, Sidi Y, et al. Small-interfering RNA targeted at antiapoptotic mRNA increases keratinocyte sensitivity to apoptosis. Br J Dermatol. 2011;164(5):947–956. doi:10.1111/j.1365-2133.2010.10191.x
- Tonel G, Conrad C. Interplay between keratinocytes and immune cells--recent insights into psoriasis pathogenesis. Int J Biochem Cell Biol. 2009;41(5):963–968. doi:10.1016/j.biocel.2008.10.022
- Zhu H, Hou L, Liu J, et al. MiR-217 is down-regulated in psoriasis and promotes keratinocyte differentiation via targeting GRHL2. Biochem Biophys Res Commun. 2016;471(1):169–176. doi:10.1016/j.bbrc.2016.01.157
- Jeon S, Song J, Lee D, et al. Inhibition of sphingosine 1-phosphate lyase activates human keratinocyte differentiation and attenuates psoriasis in mice. J Lipid Res. 2020;61(1):20–32. doi:10.1194/jlr.RA119000254
- Hong JH, Youm JK, Kwon MJ, et al. K6PC-5, a direct activator of sphingosine kinase 1, promotes epidermal differentiation through intracellular Ca2+ signaling. J Invest Dermatol. 2008;128(9):2166–2178. doi:10.1038/jid.2008.66
- Xu D, Wang J. Downregulation of cathepsin B reduces proliferation and inflammatory response and facilitates differentiation in human HaCaT keratinocytes, ameliorating IL-17A and SAA-induced psoriasis-like lesion. Inflammation. 2021;44(5):2006–2017. doi:10.1007/s10753-021-01477-0
- Lian N, Chen Y, Chen S, et al. Gasdermin D-mediated keratinocyte pyroptosis as a key step in psoriasis pathogenesis. Cell Death Dis. 2023;14(9):595. doi:10.1038/s41419-023-06094-3
- Yamamoto T. Angiogenic and inflammatory properties of psoriatic arthritis. ISRN Dermatol. 2013;2013:630620. doi:10.1155/2013/630620
- Patel AB, Tsilioni I, Weng Z, et al. TNF stimulates IL-6, CXCL 8 and VEGF secretion from human keratinocytes via activation of mTOR, inhibited by tetramethoxyluteolin. Exp Dermatol. 2018;27(2):135–143. doi:10.1111/exd.13461
- Benhadou F, Glitzner E, Brisebarre A, et al. Epidermal autonomous VEGFA/Flt1/Nrp1 functions mediate psoriasis-like disease. Sci Adv. 2020;6(2):eaax5849. doi:10.1126/sciadv.aax5849
- Nicchia GP, Stigliano C, Sparaneo A, et al. Inhibition of aquaporin-1 dependent angiogenesis impairs tumour growth in a mouse model of melanoma. J Mol Med. 2013;91(5):613–623. doi:10.1007/s00109-012-0977-x
- Zhang J, Ma WY. Nerve growth factor regulates the expression of vascular endothelial growth factor in human HaCaT keratinocytes via PI3K/mTOR pathway. Genet Mol Res. 2014;13(4):9324–9335. doi:10.4238/2014.January.24.14
- Niu X, Han Q, Li X, et al. EDIL3 influenced the αvβ3-FAK/MEK/ERK axis of endothelial cells in psoriasis. J Cell Mol Med. 2022;26(20):5202–5212. doi:10.1111/jcmm.17544
- Ghosh D, Ganguly T, Chatterjee R. Emerging roles of non-coding RNAs in psoriasis pathogenesis. Funct Integr Genomics. 2023;23(2):129. doi:10.1007/s10142-023-01057-5
- Grän F, Kerstan A, Serfling E, et al. Current developments in the immunology of psoriasis. Yale J Biol Med. 2020;93(1):97–110. doi:10.1038/jid.2012.339
- Ding Y, Gong P, Jiang J, et al. Mesenchymal stem/stromal cells primed by inflammatory cytokines alleviate psoriasis-like inflammation via the TSG-6-neutrophil axis. Cell Death Dis. 2022;13(11):996. doi:10.1038/s41419-022-05445-w
- Chen F, Wu S, Zhan J, et al. IL-22-induced ubiquitin-specific protease 15 promotes proliferation and inflammation of keratinocytes through stabilization of squamous cell carcinoma antigen 2. J Invest Dermatol. 2024;144(1):63–72.e4. doi:10.1016/j.jid.2023.07.006
- Xiao CY, Zhu ZL, Zhang C, et al. Small interfering RNA targeting of keratin 17 reduces inflammation in imiquimod-induced psoriasis-like dermatitis. Chin Med J. 2020;133(24):2910–2918. doi:10.1097/CM9.0000000000001197
- Bertelsen T, Ljungberg C, Boye Kjellerup R, et al. IL-17F regulates psoriasis-associated genes through IκBζ. Exp Dermatol. 2017;26(3):234–241. doi:10.1111/exd.13182
- Bertelsen T, Iversen L, Johansen C. The human IL-17A/F heterodimer regulates psoriasis-associated genes through IκBζ. Exp Dermatol. 2018;27(9):1048–1052. doi:10.1111/exd.13722
- Johansen C, Mose M, Ommen P, et al. IκBζ is a key driver in the development of psoriasis. Proc Natl Acad Sci U S A. 2015;112(43):E5825–E5833. doi:10.1073/pnas.1509971112
- Wang A, Wei J, Lu C, et al. Genistein suppresses psoriasis-related inflammation through a STAT3-NF-κB-dependent mechanism in keratinocytes. Int Immunopharmacol. 2019;69:270–278. doi:10.1016/j.intimp.2019.01.054
- Fan T, Wang S, Yu L, et al. Treating psoriasis by targeting its susceptibility gene Rel. Clin Immunol. 2016;165:47–54. doi:10.1016/j.clim.2016.03.009
- Gao Y, Lu J, Bao X, et al. Inhibition of phospholipases suppresses progression of psoriasis through modulation of inflammation. Exp Biol Med. 2021;246(11):1253–1262. doi:10.1177/1535370221993424
- Xiang X, Tu C, Li Q, et al. Oxymatrine ameliorates imiquimod-induced psoriasis pruritus and inflammation through inhibiting Heat Shock Protein 90 and Heat Shock Protein 60 expression in keratinocytes. Toxicol Appl Pharmacol. 2020;405:115209. doi:10.1016/j.taap.2020.115209
- Hsieh WL, Huang YH, Wang TM, et al. IFI27, a novel epidermal growth factor-stabilized protein, is functionally involved in proliferation and cell cycling of human epidermal keratinocytes. Cell Prolif. 2015;48(2):187–197. doi:10.1111/cpr.12168
- Deng Y, Chen J, Zhao Y, et al. Transdermal Delivery of siRNA through Microneedle Array. Sci Rep. 2016;6(1):21422. doi:10.1038/srep21422
- Dharamdasani V, Mandal A, Qi QM, et al. Topical delivery of siRNA into skin using ionic liquids. J Control Release. 2020;323:475–482. doi:10.1016/j.jconrel.2020.04.038
- Mandal A, Kumbhojkar N, Reilly C, et al. Treatment of psoriasis with NFKBIZ siRNA using topical ionic liquid formulations. Sci Adv. 2020;6(30):eabb6049. doi:10.1126/sciadv.abb6049
- Hsu T, Mitragotri S. Delivery of siRNA and other macromolecules into skin and cells using a peptide enhancer. Proc Natl Acad Sci U S A. 2011;108(38):15816–15821. doi:10.1073/pnas.1016152108
- Desmet E, Van Gele M, Grine L, Remaut K, Lambert J. Towards the development of a RNAi-based topical treatment for psoriasis: proof-of-concept in a 3D psoriasis skin model. Exp Dermatol. 2018;27(5):463–469. doi:10.1111/exd.13414
- Feng AP, He YM, Liu XX, et al. Expression of USP15, TβR-I and Smad7 in psoriasis. J Huazhong Univ Sci Technolog Med Sci. 2014;34(3):415–419. doi:10.1007/s11596-014-1293-1
- Vaher H, Kivihall A, Runnel T, et al. SERPINB2 and miR-146a/b are coordinately regulated and act in the suppression of psoriasis-associated inflammatory responses in keratinocytes. Exp Dermatol. 2020;29(1):51–60. doi:10.1111/exd.14049
- Rooney P, Connolly M, Gao W, et al. Notch-1 mediates endothelial cell activation and invasion in psoriasis. Exp Dermatol. 2014;23(2):113–118. doi:10.1111/exd.12306
- Ji X, Chen H, Xie L, et al. The study of GSDMB in pathogenesis of psoriasis vulgaris. PLoS One. 2023;18(1):e0279908. doi:10.1371/journal.pone.0279908
- Li R, Wang J, Wang X, et al. Increased βTrCP are associated with imiquimod-induced psoriasis-like skin inflammation in mice via NF-κB signaling pathway. Gene. 2016;592(1):164–171. doi:10.1016/j.gene.2016.07.066
- Kaikai S, Yuchen S, Lili J, Zhengtao W. Critical role of c-Jun N-terminal kinase in regulating bFGF-induced angiogenesis in vitro. J Biochem. 2011;150(2):189–197. doi:10.1093/jb/mvr060
- Ezure T, Amano S. Stanniocalcin-1 mediates negative regulatory action of epidermal layer on expression of matrix-related genes in dermal fibroblasts. Biofactors. 2019;45(6):944–949. doi:10.1002/biof.1547
- Qiu X, Zheng L, Liu X, et al. ULK1 inhibition as a targeted therapeutic strategy for psoriasis by regulating keratinocytes and their crosstalk with neutrophils. Front Immunol. 2021;12:714274. doi:10.3389/fimmu.2021.714274
- Xu M, Zhang Y, Cheng H, et al. Transcription factor 7-like 1 dysregulates keratinocyte differentiation through upregulating lipocalin 2. Cell Death Discov. 2016;2(1):16028. doi:10.1038/cddiscovery.2016.28
- Vegfors J, Ekman AK, Stoll SW, Bivik Eding C, Enerbäck C. Psoriasin (S100A7) promotes stress-induced angiogenesis. Br J Dermatol. 2016;175(6):1263–1273. doi:10.1111/bjd.14718
- Wang H, Ran LW, Hui K, et al. Survivin 和PI3K、AKT在寻常型银屑病皮损角质形成细胞中的表达及其相关性 [Expressions of survivin, PI3K and AKT in keratinocytes in skin lesions and their pathogenic role in psoriasis vulgaris]. Nan Fang Yi Ke Da Xue Xue Bao. 2017;37(11):1512–1516. Chinese. doi:10.3969/j.issn.1673-4254.2017.11.14
- Tu C, Wang S, Hu X, et al. Lipopolysaccharide induces TREM-1-dependent HIF-1α expression in human keratinocyte cell line. Cell Biol Int. 2016;40(12):1357–1365. doi:10.1002/cbin.10693
- Koegel H, von Tobel L, Schäfer M, et al. Loss of serum response factor in keratinocytes results in hyperproliferative skin disease in mice. J Clin Invest. 2009;119(4):899–910. doi:10.1172/JCI37771
- Gargalionis AN, Malakou LS, Adamopoulos C, et al. Polycystin-1 downregulation induces ERK-dependent mTOR pathway activation in a cellular model of psoriasis. Biochim Biophys Acta Mol Basis Dis. 2018;1864(10):3468–3476. doi:10.1016/j.bbadis.2018.07.036
- Tian R, Li Y, Yao X. PGRN Suppresses Inflammation and Promotes Autophagy in Keratinocytes Through the Wnt/β-Catenin Signaling Pathway. Inflammation. 2016;39(4):1387–1394. doi:10.1007/s10753-016-0370-y
- Shan Y, Zhao J, Wei K, et al. A comprehensive review of Tripterygium wilfordii hook. f. in the treatment of rheumatic and autoimmune diseases: bioactive compounds, mechanisms of action, and future directions. Front Pharmacol. 2023;14:1282610. doi:10.3389/fphar.2023.1282610
- Camela E, Potestio L, Fabbrocini G, Pallotta S, Megna M. The holistic approach to psoriasis patients with comorbidities: the role of investigational drugs. Expert Opin Investig Drugs. 2023;32(6):537–552. doi:10.1080/13543784.2023.2219387
